Abstract
The abundance and proportion of specific fatty acids differ among fundamental groups of lower trophic levels. Some prey fatty acids are transferred directly to predators. In this study, fatty acid compositions of juvenile, young, and adult anchovies (Engraulis japonicus) were evaluated as trophic markers for feeding environments in four regions (Tongyeong, Namhaedo, Yeosu, and Jindo) of the southern coastal waters of Korea. The condition factor (CF) increased with advancing life stage, and the gonad somatic index (GSI) peaked in May. C14:0, C16:0, C18:0, C16:1n-7, C18:1n-9, C20:1n-9, C20:5n-3, and C22:6n-3, among other saturated fatty acids (SFA), monounsaturated fatty acids (MUFA), and polyunsaturated fatty acids (PUFA) were predominant in all life stages. We observed geographic eastward and life-stage dependent trends wherein C14:0, C16:1n-7 C18:1n-9, and C20:5n-3 fatty acids increased, while C22:6n-3 decreased during development from juvenile to adult in most season. Indicating Calanus copepod trophic markers, MUFA 20:1n-9 and 22:1n-9, were high in the upwelling area of the southwestern sea, but low in southern coastal areas. High MUFA 18:1n-9 and SFA 16:0 in all areas suggests a significant dietary input from non-calanoid copepods in the southern coastal waters of Korea. High dependence on dinoflagellate harmful algal blooms (HABs) was detected in the ratios of major fatty acids (C20:5n-3/C22:6n-3 and C16:1n-7/C16:0) in juvenile and young fish. These results indicate that life stage- and area-specific fatty acid compositions may be explained by regional feeding environments, which influence juvenile and young fish growth and adult maturation in the southern coastal waters of Korea.
Introduction
The anchovy, Engraulis japonicus, is an important short-lived pelagic species inhabiting Korean waters, which comprises over 20% of recent Korean commercial fishery catches with 120,000–250,000 mt annually since 1974. Anchovy fishing grounds are distributed mostly in the southern coastal waters of Korea and waters adjacent to islands; various fisheries operate in these areas, targeting juvenile or adult fish depending on reproduction and recruitment processes (Kim & Lo Citation2001; Jung et al. Citation2008; Kim et al. Citation2013a). The anchovy occurs widely in the warm waters of the Korea Strait in the winter season and moves to coastal areas to spawn during early spring–autumn, mainly April–August (Choi et al. Citation2001; Kim & Lo Citation2001). After the spawning season, larvae develop into juveniles (3–4 cm fork length, FL) in 1–2 months and move to coastal areas and to estuaries. Young fish (6–7 cm FL) develop in 3–5 months and approach the adult stage (8–10 cm FL) in a year (Kim & Lo Citation2001; Jung et al. Citation2008).
The southern coastal waters of Korea have spatially and temporally heterogeneous environmental conditions due to the variation of freshwater inputs and the strength of major currents. The Seomjin Estuary showed high productivity as a result of inflows of nutrients originating from temporary terrestrial sources, with large spatial variation (Min et al. Citation2012). The Tsushima warm currents are distributed throughout southern waters of Korea with expansion or reduction, affecting temporal front movement and shift of the nursery ground of anchovy (Lie et al. Citation1995; Kim & Lo Citation2001). Coastal waters off Jindo are characterized by a wind-driven (upwelling) and strong tidal current in the shallow southwestern area (Jeong et al. Citation2009). Phytoplankton, composed mainly of Bacillariophyceae, Dinophyceae, Chrysophycea, and Cryptophycea blooms during summer often have a serious impact on coastal fisheries and the surrounding ecosystem via harmful algal blooms (HABs; Baek et al. Citation2010; Lee & Kim Citation2012).
