Abstract
The long-beaked common dolphin is one of the most abundant cetaceans in Korean waters, and their population has been estimated to comprise of more than 35,000 individuals. Delphinus capensis generally appear close to the coast and primarily feed on epipelagic small fishes and cephalopods. Thirty long-beaked common dolphins were collected from the East Sea from February to September in 2012. For stomach content analysis (SCA), the fresh prey items were identified to their lowest taxonomic level, and unidentified preys due to digestion were identified using remnants such as fish otoliths and cephalopod beaks. Fatty acid (FA) patterns of 20 dolphins from the inner layer of blubber were compared with those in samples of prey items. Enoploteuthis chunii was the dominant prey in SCA, representing 55.8% by number and 75.9% by occurrence. Common squid (Todarodes pacificus) and Pacific herring (Clupea pallasii) were the next major preys at more than 80% occurrence. Even though a distinctive difference was not observed between genders, there was a significant diet variation related to maturity. Immature dolphins consumed a higher diversity of prey, and consumed more equally than the sexually mature group, who showed a high dominance of cephalopods. Furthermore, this result fairly corresponded to FAs composition of mature dolphins with the raised percent of 20:6n–3, which is relatively abundant in T. pacificus.
Introduction
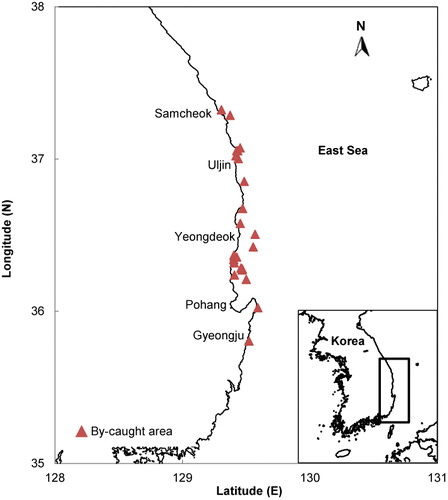
The long-beaked common dolphin, Delphinus capensis, is the member of the Delphinidae family of cetaceans. This species is distributed across world oceans, mostly in the tropical and temperate seas of the continental shelves in the Atlantic and the Pacific oceans (Perrin Citation2002). D. capensis generally appears within approximately 150 km of the coastline (Heyning & Perrin Citation1994) and exhibits long-term local residency compared to the short-beaked common dolphin (Bernal et al. Citation2003). Young and Cockcroft (Citation1994) elucidated that the distribution of common dolphins (Delphinus sp.) correlates with the distribution of their preferred prey species. Osnes-Erie (Citation1999) also demonstrated that D. capensis primarily feeds on epipelagic small fishes and cephalopods from by-caught data. In Korea, D. capensis is encountered mostly in the south-western part of the East Sea in all seasons and migrates in the form of a small subunit composed of about 20–30 or schools of 2000–3000 (Sohn et al. Citation2012). The by-catch of D. capensis frequently occurred around south region of the East Sea (An et al. Citation2004) and average of 220 common dolphins have been by-caught annually for the last 10 years (Unpublished data, the Cetacean Research Institute). Despite the high rate of annual by-catch in Korean waters, limited information was available because by-caught dolphins were generally used as food in Korea.
As one of the top predators, assessing the diet of dolphins is important to build our understanding of the ecological significance of both predator and prey in the marine food webs. Furthermore, knowledge of the diet of marine mammals gives us a better understanding of the potential interactions with fisheries. The traditional method of performing a diet study is a stomach content analysis (SCA), which allows a direct observation of prey. However, this method has provided limited information that only shows a representative of recent meals, owing to the inherent delay between feeding and a by-catch event (Hooker et al. Citation2001). Otoliths and beaks can be used to identify prey items in the case of severely digested stomach contents, because these tissues are relatively resistant to digestion and the morphological features of these are species-specific (Harkonen Citation1986).
