Abstract
γ-amino butyric acid (GABA) is the main transmitter mediating inhibitory synaptic transmission in the brain and is released not only from a subset of neurons but also from astrocytes. It has been well established that GABA is released via Ca2+- dependent exocytosis of GABA-containing vesicles in neurons. However, a novel form of GABA release in astrocytes via the Ca2+-activated anion channel, Bestrophin-1 (Best1), has been recently reported. In here, we reveal a novel anion channel-mediated GABA release mechanism in cultured cortical astrocytes pretreated with GABA. We have observed that cultured cortical astrocytes do not contain much GABA. We demonstrate in these same astrocytes, pretreated with GABA, that activation of the protease-activated receptor 1 (PAR1) produces an increase in intracellular Ca2+ concentration that leads to opening of Best1 channels and the subsequent release of GABA. These results provide strong molecular evidence for a potential astrocyte–neuron interaction via PAR1-induced and Best1-mediated GABA release.
Keywords:
Introduction
Astrocytes make direct contact with neurons and interact with them via tripartite synapses, in which astrocytic processes are in close association with the pre- and post-synapse at the synaptic junction (Araque et al. Citation1999; Grosche et al. Citation1999). Astrocytes respond to neuronal activity with an increase in intracellular Ca2+ ([Ca2+]i) (Wang et al. Citation2002; Haydon & Carmignoto Citation2006). The synaptically released neurotransmitters activate the corresponding Gαq-protein-coupled receptors expressed in astrocytes, resulting in an increase in [Ca2+]i and Ca2+-dependent release of gliotransmitters from astrocytes (Haydon & Carmignoto Citation2006; Halassa et al. Citation2007). It has recently been reported that GABA is enriched in Bergmann glia and lamellar astrocytes of the cerebellum and is released via the Best1 channel to cause tonic inhibition (Lee et al. Citation2010; Yoon et al. Citation2011). Even more remarkable is that the quantity of glial GABA content varies depending on brain region (Yoon et al. Citation2011). Bestrophin is responsible for Best vitelliform macular dystrophy (Best disease), a genetic eye disorder, and has been shown to encode a functional Ca2+-activated anion channel (CAAC; Hartzell et al. Citation2008). Best1 is directly activated by submicromolar concentrations of intracellular Ca2+ and has an anion selective pore with single channel activities (Hartzell et al. Citation2008). Best1 encodes for the CAAC in hippocampal astrocytes (Park et al. Citation2009) and channel-mediated astrocytic glutamate release via Best1 targets synaptic N-methyl-D-aspartate receptors (NMDARs) (Han et al. Citation2013) with high glutamate permeability and distal localization in CA1 hippocampal astrocytes (Park et al. Citation2013). In this study, we aim to determine the GABA content of cultured cortical astrocytes and to test whether Best1 is a Ca2+ -dependent GABA release mechanism in these cultured cells. To stimulate astrocytes in a physiological manner, we activated endogenous G-protein coupled receptors (GPCRs) by applying TFLLR (Thr-Phe-Leu-Leu-Arg peptide) (Hollenberg et al. Citation1997), a selective peptide agonist of the protease-activated receptor 1 (PAR1). Recent reports show that PAR1-induced glutamate release in cultured astrocytes is mediated by the Best1 channel (Oh et al. Citation2012). We demonstrate, in cultured cortical astrocytes, PAR1-induced GABA release following pretreatment with GABA is mediated by the activation of Best1, the GABA-permeable anion channel.
Materials and methods
Primary culture of cortical astrocytes
Mouse astrocyte cell cultures were prepared as previously described (Lee et al. Citation2007). The cerebral cortex from P0 to P2 postnatal C57BL/6 mice was dissected free of adherent meninges, minced and dissociated into a single cell suspension by trituration. Dissociated cells were plated onto 12-mm glass coverslips coated with 0.1 mg/ml poly-d-lysine. Cells were grown in Dulbecco Modified Eagle Medium (DMEM) supplemented with 25 mM glucose, 10% heat-inactivated horse serum, 10% heat-inactivated fetal bovine serum, 2 mM glutamine, and 1000 units ml−1 penicillin–streptomycin. Cultures were maintained at 37°C in a humidified atmosphere containing 5% CO2.