The prey–predator relationship is one of important parameters to understand the energy flow and community population dynamics (top-down or bottom-up control) of marine ecosystem (Cury et al. Citation2005). In order to understand the relationship, the primary tools have been stomach content analysis and feeding experiments. Because of technical challenges and to better understand predator–prey interactions, ecologists developed a variety of biomarkers and tracers, e.g., fatty acids, DNA markers, allozymes, and stable isotopes (Gorohhova & Lehtiniemi Citation2007). Among them, fatty acids integrate the trophic history of consumers over several weeks, while stomach contents analysis and feeding experiments reflect only recently ingested diets (Kreibich Citation2007; Park et al. Citation2013). Particularly, some fatty acids (called fatty acid trophic markers; FATM) are origin-specific and more conservative than others. All organic matter, especially fatty acids, is transformed in the pelagic plankton food web before reaching fish or benthic organisms (Dalsgaard et al. Citation2003; Wan et al. Citation2010; Righoux Citation2011). Therefore, FATM can be used to identify key interactions in marine food webs as qualitative markers in marine ecosystems (Dalsgaard et al. Citation2003). However, no reports have been applied to FATM to understand the feeding ecology of planktivorous fish in Korean waters with the exception of a few studies on cetaceans and zooplankton (Ko et al. Citation2009, Citation2011; Kim et al. Citation2010; Park et al. Citation2013).
The reproductive potential of anchovies in the southern coastal waters of Korea is affected by changes in feeding environments. This is reflected in their energy reserves, which show yearly and regional variation via Fulton's K (condition factor, CF) and the gonad somatic index (GSI), as evidence of bottom-up effects (Kim et al. Citation2013a). Maternal fat reserves affect the fatty acid composition of pelagic fish oocytes during maturation (Garrido et al. Citation2007). The accumulation of essential fatty acids; i.e., polyunsaturated fatty acids (PUFA), in the early developmental stages of fish may be a key reason for recruitment fluctuations; they are essential for larval development and fitness, influence behavior, and are necessary for the development of the brain and nervous system (Sargent et al. Citation1999; Rossi et al. Citation2006). In Korean waters, any studies have not yet been conducted to understand the foraging ecology of the anchovy using fatty acid profiles, although it has been known that they could closely associate with the change of their feeding environment.
The aims of this study were to understand (1) lipid and fatty acid compositions during growth and maturation among seasons and regions, and (2) feeding ecology of anchovy using FATM, and (3) the trophodynamics in light of recent environmental changes in estuaries, upwellings, and coastal areas. We evaluated the spatial and temporal heterogeneity of anchovy's diet in the southern coastal waters of Korea using references on the FATM.
Materials and methods
Sampling and morphological measurement
Anchovies were sampled off Yeosu and Tongyoung using anchovy drag nets. In addition, Jukbang fisheries off Namhaedo and Nangjang net fisheries off Jindo were used for sampling (). In this way, we investigated regional differences in feeding environments: coastal waters affected by warm offshore currents (Yeosu and Tongyoung), a bay area near an estuary influenced by rivers (Namhaedo), and an upwelling area (Jindo). We obtained young (6–8 cm FL) and adult (> 9 cm FL) anchovies in July, September, October, and November 2011, and January, February, May, and June 2012. Juveniles (3–4 cm FL) were obtained twice, in July 2011 and in May 2012. We divided adults into adult I (9–11cm FL) and adult II (>11cm FL) groups.
Fork length (FL, cm), body weight (BW, g), and gonad weight (GW, g) were measured in 2,945 specimens. The GSI and Fulton's K (CF) were measured to assess the reproductive potential and body conditions of fish under different environmental or feeding conditions (Rätz & Lloret Citation2003; Kim et al. Citation2013a). Therefore, we calculated the CF as (BW–GW)/FL^3, and the reproductive potential (GSI) as GSI = (GW × 1000)/BW.
Lipid and fatty acid analysis
Lipid contents and compositions (26 fatty acids) of anchovies were evaluated intermittently as trophic markers of the feeding environments in four regions and four size groups. Samples were kept frozen with dry ice for transportation to the lab and kept them in a freezer (–20°C) before freeze drying and analysis.
Samples were dried to a constant weight at 105°C to determine the water content. We determined ash using incineration at 550°C, crude lipids by Soxhlet extraction in a Soxtec system 1046 (TecatorAB, Hoganas, Sweden), and crude protein using the Kjeldahl method (N × 6.25) after acid digestion. Lipid extraction for fatty acid analysis (2:l v/v) was performed according to the method of Folch et al. (Citation1957), and fatty acid methyl esters (FAMEs) were prepared by transesterification with 14% BF3-MeOH (Sigma, USA). The FAME composition was determined using gas chromatography (Trace GC, Theromo Finnigan, USA) with a flame-ionization detector, equipped with a Carbowax 007 capillary column (30 m × 0.25 mm i.d., film thickness 0.25 μm, QUADREX, USA). FAMEs were identified by comparing the retention times with a standard (Supelco 37 Component FAME Mix, 18919-1AMP). Injector and detector temperatures were 230°C and 250°C, respectively. The column temperature was programmed from 100°C to 220°C at a rate of 5°C/min and 220°C to 240°C at a rate of 3°C/min. Nitrogen (99.99%) was used as the carrier gas. One internal standard (C21:0) was used for quantification.