Recently, the analysis of fatty acids (FAs) as a dietary tracer has increased in cetacean diet studies (Hooker et al. Citation2001). FA analysis for dietary study is based on the principle that the FA composition of prey will match that of the predator (Iverson Citation1993; Budge et al. Citation2006). Budge et al. (Citation2006) claimed that de novo synthesis, biosynthesis-like elongation or desaturation of FAs, is restricted to mammals foraging for a high-fat diets or fasting feeding. The FAs consumed by these predators are directly deposited into adipose tissues with little modification, so can ultimately provide an integrated record of diet (Iverson Citation1993; Budge et al. Citation2006). The essential FAs such as 20:5n–3 and 22:6n–3 are especially important dietary FAs, as marine mammals must obtain essential FAs from their diet, much like humans. Moreover, this method complements the disadvantages of SCA, because a long-term indication of diets taken over periods of up to months can be derived from FA analysis (Kirsch et al. Citation1998).
The overall aim of this study was to describe the diet of the long-beaked common dolphin in the East Sea, Korea. More specifically, the first objective was to document stomach contents, including the identification of otoliths and beaks. The secondary objective of this work was to determine whether the FAs obtained from blubber can be used as a diet indicator and to detect intra-specific variations in diet. Additionally, this study examined the difference in diet composition between the genders, ages, and reproductive states of D. capensis.
Material and methods
Sample collection
Thirty long-beaked common dolphins (16 males and 14 females) were collected from the Ganggu, Jukbyeon, Hupo, and Samcheock of the East Sea (). The sampled D. capensis were by-caught in gillnet, set net, and bladderwort from February to September in 2012. After a necropsy, SCA was conducted immediately. Stomach contents were collected and frozen at −19°C in a polyethylene bag. In case of otoliths and beaks, samples were stored in 70% ethanol for further identification. Teeth were sampled from the left mandibular ramus and were kept in 70% ethanol until age estimation. Blubber was taken at the mid-lateral region of the dorsal fin and approximately 5 cm × 5 cm in size was stored at −19°C in a polyethylene bag. The reproductive condition of D. capensis was determined by examining its gonads. Females were considered sexually mature if corpus luteum or corpus albicans was present in the ovaries (Lee Citation2011). Male specimens whose right testis weighed less than 150 g were classified as immature (Perrin et al. Citation1977).
Stomach content analysis
Stomach contents were placed in a large tray and then were separated into identifiable components. The residuals were washed gently through a sieve with a 1.0 mm mesh size in order to recover all fish otoliths and cephalopod beaks. The standard length of fishes and the mantle length of cephalopods were measured only for undigested items that were whole or nearly whole. Fresh prey items were identified to the lowest taxonomic level using published guides (Nakabo Citation2002; Youn Citation2002). Additional prey species were identified from the diagnostic hard parts such as fish otoliths and cephalopod beaks. The identification of fish otoliths was confirmed by the National Fisheries Research and Development Institute (NFRDI)'s otolith collection and specimens bought in the local market. To raise the reliability of otolith identification by direct comparison, DNA analysis was also conducted on uncontaminated muscle tissue of the fishes in stomach. The number of fishes in stomach was determined as a greater number of right or left otoliths (Pierce & Boyle Citation1991; Wang Citation2003). In the case of eroded but specifically identifiable otoliths, they were counted as half an otolith each. To prevent any bias, badly eroded and broken otoliths were not counted. Cephalopod beaks were identified following the manual and keys presented in National Museum of Natural Science (NMNS Citation2005; http://research.kahaku.go.jp/zoology/Beak-/index.htm). This was checked twice in the case of existence of the collected specimens. The higher number of upper or lower beaks was used to estimate the number of cephalopods (Pierce & Boyle Citation1991). The lower rostral length and lower hood length of the cephalopods were measured to the nearest 0.01 mm with digimatic calipers following the standards (Clarke Citation1986; Xavier & Cherel Citation2009) for identification.
Data analysis on prey importance
The percentage frequency of occurrence (%FO) and the percentage number (%N) were calculated. Additionally, the diet indices for prey diversity and evenness were analyzed using a mean N% of prey items (Krebs Citation1989). Percentage by number (%N), which is a measure of the numerical abundance of each prey species (%Ni) in the diet, was calculated as:
The Shannon–Wiener diversity index (H′) was calculated as:
Evenness (J′) was calculated as:
FA analysis
The 20 samples collected prior to August were used for FA analysis (). Lipids from the inner layer of blubber were extracted according to Folch et al. (Citation1957). FA methyl esters were prepared directly from 100 mg of the pure extracted lipid using 5 mL 14% boron trifluoride in methanol and 10 mL hexane. Analyses of FA methyl esters were performed using temperature-programmed gas liquid chromatography fitted with a Supelco 2560 column (100 m × 0.25 mm). The injection and detector temperatures were held at 250°C and 280°C, respectively. The FA composition of the three prey species that were abundant in the stomach and stand for each distribution zone and class (Clupea pallasii for pelagic fishes, Todarodes pacificus for cephalopods and Arctoscopus japonicus for mesopelagic fishes) was obtained from the reference of the NFRDI (Unpublished data, NFRDI). FAs that occurred at less than 1% were not included in the statistical analysis, because their determination was uncertain (Walton & Pomeroy Citation2003). The selected FAs were arcsin transformed prior to analysis for the requirements of multivariate analysis of variance (MANOVA). The SPSS program was used to identify the distinct difference among the groups based on the FA profiles.