Immunocytochemistry
Cultured cortical astrocytes were fixed overnight in 4% paraformaldehyde at 4°C. After fixation, cells were washed three times in phosphate buffered saline (PBS). Cells were blocked in 0.1 M PBS containing 0.3% Triton X-100 (Sigma) and 2% serum from species of the secondary antibody for 1 h. Primary antibody was then applied at the appropriate dilution: Guinea pig anti-GABA antibody 1:1000 (Millipore Bioscience Research Reagents) and incubated overnight at 4°C. After incubation, cells were washed three times in PBS and then incubated in secondary antibody Alexa 555 goat anti-guinea pig IgG at 1:400 (Invitrogen) for 2 h. After three rinses in PBS, the cells on coverslips were mounted onto glass slides. Images were visualized with an Olympus FluoView FV1000 confocal microscope.
Sniffer-patch assays
Sniffer-patch assays were performed with calcium-sensitive Fura-2 imaging techniques in cultured cortical astrocytes on 3rd day after culture from P0 to P2 C57BL/6 mice and current recordings were made from Human Embryonic Kidney 293 T (HEK293T) cells expressing GABACR. In case of virus infection, astrocytes on 3rd day after culture are infected virus and incubated 3 days more, then 6th–7th days of culture were used to sniffer-patch assays. To induce GABA release from astrocytes, PAR1 was activated by pressure application of the peptide TFLLR. On the day of the experiment, cultured mouse astrocytes (HEK293T cells added) were incubated with 5 mM Fura-2 AM (mixed with 5 ml of 20% pluronic acid: Invitrogen) for 40 min, washed at room temperature, and subsequently transferred to the microscope stage for imaging. External solution contained (in mM): 150 NaCl, 10 HEPES, 3 KCl, 2 CaCl2, 2 MgCl2, and 5.5 glucose (pH adjusted to pH 7.3 and osmolarity adjusted to 325 mOsmol/kg). Images detected at a wavelength of 510 nm (emission wavelength) were taken at excitation wavelengths 340 nm and 380 nm, using an iXon high speed electron multiplying charge coupled device (EMCCD) camera (Andor). Two of the resultant images were used for ratio calculations with Imaging Workbench 6.2 software (INDEC Systems). GABACR-mediated currents were acquired from HEK293T cells under voltage clamp (Vh = −70 mV) using a MultiClamp 700B Amplifier and currents were digitized with pCLAMP 9.2 software (Molecular Devices). Recording electrodes (4–7 MΩ) were filled with (in mM): 110 Cs-gluconate, 30 CsCl, 0.5 CaCl2, 10 HEPES, 4 Mg-ATP, 0.3 Na3-GTP, and 10 BAPTA (pH adjusted to 7.3 with CsOH and osmolarity adjusted to 290–310 mOsmol/kg with sucrose). For simultaneous recordings, Imaging Workbench software use was synchronized with that of pCLAMP 9.2.
Measurement of the percentage of GABA-evoked current
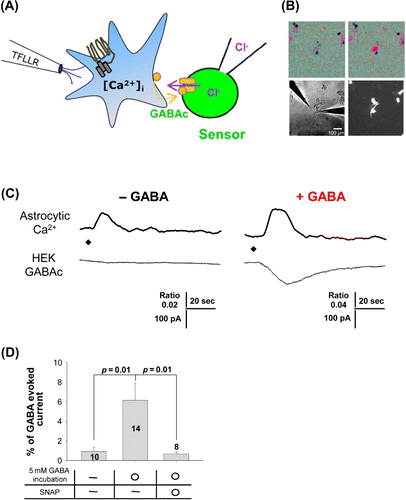
On completion of sniffer-patch experiments, different quantities of expression of GABAC receptors in sensor cells were normalized by adding 100 µM GABA to the bath to maximally activate GABA receptors on sensor cells. Measurement of this normalization was accomplished by dividing the response to GABA released from source cells by the current induced by bath application of GABA.