The fatty acid biomarkers of the major potential food sources were identified by comparing with the published literature on the diet composition of the same periods and regions (Kim et al. Citation2013b) and fatty acid composition of anchovy food sources (Zhucova Citation2000; Morais et al. Citation2002; Alfaro et al. Citation2006; Lee et al. Citation2006; Kreibich Citation2007; Dodds et al. Citation2009; Ko et al. Citation2009). Average data for the individual fatty acids are expressed as a mass percentage of total fatty acids.
Statistics
Mean CF values were determined for juveniles, young fish, and two adult groups. To identify the spawning season for adults, we calculated monthly mean values and standard deviations (SD) of the CF and GSI. Although 26 fatty acids were identified, only 16 major fatty acids (> 1.0% of total values) were used for this study. Regional and monthly mean values (±SD) of CF, GSI, lipid contents, and fatty acid compositions of juvenile, young, and adult anchovies were compared with statistical significance as biomarkers of feeding environments from July 2011 to June 2012. Fatty acid data were tested after logarithmic transformations assuming homogeneity of variance. We used one-way and two-way analysis of variance (ANOVA) to test differences in the fatty acid among four life stages and four regions. Data were analyzed using SPSS version 18.0 (Chicago, IL, 2010).
Results
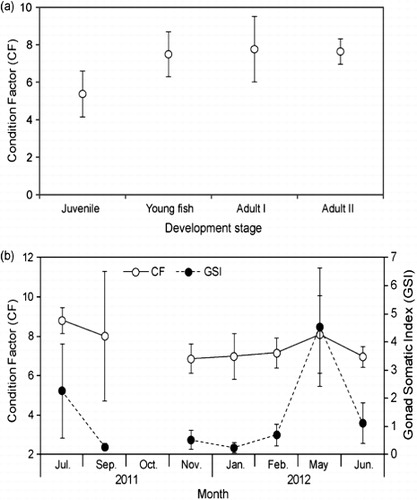
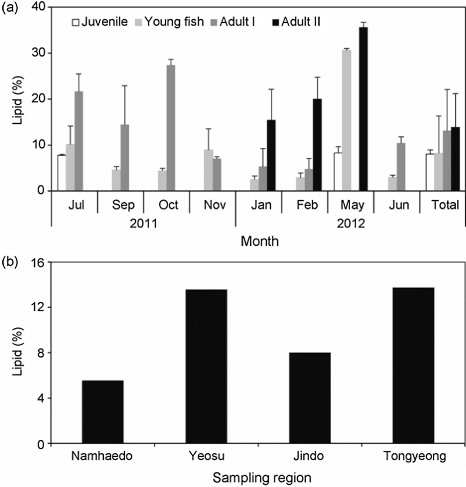
The CF showed an increasing trend with advancing anchovy life stage. Mean values (±SD) were 5.37 ± 1.23 in juveniles, 7.49 ± 1.20 in young fish, and 7.63–7.76 ± 0.68–1.75 in adults (). The GSI and CF of adults decreased gradually in July 2011, increased in February, and peaked in May 2012 (). Lipid contents tend to increase with advancing anchovy life stage from juvenile to Adult II during a year with a peak in May 2012. Mean values (±SD) of adults (13.11 ± 1.65–13.89 ± 2.54) were higher than juveniles (8.01 ± 0.92) and young fishes (8.20 ± 4.04), respectively (one-way ANOVA, F = 3.306, P < 0.05; ). Regional mean levels (±SD) of young anchovy lipids in Yeosu and in Tongyoung during July 2011 were 13.58 ± 1.06 and 13.75 ± 1.99, twice those in Jindo and in Namhaedo (7.99 ± 0.44 and 5.54 ± 1.13), respectively (one-way ANOVA, F = 23.04, P < 0.001; ).
