Abstract
The pathophysiology underlying anxiety disorders involves stress and associated increases in glucocorticoid levels in the blood. The present study investigated the effects of the administration of the crude extract of Polygala tenuifolia (EPT) on repeated restraint stress–induced anxiety-like behavioral responses in mice. Additionally, possible changes in the central noradrenergic and brain-derived neurotrophic factor (BDNF)–signaling systems were assessed in the hippocampus of these mice. For 14 consecutive days, mice were orally administrated EPT (50 or 250 mg/kg) 30 min prior to the restraint stress procedure (2 h/day). Dysregulation within the hypothalamic-pituitary-adrenal (HPA) axis in response to the repeated restraint stress was confirmed by measuring serum levels of corticosterone and the expression of corticotrophin-releasing factor in the hypothalamus. Compared with control subjects, the daily administration of higher doses of EPT (250 mg/kg) increased open-arm exploration in an elevated plus maze and the total number of line crossings in an open-field test. EPT also reversed the increased expression of tyrosine hydroxylase in the locus coeruleus and the decreased expression of BDNF mRNA in the hippocampus. Together, these findings demonstrate that the administration of EPT prior to repeated restraint stress significantly reduced anxiety-like behaviors in mice. These changes were associated with a modification of the central noradrenergic system and the upregulation of BDNF expression, which in turn attenuated activity in the HPA axis.
Introduction
The hypothalamic-pituitary-adrenal (HPA) axis is a key endocrine adaptor activated by various stressors that result in the increased concentration of glucocorticoids (GC) in circulating blood (Huang et al. Citation2011). Under normal conditions, blood GC levels are regulated tightly by negative feedback mechanisms within the HPA axis, but patients with anxiety disorders typically exhibit high blood GC concentrations due to dysfunction in this system caused by chronic stress (Watson and Mackin Citation2007). Thus, the HPA axis and its negative feedback mechanisms play an important role in the pathophysiology of stress-related psychiatric diseases, including anxiety disorders (Lim et al. Citation2012). Alterations within this system have a serious influence on affective behaviors in humans, which are mimicked by the presence of anxiety-like symptoms in rodents exposed to stressful situations (Chiba et al. Citation2012). Repeated restraint stress in rodents increases plasma corticosterone (CORT) concentrations, inhibits exploratory behavior, enhances freezing behavior during fear conditioning, and induces changes in brain-derived neurotrophic factor (BDNF) mRNA and protein expression in the hippocampus (Garcia et al. Citation2009). Thus, repeated restraint stress–induced physiological stress resulting from HPA axis dysregulation increases anxiety-like behaviors (Garcia et al. Citation2009).
Herbal extracts and their pharmacological components have been known to alleviate anxiety-related behaviors (Mizoguchi et al. Citation2009), but the mechanisms underlying their ameliorative activity are unclear. The roots of Polygala tenuifolia (PT), which is a traditional medicinal herb in Korea, have been widely reported to benefit multiple physiological actions and to produce a variety of biological effects in the peripheral nervous system and the central nervous system (CNS; Kawashima et al. Citation2004; Kim et al. Citation2013). Experimental animal models have shown that PT also exerts antidepressant-like effects and that its underlying mechanisms are likely involved in modulations of the BDNF signaling pathway in the hippocampus (Kawashima et al. Citation2004; Hu et al. Citation2010). Furthermore, PT improves hippocampal-dependent learning and memory impairments following scopolamine-induced amnesia via modulation of the HPA axis and activation of BDNF signaling pathways in mice (Sun et al. Citation2007; Choi et al. Citation2011). Thus, the crude extract of PT (EPT) may alleviate anxiety-like behaviors in a stress-induced anxiety animal model. However, the degree to which EPT alleviates the anxiety-like symptoms induced by repeated restraint stress via regulation of the central noradrenergic system and the BDNF signaling pathway in mice remains unclear.
Thus, the aim of the present study was to investigate the influence of EPT administration on anxiety-related symptoms in mice exposed to repeated restraint stress using the elevated plus maze (EPM) test and the open-field test (OFT). Additionally, the underlying neurobiological mechanisms of these behavioral effects within the central noradrenergic and BDNF signaling systems in the CNS have also been investigated.