Best1 shRNA production
The Best1 nucleotide sequence from 774 to 793 (5-tttgccaacttgtcaatgaa-3) was selected for the target region of mouse Best1 (mBest1) shRNA. For lentivirus-based shRNA expression, mBest1 shRNA was synthesized as follows: 5-tttgccaacttgtcaatgaattcaagagatcattgacaagttggcaattttttc-3 and 5-tcgagaaaaaatcgcatagcgtatgccgtttctc ttgaaaacggcatacgctatgcgaa-3. The annealed double-stranded oligonucleotide was inserted into HpaI-XhoI restriction enzyme sites of the pSicoR lentiviral vector (Addgene) and verified by sequencing.
Results
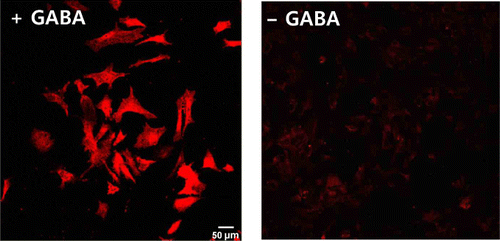
To determine the GABA content in cultured cortical astrocytes, we performed immunocytochemistry using an anti-GABA antibody. In a normal medium, cortical astrocytes have been shown to have low levels of GABA. However, following pretreatment with GABA, the GABA content increased excessively (). Therefore, we can guess that cortical astrocytes contain only a little GABA, without GABA uptake, and that they can accumulate extracellular GABA via the GABA transporter.
Next, we tested GABA release from cortical astrocytes when intracellular Ca2+ was increased by PAR1 agonist, TFLLR, with and without GABA pretreatment. We used the sniffer-patch technique in cultured cortical astrocytes and GABAC receptor-expressing HEK293T cells (). We monitored intracellular calcium signals from cortical astrocytes using Fura-2 AM imaging () and recorded GABAC receptor-mediated currents simultaneously. Without prior GABA treatment, TFLLR-induced intracellular Ca2+ increased but there was no GABA release from cortical astrocytes (, left). After pretreatment with 5 mM GABA for 20 min, the TFLLR-induced Ca2+ increase triggered GABA release from cortical astrocytes. This released GABA activated the GABAC receptor showing an inward current (, right). We also tested whether the GABA transporter was involved in GABA uptake by using the GABA transporter inhibitor, SNAP5114. When SNAP5114 was added to the culture medium prior to pretreatment with GABA, no significant GABA release was observed (). From these results, we confirmed that cortical astrocytes must uptake GABA from the extracellular region using the GABA transporter in order to induce the release of GABA when [Ca2+]i increases.
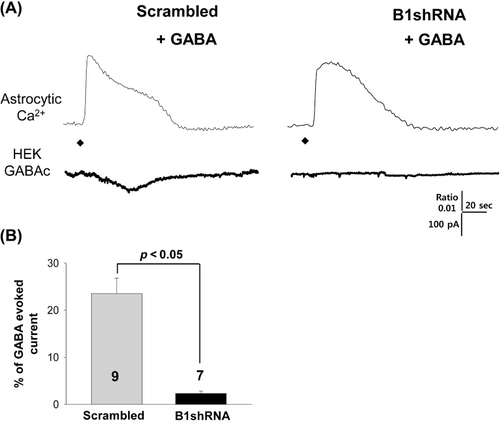
Finally, we investigated a possible mechanism for GABA release mediated by the Best1 channel in cortical astrocytes by gene silencing of Best1 using Best1 shRNA containing virus infection. Even after GABA pretreatment, Best1 shRNA-infected astrocytes do not release GABA when [Ca2+]i increases, while scrambled shRNA-infected astrocytes showed a huge release of GABA-induced current induced by TFLLR puffing (, ). It seems clear that cortical astrocytes employ the Best1 channel to release GABA. This Best1-mediated GABA release mechanism is consistent with our previous reports in cerebellar glial cells.