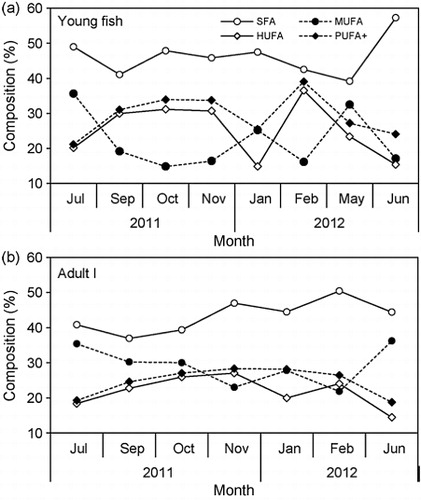
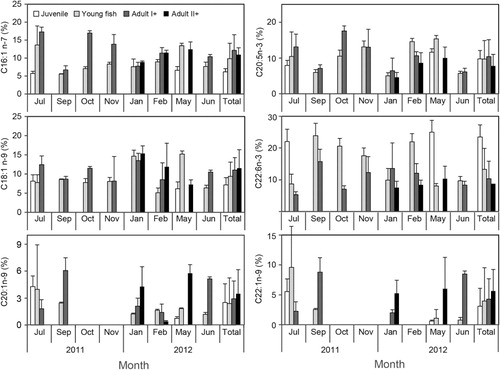
Mean levels of the 16 major fatty acids and three groups (0, 1, and multiple double bonds; saturated fatty acids (SFA), monounsaturated fatty acids (MUFA), and PUFA, respectively) showed marked variation among life stages (). C14:0, C16:0, C18:0, C16:1n-7, C18:1n-9, C20:1n-9, C20:5n-3, and C22:6n-3 were the predominant fatty acids in juvenile, young, and adult anchovies. Levels of SFA were comparatively stable from 39.5 to 45.23 and MUFA increased from 19.55 to 32.54. PUFA decreased from 34.72 to 18.87, depending on the life stage (). Young and adult anchovies showed an increasing PUFA content and a decreasing MUFA content during September–February, and a decrease in May, with a higher PUFA/SFA ratio in young anchovies ( and ).
Table 1. Major fatty acid groups that comprised >1.0% of total fatty acids in anchovies (E. japonicus) of four size groups in the southern waters of Korea.
The major fatty acids showed general variation according to season and life stage (). C16:1n-7, C20:5n-3, C22:1n-9, and C22:6n-3 levels were lower in January than in other periods, while the C18:1n-9 level was higher in January. Mean C16:1n-7 and C18:1n-9 levels increased, while that of C22:6n-3 decreased, with advancing life stage in most seasons.
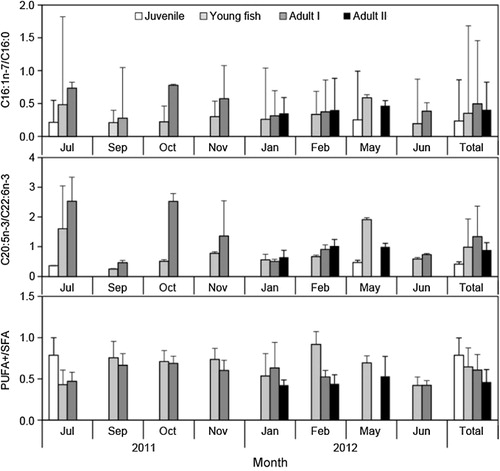
The C16:1n-7/C16:0 and C20:5n-3/C22:6n-3 ratios generally increased with life stage, and seasonal changes in fatty acid ratios were detected during young fish and adults periods with the low mean ratios (<1) for the C16:1n-7/C16:0 during all season and the C20:5n-3/C22:6n-3 during most season and life stages except July and October 2011 and May 2012 ().