Materials and methods
Animals
The present study utilized 12-week-old male BALB/c mice weighing 28–30 g (Samtako Animal Company, Seoul, Korea). All mice were housed in a limited-access rodent facility with seven or eight mice per polycarbonate cage. The room controls were set to maintain the temperature at 22°C ± 2°C and the relative humidity at 55% ± 15%, the cages were lit by artificial light for 12 h each day, and sterilized drinking water and a standard chow diet were supplied ad libitum throughout the study. All animal experiments began a minimum of 7 days after the animals arrived.
Experimental groups
The mice were randomly divided into five groups of eight animals as follows: unstressed group treated daily with 0.9% saline (NOR; n = 8), restraint-stressed group treated daily with saline (STR; n = 8; negative control), restraint-stressed group treated daily with 50 mg/kg EPT (STR + EPT50; n = 8), restraint-stressed group treated daily with 250 mg/kg EPT (STR + EPT250; n = 8), and restraint-stressed group treated daily with 10 mg/kg fluoxetine (FLX) hydrochloride (Sigma-Aldrich Chemical Co, St. Louis, MO, USA; STR + FLX; n = 8; positive control). EPT was supplied by Dr. Shim in Kyung Hee University. Saline and EPT were orally administered 30 min prior to the daily restraint stress for 14 consecutive days. FLX was administered intraperitoneally (i.p.) 30 min prior to the daily restraint stress for 14 consecutive days. EPT and FLX were dissolved in a 0.9% physiological saline solution.
The restraint stress procedure was conducted once daily for 14 consecutive days for 2 h from 10:00 am to 12:00 pm using rodent immobilization tubes. The mice were forcibly placed into transparent plastic tubes (3 × 11 cm) that had one open end and one conical end with breathing holes. The animals received ample air but were unable to move within the tubes. Changes in body weight gain (measured at the initial step of the restraint stress) and serum CORT levels (measured subsequent to the expression of repeated restraint stress–induced depression-like symptoms) were assessed throughout the experiment to monitor the development of psychosomatic symptoms. Behavioral testing for anxiety-like behaviors was done 24 h after the end of the chronic physiological stress protocol.
EPM test
The EPM is a widely used behavioral measure that assesses the anxiogenic or anxiolytic effects of pharmacological agents. Animals exhibiting anxiety-like behaviors typically experience a reduced number of entries and amount of time spent in the open arms of the maze, and thus, an increased amount of time in the closed arms of the maze. The EPM apparatus used in the present study consisted of two open arms (16.5 × 5.5 cm), two closed arms (16.5 × 5.5 × 12.5 cm), and a central platform (5.5 × 5.5 cm) arranged such that the open arms and closed arms were directly opposite each other. The EPM apparatus was constructed from black Plexiglas and elevated 20 cm above the floor. At the beginning of each trial, the subjects were placed in the center of the maze facing a closed arm, and the following parameters were recorded during the 5-min test period: number of open-arm entries, number of closed-arm entries, time spent in open arms, and time spent in closed arms. Behavior in the maze was recorded by a video camera mounted on the ceiling above the center of the maze with the S-MART program (PanLab Co., Barcelona, Spain).
Open-field test
Prior to the EPM test, all mice were placed individually in a rectangular container made of dark polyethylene (40 × 40 × 25 cm), which provided the best contrast for the white mice in a dimly lit room, and all locomotor activity was measured by a video camera mounted above the center of the maze. The speed and distance of the movements of the subject were monitored by a computerized video-tracking system using the S-MART program (Panlab Co.). Following a habituation period of 5 min, the total distance traveled in the container (measured in cm) and the number of line crossings (with all four paws) across defined squares for an additional 5 min were recorded.