Table 1. Biological data of by-caught D. capensis, East Sea, Korea.
Results
Stomach content analysis
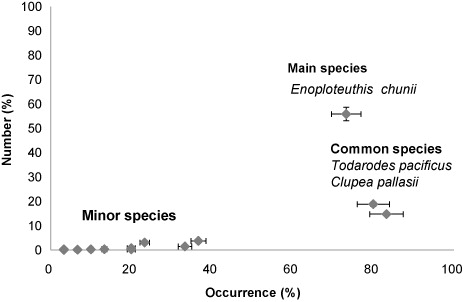
The stomach contents were collected from 14 female and 16 male long-beaked common dolphins. Most prey items were fairly well digested; therefore, hard parts (cephalopods beaks and fish otoliths) accounted for 90.3% of the total prey number. A total of 2954 prey items comprising eight species of fish and six species of cephalopods were identified by fish otoliths, squid beaks, and fresh fractions (). Cephalopods represented the most abundant prey group, accounting for 71.8% of the total number of prey items with a 96.6% occurrence. Fishes comprised 28.2% by number of the prey consumed and were found in 26 (82.8%) of the 29 stomachs. Enoploteuthis chunii was the dominant prey, representing 55.8% by number and 75.9% by occurrence. Common squid (T. pacificus) was the next major cephalopod prey, comprising 14.7% by number and occurred in 86.2% of the stomachs. Pacific herring (C. pallasii) was found predominantly among the fishes consumed, accounting for 18.8% by number and 82.8% by occurrence (). Only eight species were identified from fresh prey items. Six fish species' fresh remains comprised 10%N of the total number of fishes, and only two species of cephalopods were found in stomachs in an undigested condition (9%N). Otherwise, six species were identified by hard remains from fish otoliths and cephalopods beaks: Konoshiro gizzard shad (Konosirus punctatus), Sailfin sand fish (Arctoscopus japonicus), Mitra squid (Uroteuthis chinensis), Swordtip squid (Uroteuthis edulis), Big fin reef squid (Sepioteuthis lessoniana), and Schoolmaster gonate squid (Berryteuthis magister).
Table 2. Composition of stomach contents for D. capensis (n = 30).
Nine species among 12 DNA samples corresponded to species identified by otoliths. Two samples failed in the amplification process, and one was expressed as pacific herring that was Konoshiro gizzard shad by otolith identification.
The scatter plot divided the different prey species into three groups (). Eleven species were rarely found in the stomachs (occurrence < 40%) and occupied relatively small portions of the total prey number (%N). T. pacificus and C. pallasii were categorized as common preys as they were observed more than 80% stomachs. A single prominent prey species, E. chunii, was found. Although the occurrence percentage was slightly lower than T. pacificus and C. pallasii, more than half the number of total prey items were E. chunii (55.8%N).