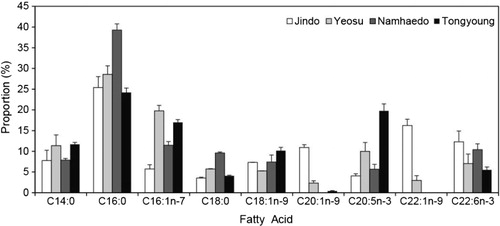
With respect to regional changes for the fatty acids of young anchovy during July 2011, C20:1n-9, C22:1n-9, and C22:6n-3 were the highest levels in the adjacent waters of Jindo. C14:0, 16:1n-7 and C20:5n-3 exhibited high levels in Yeosu and in Tongyoung. C16:0 and C18:0 showed high levels in Namhaedo. An eastward trend was observed in which levels of C16:1n-7, C18:0, and C20:5n-3 fatty acids increased, while those of C20:1n-9, C22:1n-9, and C22:6n-3 decreased (). There were significant differences in the four regions for C16:1n-7, C20:1n-9, C22:1n-9, C20:5n-3, C16:1n-7/C16:0, and C20:5n-3/C22:6n-3 ratios and in life stages for C22:6n-3 and C16:1n-7/C16:0 (: two-way ANOVA, F = 4.52–7.21, and P < 0.01–0.005, respectively).
Table 2. Two-way ANOVA for the major fatty acids and their ratios. Main factors were region (Jindo, Yeosu, Namhaedo and Tongyoung) and life stage (juvenile, young fish, adult I, and adult II).
Discussion
Our study revealed significant differences in crude lipids among anchovy groups in four regions and four life stages (). Crude lipid contents increased with life stage; the highest content was in adults and the lowest in juveniles, in agreement with Lee et al. (Citation1986). The fat content of female anchovies peaks during the prespawning period and decreases dramatically thereafter (Wan et al. Citation2010). We identified a similar seasonal variation in lipid content, the GSI and CF; lipid contents peaked in May and decreased in June; the GSI also reflected the early and late spawning periods (). Condition indices of fish are influenced by annual and seasonal variability in plankton production cycles (Rätz & Lloret Citation2003). Anchovy reproductive potential is closely linked to energy reserves that are themselves influenced by environmental and/or feeding conditions (Hunter & Leong Citation1981; Kim et al. Citation2013a). Therefore, the association of anchovy lipid content with the GSI and CF in Korean waters suggests reservation of energy for spawning since lipids are used as a source of energy for reproduction.
Mean anchovy lipid levels in Yeosu and in Tongyoung were twofold higher than those in Jindo and in Namhaedo, respectively. The lipid levels in planktivorous fishes (e.g., anchovy, sprats) are considered as an indicator of nutritional and food supply condition because a fish containing high levels of lipid likely has good nutritional condition with sufficient food supply (Nikolsky et al. Citation2012). Occurrence of frontal area between the southern coastal waters of Korea and the Tsushima warm currents off Yeosu and Tongyoung influenced concentration of chlorophyll and zooplankton (Choo Citation2002; Moon et al. Citation2010). Oceanographic data showed strong upwelling by tidal currents around Jindo and a fast current speed by a wide range of the ebb and flow of the tide and its narrow width in the Jijok Strait distributing Jukbang set net in Namhaedo, which seems to consume more lipids as energy supply to seek food and maintain their position (Lie et al. Citation1995; Jeong et al. Citation2009). Therefore, habitat can influence the crude lipid content by physical and biological oceanographic factors.
The C20:5n-3/C22:6n-3 and C16:1n-7/C16:0 ratios of major fatty acids generally increased with advancing life stage (). Since diatoms contain high levels of 16:1n-7 and 20:5n-3 (eicosapentaenoic acid; EPA), dinoflagellates and phaeocystis are usually rich in 22:6n-3 (docosahexanenoic acid; DHA), the ratios of 16:1n-7/16:0 and EPA/DHA allow differentiation of diatom- and flagellate-based diets (; Auel et al. Citation2002; Alfaro et al. Citation2006). DHA decreased with body length and is more essential than EPA during the earlier stages of anchovy development (Wan et al. Citation2010). In this study, the C20:5n-3/C22:6n-3 and C16:1n-7/C16:0 ratios of juvenile and young fish indicated a marked dependence on dinoflagellate HABs.
Table 3. Comparison with diet composition and dominat fatty acids of E. japonicus using references on the feeding characteristics. Percentage abundances such as (No, %) were analyzed for the seasonal data in Yeosu and the regional data in Jindo, Yeosu, and Tongyoung.