Discussion
In this study we show direct evidence for intracellular Ca2+-dependent Best1-mediated GABA release by PAR1 activation in cultured cortical astrocytes. However, in normal medium, cultured cerebellar glial cells possess a considerable amount of GABA and this astrocytic GABA can be released upon PAR1 activation without pretreatment of GABA (data not shown). This inconsistency may come from a natural variation in GABA content between glial cells and/or from different molecular machinery in different brain regions (Yoon et al. Citation2011).
We utilized the native GPCR, PAR1, which has been extensively used in numerous studies to selectively activate astrocytes. PAR1 has been shown to be expressed exclusively in astrocytes from human and rodent brain (Junge et al. Citation2004; Lee et al. Citation2007; Shigetomi et al. Citation2008) and to mediate neuron–glia interactions. Therefore, the source of PAR1-induced GABA is most likely the astrocyte, as a direct consequence of increased [Ca2+]i. Although to date, there is no direct evidence of the way in which PAR1 is activated in a physiological setting, recent studies show that the tPA-plasmin pathway is an endogenous PAR1 agonist (Mannaioni et al. Citation2008), suggesting that physiological PAR1 activation is initiated by the activation of the tPA-plasmin pathway under physiological conditions such as synaptic plasticity (Tomimatsu et al. Citation2002; Pang & Lu Citation2004).
Although the quantity of GABA in cortical astrocytes is small or absent, these astrocytes express GABA transporters that can take up extracellular GABA. Thus, if extracellular GABA is increased, more GABA can be taken up through these GABA transporters and be accumulated in cortical astrocytes, for subsequent release via the Best1 channel. A recent report shows that a high GABA content in reactive astrocytes in the dentate gyrus of a mouse model for AD results in increased tonic inhibition and memory deficit (Wu et al. Citation2014). In this AD model mouse, the increased GABA content comes from an enhanced activity of monoamine oxidase B (MAO-B), the GABA synthesizing enzyme of glia (Jo et al. Citation2014). Therefore, astrocytic GABA content may be increased through increased enzyme activity of MAO-B under certain pathological conditions.
In summary, we reveal a novel anion channel-mediated GABA release mechanism in cultured cortical astrocytes pretreated with GABA. The ideas and tools developed in this study should prove helpful to further our understanding of the physiological and pathological role of GABA release mechanisms and their functional significance.
Acknowledgements
This work was supported within the framework of the International Cooperation Program managed by the National Research Foundation of Korea (2013K2A2A4003724), the World Class Institute (WCI 2009-003) programs of the National Research Foundation (NRF) funded by the Korean Ministry of Science, Education and Technology (MEST), by the National Agenda Project (NAP) of the Korea Research Council of Fundamental Science and Technology (KRCF: NAP-09-04), and by an Internal Grant (2E24480) from the Korea Institute of Science and Technology (KIST).
References
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. 1999. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 22:208–215.10.1016/S0166-2236(98)01349-6
- Grosche J, Matyash V, Moller T, Verkhratsky A, Reichenbach A, Kettenmann H. 1999. Microdomains for neuron-glia interaction: parallel fiber signaling to Bergmann glial cells. Nat Neurosci. 2:139–143.10.1038/5692
- Halassa MM, Fellin T, Haydon PG. 2007. The tripartite synapse: roles for gliotransmission in health and disease. Trends Mol Med. 13:54–63.10.1016/j.molmed.2006.12.005
- Han KS, Woo J, Park H, Yoon BJ, Choi S, Lee CJ. 2013. Channel-mediated astrocytic glutamate release via Bestrophin-1 targets synaptic NMDARs. Mol Brain. 6:4.10.1186/1756-6606-6-4
- Hartzell HC, Qu Z, Yu K, Xiao Q, Chien LT. 2008. Molecular physiology of bestrophins: multifunctional membrane proteins linked to best disease and other retinopathies. Physiol Rev. 88:639–672.10.1152/physrev.00022.2007
- Haydon PG, Carmignoto G. 2006. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev. 86:1009–1031.10.1152/physrev.00049.2005
- Hollenberg MD, Saifeddine M, al-Ani B, Kawabata A. 1997. Proteinase-activated receptors: structural requirements for activity, receptor cross-reactivity, and receptor selectivity of receptor-activating peptides. Can J Physiol Pharmacol. 75:832–841.10.1139/y97-110
- Jo S, Yarishkin O, Hwang YJ, Chun YE, Park M, Woo DH, Bae JY, Kim T, Lee J, Chun H, et al. 2014. GABA from reactive astrocytes impairs memory in mouse models of Alzheimer's disease. Nat Med. Advanced Online Publication [E pub ahead of print].