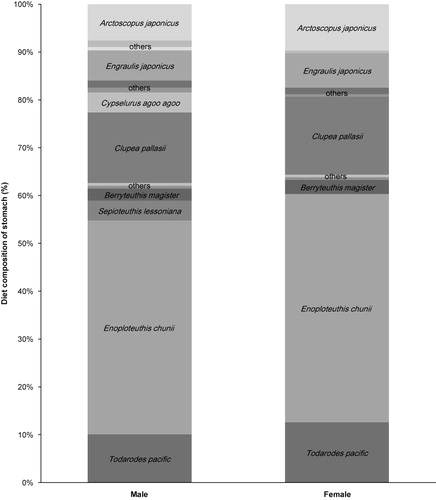
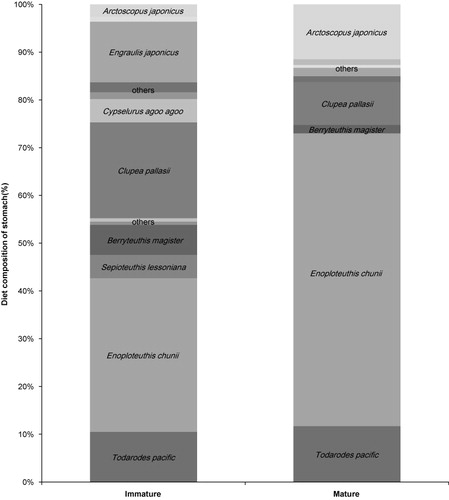
The factors “sex,” “maturity,” and “age” were tested to investigate dietary variation among the individuals. The prey composition for males was more diversified (14 taxa) than the females (11 taxa). Immature dolphins consumed 13 taxa of prey species, which were 4 more taxa than the mature dolphins (i.e. 9 taxa), and the diet composition of the mature groups showed a high dominance of E. chunii (). The prey diversity (H′) of the long-beaked common dolphin was 0.5632, while evenness (J′) was calculated to be 0.468. No significant difference was found between the males and females (t-test, p = 0.274). In the case of sexually maturity, immature common dolphins (H′ = 0.616) had higher prey diversity than mature individuals (H′ = 0.509), although small sample sizes prohibited the statistical difference (t-test, p = 0.254). Prey diversity was weakly correlated with age (r = −0.400, p = 0.041, n = 26). The significant difference of evenness (J′) was not found to be related to sex (t-test, p = 0.356). Likewise, the evenness difference between mature and immature, male for 0.4643 and female for 0.5311, was not significantly different (t-test, p = 0.7553). However, there was a significant correlation between age and evenness (r = −0.488, p = 0.011, n = 26).
Diet composition (number of prey species and number of individuals) of stomach in groups related to sex and maturity.FA analysis
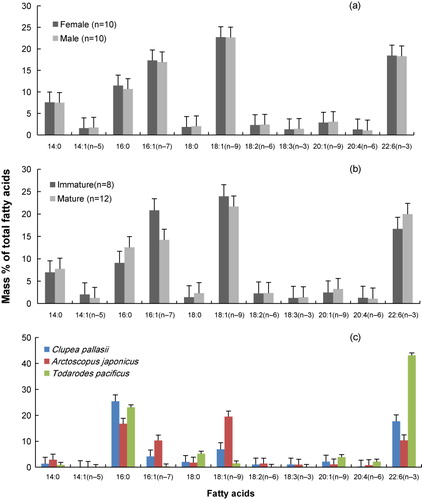
Thirty-six individual FAs were identified in the inner layer of the blubber samples. The major FAs were classified as present in amounts ≥1% by mass and only 12 major FAs were present: 14:0, 14:1n−5, 16:0, 16:1n−7, 18:0, 18:1n−9, 18:2n−6, 18:3n−3, 20:1n−9, 20:4n−6, 22:6n−3, and 24:0. ΣMUFA (monounsaturated unsaturated fatty acids) accounted for the greatest proportion of all FA examined, 44.36 ± 1.29% by mass. ΣSFA (saturated fatty acids), Σ(n−3)PUFA (polyunsaturated fatty acids), and Σ(n−6)PUFA each accounted for 27.67 ± 0.71, 20.01 ± 0.61, and 3.49 ± 0.12%, respectively, by mass of all the FAs. Σ(n−3)PUFA/(n−6)PUFA ratios were 5.74 ± 0.11 in the inner layer ().
Table 3. FAs commonly found in quantities ≥0.5% by mass.
No significant difference was observed in the proportions of the major FAs between the male and female the long-beaked common dolphins (MANOVA, p = 0.114; ). However, immature groups notably differed in FA composition and could be readily separated from mature groups using MANOVA analyses (p = 0.006). At the prey items, three species showed a large difference in FA composition, especially in 16:1n–7, 18:1n–9, and 22:6n–3 (). 22:6n–3 was predominately higher in T. pacificus than the other preys. Arctoscopus japonicus contained high levels of the FAs 16:1n–7 and 18:1n–9, while T. pacificus contained low levels of these FAs but high levels of 22:6n–3.