C16:1n-7, C20:5n-3, C22:1n-9, and C22:6n-3 levels were lower in January than during other periods (). The stomach content analysis of anchovies indicated that dominant prey items were Cirriped (barnacle) larvae and copepods in summer, phytoplankton and Pseudodiaptomus marinus in autumn, and Paracalanus parvuss and the cold-water copepod Centropages abdominalis in winter, suggesting that prey selectivity is highly flexible and adaptable in the waters (; Kim et al. Citation2013b). The fatty acid composition of the barnacles Hesperibalanus hesperius and Balanus rostratus strongly indicates a diet containing diatoms (Zhucova Citation2000). Dominant consumers preying upon diatoms have been reported to contain high levels of biomarker fatty acids (, 16:1n-7 and 20:5n-3). Anchovies spawn in spring–summer in the southern coastal waters of Korea and juveniles occur in summer–autumn (Kim & Lo Citation2001; Jung et al. Citation2008). These results indicate that life stage- and season-specific fatty acid compositions may be explained by the influence of seasonal feeding environments on the growth of juvenile and young fish, and adult maturation, in the southern coastal waters of Korea in terms of ontogenetic and physiological condition of the anchovy.
In this study, the C18:1n-9 level was higher in January than during other periods, suggesting considerable dietary input of carnivorous. The n-9 isomer of 18:1 is a common fatty acid in metazoans and an indicator of carnivory (Stübing & Hagen Citation2003). Anchovy possess a relatively coarse branchial apparatus to feed predominantly particulate-feeding as carnivory and maximize their net energy gain (Lingen et al. Citation2006). Therefore, seasonal differences in the availability of carnivorous prey for anchovies in the southern coastal waters of Korea seem to be influenced by differences in the 18:1n-9 fatty acid level. A distinct change in fatty acid signature was observed for the Japanese anchovy with the increase in body length, which is independent of geographic location (Wan et al. Citation2010). In this study, we observed a significant difference in C22:6n-3 of each life stages and eastward trend regionally, in which levels of the C16:1n-7 and C20:5n-3 fatty acids increased, while those of C20:1n-9 and C22:1n-9 decreased (, ). High levels of the C20:1n-9 fatty acid indicates predation of herbivorous calanus copepods (Auel et al. Citation2002; Dodds et al. Citation2009). The main prey items of anchovies in the coastal waters of Yeosu and Jindo in July were the cyprii stage of barnacles and the copepod Calanus sinicus. However, the predominant prey items near Tongyeong were the small copepods Paracalanus parvuss and Corycaeus affinis based on the stomach contents (; Kim et al. Citation2013b). Righoux (Citation2011) examined differences in food web structures from north to south, based on the average spatial differences in primary productivity, and reported that zooplankton in the more productive region show enhanced herbivorous feeding. Levels of the calanus-specific copepod lipid biomarkers C20:1n-9 were high in the upwelling area around Jindo, but low in the Jukbang fishing ground (Namhaedo) in the southern narrow bay and estuary area. These results show that anchovies are dependent on calanus copepods and other zooplankton larvae related with variation among regional feeding environments and the trophic dynamics/feeding habits of juvenile, young and adults anchovies.
Three groups (SFA, MUFA, and PUFA, respectively) of fatty acids showed considerable variation among life stages (, Figures and ). Because fatty acids integrate the feeding history of consumers over several weeks, fatty acids could provide better information on the linkage between zooplankton secondary production and higher trophic levels, which are apparently linked to differences in diet during each life stage (Auel et al. Citation2002; Wan et al. Citation2010; Righoux Citation2011). Anchovy larvae recruits in 1–2 months after the spawning season and develop to young fish in the adjacent coastal areas and estuaries (Kim & Lo Citation2001). Therefore, variation of PUFA and MUFA contents in each life stages of anchovy off Korea also seems to be related with the seasonal changes of the feeding environment during migration which could related with the recruitment process and consequently population fluctuation.
Acknowledgments
This work was funded by the National Fisheries Research and Development Institute (RP-2014-FR-015, Survey of coastal fisheries resources and marine environmental ecology in the South Sea). We extend our gratitude to the two anonymous reviewers, who improved the quality of the original manuscript greatly.