CORT measurement
For this, the unanesthetized mice were rapidly decapitated, and blood was quickly collected via the abdominal aorta. Mice were randomly divided into five groups consisting of four individuals each, the same as above. The blood samples were centrifuged at 4000g for 10 min, and serum was collected. The CORT concentration was measured by a competitive enzyme-linked immunosorbent assay (ELISA) using a rabbit polyclonal CORT antibody (Novus Biologicals Corticosterone kit; Novus Biologicals, LLC, Littleton, CO, USA) according to the manufacturer's protocol.
Immunohistochemical analyses of corticotropin-releasing factor and tyrosine hydroxylase
To conduct the immunohistochemical analyses, four mice from each group were deeply anesthetized with sodium pentobarbital (80 mg/kg, i.p.) and perfused through the ascending aorta with 0.9% saline followed by 300 mL of 4% paraformaldehyde in 0.1 M phosphate-buffered saline (PBS). The brains were removed in a randomized order, post-fixed overnight, and cryoprotected with 20% sucrose in 0.1 M PBS at 4°C. Coronal sections (30 μm) were cut through the hypothalamus and locus coeruleus (LC) using a cryostat (Leica CM1850; Leica Microsystems Ltd., Nussloch, Germany). Briefly, the sections were incubated with primary goat anti-corticotrophin-releasing factor (CRF) antibody (1:500 dilution; Santa Cruz Biotechnology Inc., Santa Cruz, California, CA, USA) and primary sheep anti-TH antibody (1:2000 dilution; Chemicon International Inc. Temecula, CA, USA) for 72 h at 4°C. Next, the sections were incubated for 120 min at room temperature with secondary antibodies (1:200 dilution; Vector Laboratories Co., Burlingame, CA, USA). To visualize immunoreactivity, the sections were incubated for 90 min in ABC reagent (Vectastain Elite ABC kit; Vector Laboratories Co.), and then in a solution containing 3,3′-diaminobenzidine (Sigma-Aldrich Chemical Co.) and 0.01% H2O2 for 1 min. The numbers of CRF- and TH-labeled cells in the stained sections of the hypothalamus and LC were quantified at a magnification of 200 × by an observer blinded to the experimental groups.
Total RNA preparation and reverse transcription–polymerase chain reaction
The mRNA expression levels of BDNF in the hippocampal tissues isolated from four mice per group were determined using reverse transcription–polymerase chain reaction (RT-PCR). Following decapitation, the brains were removed quickly and stored at −80°C until use. Total RNA was prepared from the brain tissue samples using TRIzol® reagent (Invitrogen Co., Carlsbad, CA, USA) according to the supplier's instructions. Briefly, complementary DNA was synthesized from total RNA using reverse transcriptase (Takara Co., Shiga, Japan). Then, RT-PCR was performed using a PTC-100 programmable thermal controller (MJ Research, Inc., Watertown, MA, USA). All primers were designed using published mRNA sequences and primer designing software (Primer 3; Whitehead Institute for Biomedical Research, Cambridge, MA, USA). The PCR products were separated on 1.2% agarose gels and stained with ethidium bromide, and the density of each band was quantified using an image-analyzing system (i-MaxTM; CoreBio System Co., Seoul, Korea). The expression levels of each were compared by calculating the relative density of the target band, such as BDNF, with that of GAPDH.
Statistical analysis
All measurements were performed by an independent investigator blinded to the experimental conditions, and the results are expressed as means ± standard error of the means. Differences within or between normally distributed data were analyzed using an analysis of variance using SPSS (Version 13.0, SPSS, Inc., Chicago, IL, USA) and a Tukey's post hoc test. A p value of <.05 was considered to indicate statistical significance.
Results
Effects of EPT on repeated restraint stress–induced changes in body weight and increased serum CORT levels
In the present study, mice exposed to repeated restraint stress began to lose body weight on the first day of the procedure. Body weight was assessed daily for 14 consecutive days to identify whether subjects in the repeated restraint stress group (STR) would exhibit body weight loss (difference between starting weight and daily weights; ). Compared with the NOR group, subjects in the STR group showed a significant gradual reduction in body weight gain over 14 days. However, subjects treated with 250 mg/kg EPT exhibited a significant inhibition of this reduction in body weight gain compared with the STR group (p < .05 on Day 11; p < .01 on Day 13).