Discussion
The prey composition of the SCA agreed with the findings of previous studies that the long-beaked common dolphin feeds on the scrolling epipelagic fishes and neritic cephalopods (Osnes-Erie Citation1999). Cephalopods mainly accounted for diet composition, and E. chunii, a neritic-oceanic mesopelagic squid, was a prevalent prey to D. capensis. It is known that this prey is distributed in the western Pacific Ocean at approximately 20°N to 40°N, 120°E to 150°E (Jereb & Roper Citation2010) and migrates vertically, ascending to the surface shallower than 300 m at night. Unfortunately, E. chunii is not a target species for commercial fishing in Korea; therefore, its habitat and biomass are uncertain. Only six E. chunii were recorded in Korean waters by Son and Hong (Citation1992), and these specimens were collected by a mid-water trawler working at 200 m depth. In Japanese waters, E. chunii was known to be abundant in the mesopelagic zone, especially at depths of 15–200 m, and found as the prey of pollock (Okiyama, Citation1993). In addition, this cephalopod was reported as the prey of the spotted dolphin, Cuvier's beaked whale, and dwarf sperm whales in Taiwanese waters (Wang Citation2003). The luminescent organs of the family Enoploteuthidae may facilitate the feeding of dolphins and the large size of schools would be advantageous to dolphins by minimizing their energy-cost for increased foraging efficiency.
The composition of the diet mostly corresponds to the abundance of prey species in the environment. Even though there are no catch statistics for E. chunii, common squid and Pacific herring were reported as the most abundant species in the East Sea, based on their catch statistics (MIFAFF, Citation2012). The small sample size (n = 30) means that there is a limit to the usefulness of what we can infer about diet variation between the genders, ages, and maturity stages. Between the sex groups, there was no statistical difference in the prey diversity index (H′) or the FAs composition (MANOVA, p = 0.114). However, Cockcroft and Ross (Citation1990) suggested that cephalopods are consumed in greater number by lactating bottlenose dolphins, and presumably the high energetic demands of pregnancy or lactation should mean that reproductive dolphins require preys that are more specific. Compared to the sample sizes of previous researches (Osnes-Erie Citation1999, p. 94; Wang Citation2003, p. 45), 20 samples are small in number to determine the effect of gender on diet. Therefore, sufficient sampling that takes into account the reproductive status of both males and females is needed to detect any diet difference between genders.
Immature dolphins revealed a higher diversity of prey than sexually mature groups, and the statistical correlation between age and diversity index (H′) suggests that there is a meaningful diet change related to maturity. The decline in evenness and diversity can be interpreted as a concentration on specific preys. For example, the prey diversity of mature dolphins reduces as they get older, and the percentage composition of E. chunii increased. This trend was also stated by Meynier et al. (Citation2008); they found that mature short-beaked common dolphins exhibited less diversity in their prey composition, and that they focused on sardine feeding. The preference for cephalopods was distinctly revealed by SCA that estimated that the ratio of cephalopods to fishes in the diet was 55:45 for immature groups and 74:26 for mature groups (). Furthermore, this result fairly corresponded to the FAs composition of the mature dolphins, with the raised percentage of 20:6n–3. The FA patterns in the mature long-beaked common dolphins exhibited similar characteristics to those of T. pacificus, which also contained a relatively high value of 20:6n–3, and these were particularly different from the immature dolphins and Arctoscopus japonicus, which contained a high level of 16:7n–1 and 18:1n–9 (). Consequently, there was a distinct inference that the mature long-beaked common dolphins preferentially consumed cephalopods. This result also implies that the FA analysis can be used as a sufficiently reliable method to detect the inter-specific difference of diet.
The varied FA composition of D. capensis related to maturity () indicates that the diets of the long-beaked common dolphin change with growth. This difference was thought to result from the change of their energy needs and the development of their foraging skills depending on growth stage (Pusineri et al. Citation2007). Adult common dolphins feed cooperatively within large groups, but the calves of common dolphins were generally separated from the group during feeding (Burgess Citation2006). The increased composition of cephalopods with growth could be derived from the ability to dive deeper and the development of cooperative feeding skills as they grow.