References
- Alfaro A, Thomas F, Sergent L, Duxbury M. 2006. Identification of trophic interactions within an estuarine food web (northern New Zealand) using fatty acid biomarkers and stable isotopes. Est Coast Shelf Sci. 70:271–286.[doi:10.1016/j.ecss.2006.06.017]
- Auel H, Harjes M, Rocha R, Stübing D, Hagen W. 2002. Lipid biomarkers indicate different ecological niches and trophic relationships of the Arctic hyperiid amphipods Themisto abyssorum and T. libellula. Polar Biol. 25:374–383.
- Baek SH, Shin K, Hyun B, Jang PG, Kim HS, Hwang OM. 2010. Distribution characteristics and community structure of phytoplankton in the different water masses during early summer of southern sea of Korea. Ocean Polar Res. 32:1–13.
- Choi SG, Kim JY, Kim SS, Choi YM, Choi KH. 2001. Biomass estimation of anchovy (Engraulis japonicus) by acoustic and trawl surveys during spring season in the Southern Korean Waters. J Korean Soc Fish Res. 4:20–29.
- Choo HS. 2002. The variations of oceanic conditions and the distributions of eggs and larvae of anchovy in the southern sea of Korea in summer. J Korean Fish soc. 35:77–85.
- Cury P, Shannon L, Roux JP, Daskalov G, Jarre A, Moloney C, Pauly D. 2005. Trophodynamic indicators for an ecosystem approach to fisheries. ICES J Mar Sci. 62:430–442.
- Dalsgaard J, John MS, Kattner G, Müller-Navarra D, Hagen W. 2003. Fatty acid trophic markers in the pelagic marine environment. Adv Mar Biol. 46:225–340.
- Dodds LA, Black KD, Orr H, Roberts JM. 2009. Lipid biomarkers reveal geographical differences in food supply to the cold-water coral Lophelia pertusa (Scleractinia). Mar Ecol Prog Ser. 397:113–124.
- Folch J, Lees M, Sloane SGH. 1957. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 226:497–509.
- Garrido S, Rosa R, Ben-Hamadou R, Cunha ME, Chícharo MA, Lingen CD. 2007. Effect of maternal fat reserves on the fatty acid composition of sardine (Sardina pilchardus) oocytes. Comp Biochem Physiol B. 148:398–409.
- Gorohhova E, Lehtiniemi M. 2007. A combined approach to understand trophic interactions between Cercopagis pengoi (Cladocera; Onychopoda) and mysids in the Gulf of Finland. Limnol Oceanogr. 52:685–695.
- Hunter JR, Leong R. 1981. The spawning energetics of female northern anchovy, Engraulis mordax. Fish Bul. 79:215–229.
- Jeong HD, Kwon CH, Kim SW, Cho KD. 2009. Fluctuation of tidal front and expansion of cold water region in the southwestern sea of Korea. J Kor Soc Mar Env Saf. 15:289–296
- Jung S, Hwang SD, Kim JY. 2008. Fecundity and growth-dependent mortality of Pacific anchovy (Engraulis japonicus) in Korean coastal waters. Fish Res. 93:39–46.
- Kim HS, Ju SJ, Ko AR. 2010. Comparisons of feeding ecology of Euphausia pacifica from Korean waters using lipid composition. Ocean Polar Res. 32:165–175.
- Kim JY, Lee SK, Kim SS, Choi MS. 2013a. Environmental factors affecting anchovy reproductive potential in the southern coastal waters of Korea. Ani Cells Sys. 17:133–140.
- Kim JY, Lo N. 2001. Temporal variation of seasonality of egg production and the spawning biomass of Pacific anchovy, Engraulis japonicus, in the southern waters of Korea in 1983–1994. Fish Oceanogr. 10:297–310.
- Kim MJ, Youn SH, Kim JY, Oh CW. 2013b. Feeding characteristics of the Japanese anchovy, Engraulis japonicus according to the distribution of zooplankton in the coastal waters of southern Korea. Korean J Environ Biol. 31:275–287.
- Ko AR, Ju SJ, Lee CR. 2009. The physiological and ecological comparisons between warm (Pleuromamma sp.) and cold water copepod species (Neocalanus plumchrus) in the Northwestern Pacific ocean using lipid contents and compositions. Ocean Polar Res. 31:121–131.