Note: *p < .05, **p < .01 versus NOR group, #p < .05 versus STR group.
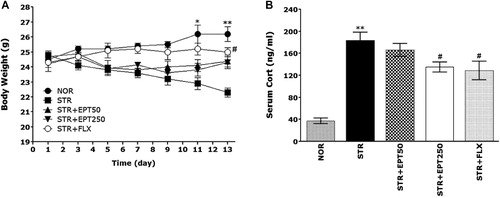
Serum CORT levels were also measured in each group following repeated restraint stress for 14 days. An ELISA revealed that repeated restraint stress significantly increased serum CORT concentrations by 434.88% compared with saline-treated mice (; p < .01). However, the administration of EPT significantly inhibited the repeated restraint stress–induced increase in serum CORT levels (p < .05).
Effects of EPT on repeated restraint stress–induced anxiety-like behavior
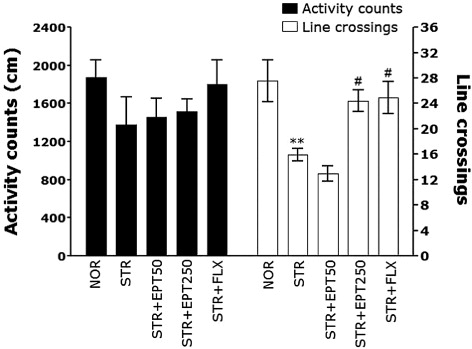
The effects of EPT administration on anxiety-like behaviors, which were characterized by decreased open-arm exploration in the EPM, were also investigated (). Post hoc comparisons revealed a significant decrease in the percentage of time spent in the open arms of the maze by mice exposed to repeated restraint stress for 14 days compared with saline-treated mice not exposed to stress (NOR; p < .001). However, although the findings were only minimally significant, the STR + EPT250 group exhibited a slight increase in the percentage of time spent in the open arms of the maze, which had previously decreased following repeated restraint stress, compared with the STR group ().
Note: ***p < .001 versus NOR group, #p < .05 versus STR group.
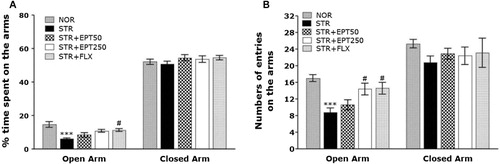
Mice in the STR + EPT250 group entered the open arms of the EPM significantly more times than did mice in the STR group (p < .05; ). Because there were no significant differences in the percentage of time spent or the number of entries into the closed arms among the groups, the observed anxiety-like behaviors of the mice exposed to repeated restraint stress were likely not attributable to differences in their locomotor activities ( and ). These findings indicate that the increase in the number of entries into the open arms of the maze in the STR + EPT250 group was comparable to the number of open-arm entries in the STR + FLX group.
Effects of EPT on motor function and exploratory behavior following repeated restraint stress
The open-field activity of mice in the present study was used to evaluate locomotor activity and exploratory behavior among subjects receiving repeated restraint stress for 14 days (). There were no significant differences in locomotor activity (motor function) during the OFT among groups, but mice exposed to repeated restraint stress (STR) displayed a significant decrease in the total number of line crossings compared with the NOR group (p < .01). However, there was a significant increase in the total number of line crossings by EPT-treated mice (250 mg/kg) compared with mice in the STR group (p < .05), indicating that anxiety-like behaviors in the STR + EPT250 group were comparable to those of the STR + FLX group.