There are possible biases in the estimation of a diet. A wrong estimate can be derived due to the variation in digestive rates between preys and the degree of acidity of stomach. Hence, only the forestomach was observed in this study to mitigate the effect of chemical digestion, because the digestive fluid was not secreted in the forestomach. Because otoliths are composed of calcium carbonate, they are easily broken, leading to the possibility of underestimation, owing to exclusion of the eroded and broken otoliths from the estimation. It is also likely that the secondary introduction of prey can also effect the diet estimation. Walker et al. (Citation2002) claimed that some otoliths that exhibited sharp angular chips and severe erosion were considered as having been secondarily introduced by cephalopods and larger fish. In this work, one isopoda and crustacean found in CRI 0015 were presumed to have been eaten by fishes or cephalopods, and then these specimens were ignored in diet estimation.
In conclusion, one of the marked results presented this study was the obvious dominance of E. chunii in the diet of the long-beaked common dolphin. However, the evaluated prey importance in the present work should not be considered a mass of preys (M%). Although E. chunii occupied 55.8% of the diet by number, their contribution to the total mass may be diminished due to their very small individual size. While the quantitative information about consumption was not suggested in this study, it is obvious that E. chunii, T. pacificus, and C. pallasii are important prey in the long-beaked common dolphin's diet. The energetic needs of D. capensis and the caloric value of prey items would require investigation in future study.
Acknowledgments
This study was supported by the NFRDI (RP-2013-FR-081). We thank Sukyong Kang at NFRDI for assistance of Otolith identification and Hyuck Joon Kwun at Pukyong National University for DNA work.
References
- An YR, Kim ZG, Sohns HS, Yang WS. 2004. By-catch of small cetaceans in the eastern coastal waters of Korea. J Korean Soc Fish Res. 6:163–172.
- Bernal R, Olabarra C, Moraga R. 2003. Occurrence and long-term residency of two long-beaked common dolphins, Delphinus capensis (Gray 1828), in adjacent small bays on the Chilean central coast. Aquat Mammal. 29:396–399. 10.1578/01675420360736587
- Budge SM, Iverson SJ, Koopman HN. 2006. Studying trophic ecology in marine ecosystems using fatty acid: a primer on analysis and interpretation. Mar Mammal Sci. 22:759–801. 10.1111/j.1748-7692.2006.00079.x
- Burgess EA. 2006. Foraging ecology of common dolphins (Delphinus sp.) in the Hauraki Gulf, New Zealand [ Master thesis]. Auckland: Massey University; p. 143.
- Clarke MR. 1986. A handbook for the identification of cephalopod beaks. Oxford: Clarendon Press.
- Cockcroft VG, Ross GJB. 1990. Food and feeding of the Indian Ocean bottlenose dolphin off southern natal, South Africa. In: Leatherwood S, Reeves RR, editors. Tthe bottlenose dolphin. San Diego (CA): Academic Press; p. 653.
- Folch J, Lees M, Sloane-Stanley GH. 1957. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 226:497–509.
- Härkönen T. 1986. Guide to the otoliths of the bony fishes of the northeast Atlantic. Hellurup (Denmark): Daubiu Aps; p. 256.
- Heyning JE, Perrin WF. 1994. Evidence for two species of common dolphins (genus Delphinus) from the eastern north Pacific. Contrib Sci. 442:1–35.
- Hooker SK, Iverson SJ, Ostrom P, Smith SC. 2001. Diet of northern bottlenose whales inferred from fatty-acid and stable-isotope analyses of biopsy samples. Can J Zool. 79:1442–1454. 10.1139/z01-096
- Iverson SJ. 1993. Milk secretion in marine mammals in relation to foraging: can milk fatty acids predict diet? Symp Zool Soc Lond. 66:263–291.
- Jereb P, Roper CFE. 2010. Cephalopods of the world: an noted and illustrated catalogue of cephalopod species known to date. Vol. 2: Myopsid and oegopsid squids. FAO Species Catalogue for Fishery Purposes. 4:186.
- Kirsch PE, Iverson SJ, Bowen WD, Kerr SR, Ackman RG. 1998. Dietary effects on the fatty acid signature of whole Atlantic cod (Gadus morhua). Can J Fish Aquat Sci. 55:1378–1386. 10.1139/f98-019
- Krebs CJ. 1989. Ecological methodology. Menlo Park (CA): Benjamin/Cummings; p. 654.