- Junge CE, Lee CJ, Hubbard KB, Zhang Z, Olson JJ, Hepler JR, Brat DJ, Traynelis SF. 2004. Protease-activated receptor-1 in human brain: localization and functional expression in astrocytes. Exp Neurol. 188:94–103.10.1016/j.expneurol.2004.02.018
- Lee CJ, Mannaioni G, Yuan H, Woo DH, Gingrich MB, Traynelis SF. 2007. Astrocytic control of synaptic NMDA receptors. J Physiol. 581:1057–1081.10.1113/jphysiol.2007.130377
- Lee S, Yoon BE, Berglund K, Oh SJ, Park H, Shin HS, Augustine GJ, Lee CJ. 2010. Channel-mediated tonic GABA release from glia. Science. 330:790–796.10.1126/science.1184334
- Mannaioni G, Orr AG, Hamill CE, Yuan H, Pedone KH, McCoy KL, Berlinguer Palmini R, Junge CE, Lee CJ, Yepes M, et al. 2008. Plasmin potentiates synaptic N-methyl-D-aspartate receptor function in hippocampal neurons through activation of protease-activated receptor-1. J Biol Chem. 283:20600–20611.10.1074/jbc.M803015200
- Oh SJ, Han KS, Park H, Woo D, Kim HY, Traynelis SF, Lee CJ. 2012. Protease activated receptor 1-induced glutamate release in cultured astrocytes is mediated by Bestrophin-1 channel but not by vesicular exocytosis. Mol Brain. 5:38.
- Pang PT, Lu B. 2004. Regulation of late-phase LTP and long-term memory in normal and aging hippocampus: role of secreted proteins tPA and BDNF. Ageing Res Rev. 3:407–430.10.1016/j.arr.2004.07.002
- Park H, Han KS, Oh SJ, Jo S, Woo J, Yoon BE, Lee CJ. 2013. High glutamate permeability and distal localization of Best1 channel in CA1 hippocampal astrocyte. Mol Brain. 6:54.10.1186/1756-6606-6-54
- Park H, Oh SJ, Han KS, Woo DH, Mannaioni G, Traynelis SF, Lee CJ. 2009. Bestrophin-1 encodes for the Ca2+-activated anion channel in hippocampal astrocytes. J Neurosci. 29:13063–13073.10.1523/JNEUROSCI.3193-09.2009
- Shigetomi E, Bowser DN, Sofroniew MV, Khakh BS. 2008. Two forms of astrocyte calcium excitability have distinct effects on NMDA receptor-mediated slow inward currents in pyramidal neurons. J Neurosci. 28:6659–6663.10.1523/JNEUROSCI.1717-08.2008
- Tomimatsu Y, Idemoto S, Moriguchi S, Watanabe S, Nakanishi, H. 2002. Proteases involved in long-term potentiation. Life Sci. 72:355–361.10.1016/S0024-3205(02)02285-3
- Wang CM, Chang YY, Kuo JS, Sun SH. 2002. Activation of P2X(7) receptors induced [(3)H]GABA release from the RBA-2 type-2 astrocyte cell line through a Cl(-)/HCO(3)(-)-dependent mechanism. Glia. 37:8–18.10.1002/glia.10004
- Wu Z, Guo Z, Gearing M, Chen G. 2014. Tonic inhibition in dentate gyrus impairs long-term potentiation and memory in an Alzhiemer's disease model. Nat Commun. 5:4159.
- Yoon BE, Jo S, Woo J, Lee JH, Kim T, Kim D, Lee CJ. 2011. The amount of astrocytic GABA positively correlates with the degree of tonic inhibition in hippocampal CA1 and cerebellum. Mol Brain. 4:42.10.1186/1756-6606-4-42