Effects of EPT on repeated restraint stress–induced CRF- and TH-like immunoreactivities
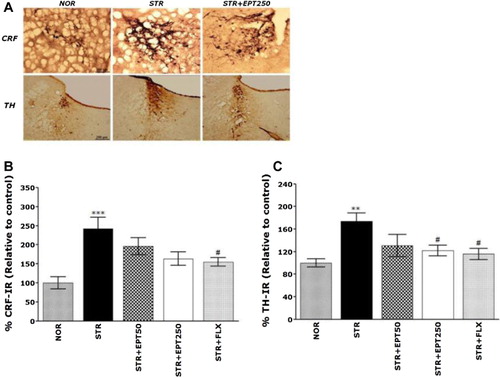
After the behavioral measures were completed, CRF-like immunoreactivity was analyzed in the cell bodies of various hypothalamic regions, including the paraventricular nucleus (PVN; ). Compared with the NOR group, CRF-immunoreactive cells in the PVN of the STR group increased by 242.56%, which indicates that repeated restraint stress results in a significant increase in CRF expression (p < .001; ). Compared with the STR group, there was a slight decrease in the number of CRF-immunoreactive neurons in the PVN region of the STR + EPT250 group, although this result was only minimally significant.
TH-like immunoreactivity was also analyzed in central noradrenergic regions, including the LC (). Compared with the NOR group, TH-immunoreactive cells in the LC of the STR group increased by 174.25%, indicating that repeated restraint stress results in a significant increase in TH expression (p < .01; ). Compared with the STR group, there was a significant decrease in the number of TH-immunoreactive neurons in the central noradrenergic region of the STR + EPT250 group (p < .05).
Effects of EPT on the repeated restraint stress–induced expression of BDNF mRNA in the hippocampus
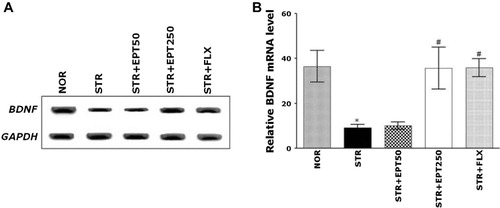
The effects of EPT administration on the expression of BDNF mRNA in the hippocampus of mice exposed to repeated restraint stress were investigated using RT-PCR (). There was a significant decrease in the hippocampal BDNF mRNA expression in the STR group compared with the NOR group (p < .05). The decreased expression of BDNF mRNA in the STR group was significantly restored to levels similar to those in the NOR group by 250 mg/kg EPT (STR + EPT250; p < .05). This indicates that the expression of BDNF mRNA in the hippocampus of mice receiving 250 mg/kg EPT was similar to that of mice receiving 10 mg/kg FLX.
Discussion
Using two behavioral measures of anxiety, the EPM and OFT, the present findings clearly demonstrate for the first time that EPT exerts anxiolytic effects. The administration of EPT prior to repeated restraint stress significantly increased open-arm exploration in the EPM and decreased the time spent in the corners of the OFT. The effects of EPT (250 mg/kg) were similar to those observed following FLX treatment. It seems likely that these behavioral effects are based on modifications of the central noradrenergic system and the prevention of attenuated BDNF expression in the hippocampus, which underlie the development of anxiety (Sun et al. Citation2007; Choi et al. Citation2011). In the present investigation of mice exposed to repeated restraint stress, EPT alleviated stress-induced behavioral abnormalities and corresponding changes in the HPA axis (Kawashima et al. Citation2004; Hu et al. Citation2010). Based on these findings, it is suggested here that that the administration of EPT prior to repeated restraint stress produces anxiolytic effects and that EPT is a candidate therapeutic agent for the treatment of psychiatric diseases, including anxiety.
Body weight and serum levels of CORT were measured to investigate the physiological responses of the body, including the HPA axis, to EPT. The present findings demonstrate that the administration of higher doses of EPT resulted in a significant inhibition of the reduced body weight gain and increased serum CORT levels. This suggests that treatment with EPT inhibits HPA axis–associated psychological dysfunction and may influence endogenous CORT levels in the CNS, which in turn would normalize behavioral and neurochemical responses. Similarly, in the present study, serum CORT levels in mice treated with FLX prior to the EPM test and OFT were lower than those of controls, consistent with the findings of previous studies (Gregus et al. Citation2005; Nacher et al. Citation2005).