- Lee YR. 2011. Age, growth and sexual maturity of the finless porpoise, Neophocaena asiaeorientalis, in the Yellow Sea, Korea [ Master thesis]. Busan (Korea): Pukyoung National University; p. 61.
- Meynier L, Pusineri C, Spitz J, Santos MB, Pierce GJ, Ridoux V. 2008. Intraspecific dietary variation in the short-beaked common dolphin Delphinus delphis in the Bay of Biscay: importance of fat fish. Mar Ecol Prog Ser. 354:277–287. 10.3354/meps07246
- MIFAFF. 2012. Statistical Database for Fisheries Production. [Internet]. Ministry for Food, Agriculture, Forestry and Fisheries, Korea; [ cited 2012 Sep 10]. Available from: http://www.fips.go.kr/
- Nakabo T. 2002. Fishes of Japan with pictorial keys to the species. ( English edition). Tokyo: Tokai University Press; p. 1749.
- NMNS. 2005. Manual for identification of cephalopods beaks in the Northwest Pacific [Internet]. Tokyo: National Museum of Nature and Science; [ cited 2012 Sep 10]. Available from: http://research.kahaku.go.jp/zoology/Beak-E/index.htm
- Okiyama M. 1993. Kinds, abundance and distribution of the oceanic squids in the Sea of Japan. In: Okutai T, O'Dor RK, Kubodera T, editors. Recent advances in cephalopod fisheries biology. Tokyo: Tokai University Press; p. 403–415.
- Osnes-Erie LD. 1999. Food habits of common dolphin (Delphinus dolphin and D.capensis) off California [ Master thesis]. San Jose (CA): San Jose State University; p. 56
- Perrin WF. 2002. Common dolphins. In: Perrin WF, Würsig B, Thewissen GM, editors. Encyclopedia of marine mammals. San Diego (CA): Academic Press; p. 245–248.
- Perrin WF, Holts DB, Miller RB. 1977. Growth and reproduction of the eastern spinner dolphin, a geographical form of Stenella longirostris in the eastern tropical Pacific. Fish Bull. 75:725–750.
- Pierce GJ, Boyle PR. 1991. A review of methods for diet analysis in piscivorous marine mammals. Oceanogr Mar Biol. 29:409–486.
- Pusineri C, Magnin V, Meynier L, Spitz J, Hassani S, Ridoux V. 2007. Food and feeding ecology of the common dolphin (Delphinus delphis) in the oceanic Northeast Atlantic and comparison with its diet in neritic areas. Mar Mammal Sci. 23:30–47. 10.1111/j.1748-7692.2006.00088.x
- Sohn HS, Park KJ, An YR, Choi SG, Kim ZG, Kim HW, An DH, Lee YR, Park TG. 2012. Distribution of whales and dolphins in Korean waters based on a sighting survey from 2000 to 2010. Kor J Fish Aquat Sci. 45:486–492.
- Son MH, Hong SY. 1992. A short note on Enoploteuthis (Paraenoploteuthis) chunii (Cephalopoda: Enoploteuthidae) from the Korean waters. Contributions of the Korea Institute of Ocean Science of National Fisheries, University of Pusan. 25:255–259.
- Walker WA, Mead JG, Brownell RL. 2002. Diets of Baird's beaked whales, Berardius bairdii, in the southern sea of Okhotsk and off the pacific coast Of Honshu, Japan. Mar Mammal Sci. 18:902–919. 10.1111/j.1748-7692.2002.tb01081.x
- Walton M, Pomeroy P. 2003. Use of blubber fatty acid profiles to detect inter-annual variations in the diet of grey seals Halichoerus grypus. Mar Ecol Prog Ser. 248:257–266. 10.3354/meps248257
- Wang MC. 2003. Feeding Habits of the Pantropical Spotted Dolphin, Stenella attenuata, off the Eastern Coast of Taiwan. Zool Stud. 42:368–378.
- Youn CH. 2002. Fishes of Korea with pictorial key and systematic list. Seoul (Korea): Academic Seojeok.
- Young DD, Cockcroft V. 1994. Diet of common dolphins (Delphinus delphis) off the south-east coast of southern Africa: opportunism or specialization? J Zool. 234:41–53. 10.1111/j.1469-7998.1994.tb06055.x
- Xavier JC, Cherel Y. 2009. Cephalopod beak guide for the southern ocean. Cambridge (UK): British Antarctic Survey; p. 129.