- Ko AR, Ju SJ, Moon DY, Choi SG, Kim ZG, Shin KH. 2011. Stratification of lipid content and composition in blubber of marine cetacean from Korean waters. Ocean Polar Res. 33:35–43.[doi:10.4217/OPR.2011.33.1.035]
- Kreibich T. 2007. Physiological adaptations of copepods from the North Sea and the North Atlantic to changing nutritional conditions [PhD thesis]. Bremen, Germany: Universität Bremen.
- Lee EH, Oh KS, Lee TH, Chung YH. 1986. Fatty acid content of five kinds of boiled-dried anchovies on the market. Bull Korean Fish Soc. 19:183–186.
- Lee R, Hagen W, Kattner G. 2006. Lipid storage in marine zooplankton. Mar Ecol Prog Ser. 307:273–306.
- Lee YS, Kim JY. 2012. Appearance and characteristics of major phytoplankton species based on long-term monitoring datasets in southern sea of Korea. J Environ Biol. 33:823–829.
- Lie HJ, Byun SK, Bang I, Cho CH. 1995. Physical structure of Eddies in the Southwestern East sea. J Kor Soc Oceanogr. 30:170–183.
- Lingen CD, Hutchings L, Field JD. 2006. Comparative trophodynamics of anchovy Engraulis encrasicolus and sardine Sardinops sagax in the southern Benguela: are species alternations between small pelagic fish trophodynamically mediated? Afr J Mar Sci. 28:465–477.
- Min JO, Ha SY, Jung MH, Choi BH, Lee YJ, Youn SH, Yoon WD, Lee JS, Shin KH. 2012. Seasonal variation of primary productivity and pigment of phytoplankton community structure in the Seomjin estuary. Korean J Limn. 45:139–149.
- Moon SK, Kim IS, Hong SN, Lim DH, Jeong BY. 2010. Changes in the proximate and fatty acid compositions of chub mackerel, Scomber japonicus muscle during cultivation. Korean J Fish Aquatsci. 43:589–597.
- Morais S, Narciso L, Calado R, Nunes ML, Rosa R. 2002. Lipid dynamics during the embryonic development of Plesionikamartia martia (Decapoda; Pandalidae), Palaemon serratus and P. elegans (Decapoda; Palaemonidae): relation to metabolic consumption. Mar Ecol Prog Ser. 242:195–204.
- Nikolsky V, Shulman G, Shchepkina A, Yuneva T, Bat L, Kaya Y, Kideyş A, Seyhan K. 2012. Assessment of food supply of small pelagic fish in the black sea based on their lipid content. Turk J Fish Aqua Sci. 12:429–434.
- Park HJ, Choy EJ, Lee KS, Kang CK. 2013. Trophic transfer between coastal habitats in a seaglass-dominated macrotidal embayment system as determined by stable isotope and fatty acid signatures. Mar Fresh Res. 64:1169–1183.
- Rätz H, Lloret J. 2003. Variation in fish condition between Atlantic cod (Gadusmorhua) stocks, the effect on their productivity and management implications. Fish Res. 60:369–380.
- Righoux NB. 2011. Trophic ecology of zooplankton at a frontal transition zone: fatty acid signatures at the subtropical convergence. South Ocean J Plank Res. 33:491–505.
- Rossi S, Sabate's A, Latasa M, Reyes E. 2006. Lipid biomarkers and trophic linkages between phytoplankton, zooplankton and anchovy (Engraulis encrasicolus) larvae in the NW Mediterranean. J Plankton Res. 28:551–562.[doi:10.1093/plankt/fbi140]
- Sargent J, McEvoy L, Estevez A, Bell G, Bell M, Henderson J, Tocher D. 1999. Lipid nutrition of marine fish during early development: current status and future directions. Aquaculture. 179:217–229.
- Stübing D, Hagen W. 2003. Fatty acid biomarker ratios – suitable trophic indicators in Antarctic euphausiids? Polar Biol. 26:774–782.
- Wan R, Wu Y, Huang L, Zhang J, Gao L, Wang N. 2010. Fatty acids and stable isotopes of a marine ecosystem: study on the Japanese anchovy (Engraulis japonicus) food web in the Yellow Sea. Deep-Sea Research II. 57:1047–1057.
- Zhucova NV. 2000. Fatty acid components of two species of barnacles, Hesperibalanus hesperius and Balanus rostratus (Cirripedia), as indicators of food sources. Crustaceana. 73:513–518.[doi:10.1163/156854000504561]