The administration of EPT produced anxiolytic-like effects in an animal model of anxiety. In the present study, the administration of a higher dose of EPT (250 mg/kg) prior to repeated restraint stress significantly reduced anxiety-like behaviors in the EPM in terms of increased entries into open arms, but not in the percentage of time spent in them. This suggests that there was an increase in locomotor activity rather than merely an increase in the time spent in the end of the open arms of the EPM. The present findings are consistent with those of previous studies showing that FLX treatment increases the percentage of total entries and time spent in the open arms of the EPM (Lee et al. Citation2012). However, in the present study, a lower dose of EPT (50 mg/kg) did not have any anxiolytic effects. Thus, the present findings suggest that the decrease in anxiety-related behaviors following the administration of a higher dose of EPT may be related to an amelioration of the dysregulation in the HPA axis induced by an increased CORT response following chronic stress.
Also, the OFT was conducted to evaluate the locomotor–exploratory behaviors of mice exposed to chronic stress and to rule out any confounding motor impairments that may have influenced performance in the EPM (Lee et al. Citation2012). However, the administration of EPT prior to repeated restraint stress significantly reduced anxiety-like behavior in the OFT, as indicated by an increase in the total number of line crossings. This increase may reflect a reduction in anxiety as well as an enhanced drive to explore. Additionally, the present findings suggest that the increase in the total number of line crossings in the OFT mirrored changes in behavioral performance in the EPM. Thus, these changes were likely due to an enhanced motivation to explore and the anxiolytic effects of EPT.
In mice subjected to repeated restraint stress in the present study, the administration of EPT slightly blocked increases in CRF immunoreactivity in the PVN but significantly decreased TH-like immunoreactivity in the LC. The TH enzyme is an important player in the stress-induced activation of the CNS and in stress-related psychopathological conditions, including anxiety (Park et al. Citation2010). Therefore, the administration of EPT may regulate increases in anxiolytic-like behaviors in the EPM and OFT via the increased expression of TH in the LC, which is consistent with previous studies (Park et al. Citation2010). Together, the present findings suggest that EPT attenuated the behaviors and neurochemical responses associated with anxiety by modulating the HPA axis and the central noradrenergic system. This suggests that the administration of EPT, like FLX, indirectly alters catecholamine synthesis in the brain and produces physiological effects (Lee et al. Citation2012). Therefore, the present results indicate that EPT stimulates the central noradrenergic system and attenuates activity in the HPA axis.
Furthermore, our previous study found that 2 weeks of repeated restraint stress decreased the expression of BDNF mRNA in the mice hippocampus and also decreased the anxiety-like behaviors (Dalle Molle et al. Citation2012). In the present study, the administration of EPT restored BDNF mRNA levels in the hippocampus of mice subjected to repeated restraint stress, which suggests that a modulation of the BDNF signaling pathway plays a role in the anxiolytic actions of EPT.
In conclusion, the present study demonstrates that the administration of EPT had an anxiolytic-like influence on performance of mice in the EPM and OFT. These effects are likely mediated by modifications in the central noradrenergic system and by an upregulation of BDNF expression, which in turn result in attenuated activity within the HPA axis. These findings indicate that EPT may be an efficacious option for the treatment of mental and psychiatric disorders resulting from increases in HPA axis activity.
Additional information
Funding
References
- Chiba S, Numakawa T, Ninomiya M, Richards MC, Wakabayashi C, Kunugi H. 2012. Chronic restraint stress causes anxiety- and depression-like behaviors, downregulates glucocorticoid receptor expression, and attenuates glutamate release induced by brain-derived neurotrophic factor in the prefrontal cortex. Prog Neuropsychopharmacol Biol Psychiatry. 39:112–119. 10.1016/j.pnpbp.2012.05.018
- Choi JG, Kim HG, Kim MC, Yang WM, Huh Y, Kim SY, Oh MS. 2011. Polygalae radix inhibits toxin-induced neuronal death in the Parkinson's disease models. J Ethnopharmacol. 134:414–421. 10.1016/j.jep.2010.12.030
- Dalle Molle R, Portella AK, Goldani MZ, Kapczinski FP, Leistner-Segal S, Salum GA, Manfro GG, Silveira PP. 2012. Associations between parenting behavior and anxiety in a rodent model and a clinical sample: relationship to peripheral BDNF levels. Transl Psychiatry. 2:e195. 10.1038/tp.2012.126
- Garcia LS, Comim CM, Valvassori SS, Réus GZ, Stertz L, Kapczinski F, Gavioli EC, Quevedo J. 2009. Ketamine treatment reverses behavioral and physiological alterations induced by chronic mild stress in rats. Prog Neuropsychopharmacol Biol Psychiatry. 33:450–455. 10.1016/j.pnpbp.2009.01.004
- Gregus A, Wintink AJ, Davis AC, Kalynchuk LE. 2005. Effect of repeated corticosterone injections and restraint stress on anxiety and depression-like behavior in male rats. Behav Brain Res. 156:105–114. 10.1016/j.bbr.2004.05.013
- Hu Y, Liao HB, Dai-Hong G, Liu P, Wang YY, Rahman K. 2010. Antidepressant-like effects of 3,6'-disinapoyl sucrose on hippocampal neuronal plasticity and neurotrophic signal pathway in chronically mild stressed rats. Neurochem Int. 56:461–465. 10.1016/j.neuint.2009.12.004
- Huang Z, Zhong XM, Li ZY, Feng CR, Pan AJ, Mao QQ. 2011. Curcumin reverses corticosterone-induced depressive-like behavior and decrease in brain BDNF levels in rats. Neurosci Lett. 493:145–148. 10.1016/j.neulet.2011.02.030
- Kawashima K, Miyako D, Ishino Y, Makino T, Saito K, Kano Y. 2004. Anti-stress effects of 3,4,5-trimethoxycinnamic acid, an active constituent of roots of Polygala tenuifolia (Onji). Biol Pharm Bull. 27:1317–1319. 10.1248/bpb.27.1317
- Kim KS, Lee DS, Bae GS, Park SJ, Kang DG, Lee HS, Oh H, Kim YC. 2013. The inhibition of JNK MAPK and NF-κB signaling by tenuifoliside A isolated from Polygala tenuifolia in lipopolysaccharide-induced macrophages is associated with its anti-inflammatory effect. Eur J Pharmacol. 721:267–276. 10.1016/j.ejphar.2013.09.026
- Lee B, Shim I, Lee H, Hahm DH. 2012. Effect of ginsenoside Re on depression- and anxiety-like behaviors and cognition memory deficit induced by repeated immobilization in rats. J Microbiol Biotechnol. 22:708–720. 10.4014/jmb.1112.12046
- Lim H, Jang S, Lee Y, Moon S, Kim J, Oh S. 2012. Enhancement of anxiety and modulation of TH and pERK expressions in amygdala by repeated injections of corticosterone. Biomol Ther. 20:418–424. 10.4062/biomolther.2012.20.4.418
- Mizoguchi K, Ikeda R, Shoji H, Tanaka Y, Jin XL, Kase Y, Takeda S, Maruyama W, Tabira T. 2009. Saikokaryukotsuboreito, a herbal medicine, prevents chronic stress-induced anxiety in rats: comparison with diazepam. J Nat Med. 63:69–74. 10.1007/s11418-008-0281-9
- Nacher J, Pham K, Gil-Fernandez V, McEwen BS. 2005. Chronic restraint stress and chronic corticosterone treatment modulate differentially the expression of molecules related to structural plasticity in the adult rat piriform cortex. Neuroscience. 126:503–509. 10.1016/j.neuroscience.2004.03.038
- Park HJ, Shim HS, Kim H, Kim KS, Lee H, Hahm DH, Shim I. 2010. Effects of Glycyrrhizae radix on repeated restraint stress-induced neurochemical and behavioral responses. Kor J Physiol Pharmacol. 14:371–376. 10.4196/kjpp.2010.14.6.371
- Sun XL, Ito H, Masuoka T, Kamei C, Hatano T. 2007. Effect of Polygala tenuifolia root extract on scopolamine-induced impairment of rat spatial cognition in an eight-arm radial maze task. Biol Pharm Bull. 30:1727–1731.
- Watson S, Mackin P. 2007. HPA axis function in mood disorders. Psychiatry. 5:166–170. 10.1383/psyt.2006.5.5.166