Abstract
In this study, we investigated the relationship between anti-inflammatory mechanisms and the combined ameliorating effects of rose hip powder (RHP) and green tea seed extract (GTSE) in a monosodium iodoacetate (MIA)-induced osteoarthritis (OA) animal model. We confirmed the individual effects of RHP (500 mg/kg bw) and GTSE (50 mg/kg bw) in the OA model. Treatment with the mixture of RHP and GTSE (Mix) resulted in significantly enhanced stance and propulsion times compared to treatment with RHP or GTSE alone. To examine the combined effects of RHP and GTSE in vivo, pro-inflammatory cytokines, such as tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), were measured. The administration of Mix significantly reduced the expression of pro-inflammatory cytokines. Additionally, cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) expressions were attenuated to a greater extent after the administration of Mix compared to the other treatments. Furthermore, we evaluated the anti-osteoarthritic effects of RHP, GTSE, and Mix on articular cartilage damage using micro-computed tomography (micro-CT) in OA in rats. After three weeks of treatment, we observed that the administration of RHP, GTSE, and Mix protected against bone destruction and reduced the number of erosion lacunae, but there was no statistical difference among RHP, GTSE, and Mix. Although additional research is warranted, our results suggest that the biological effects of GTSE were enhanced by RHP supplementation to include anti-inflammatory effects, with the potential ability of offering a benefit to OA patients.
Introduction
Osteoarthritis (OA) is a degenerative disease that is accompanied by inflammation and cartilage damage, and it has been suggested that this joint disease results from a combination of mechanical damage and biomechanical stress (Goldring & Goldring Citation2007). The progression of mechanical damage is associated with the destruction of articular cartilage, thickening of the subchondral bone, and increased pressure on joints (Simon et al. Citation2000; Saklatvala Citation2007). The biomechanical pathway of OA includes the expression of cytokines (interlukin-1b [IL-1b], tumor necrosis factor-α [TNF-α], and IL-15) and a number of other genes that enhance or modulate inflammatory and catabolic responses, including cyclooxygenase (COX-2) and inducible nitric oxide synthase (iNOS or NOS-2) (Goldring & Goldring Citation2007).
Rose hip, produced by Rosa canina L., is the best-known and most popular functional food used worldwide for the treatment of OA; it is also used as a diuretic and laxative, and in gout treatment (Nagatomo et al. Citation2013). Rose hip has been studied as an anti-oxidant and has a particularly high vitamin C content compared to orange fruit (Haas Citation1995; Stralsjö et al. Citation2003). Studies on the photochemical compounds found in rose hip have shown that the fruits contain phenolic acids, proanthocyanidins, tannins, flavonoids, fatty acids, pectines, carotenoids, and fruit acids (Hvattum Citation2002; Kumarasamy et al. Citation2003; Uggla et al. Citation2003; Chrubasik et al. Citation2008). Moreover, it has been shown that rose hip fruit extract possesses anti-inflammatory and anti-oxidant properties both in vitro and in vivo (Kharazmi & Winther Citation1999; Larsen et al. Citation2003; Lattanzio et al. Citation2011; Deliorman et al. Citation2007). Previous studies have investigated the anti-inflammatory effects of rose hip (Orhan et al. Citation2007; Francesca & Emanuela Citation2011) and the inhibitory role of rose hip in pain models. Several clinical trials in arthritis patients have shown an improvement in pain, stiffness, and active hip joint movement (Chrubasik et al. Citation2008). Although many studies on the nutritional value, chemical composition, and clinical significance of R. canina L., especially rose hip powder (RHP), have been published, there is no detailed study concerning the in vivo role of cytokine in this process.
Green tea seed contains many biologically active compounds such as saponin, flavonoids, vitamins, and tannins; for example, two flavonol glycosides from seeds of Camellia sinensis and inactivation of human type A and B influenza viruses by tea-seed saponins have been reported. In a recent study, the green tea seed coat was shown to inhibit inflammatory mediator in a HUVEC cell model (Noh et al. Citation2011). However, its effects on OA inflammation have not been investigated.
Therefore, the goal of this study was to evaluate the chondroprotective and anti-inflammatory effects of rose hip combined with green tea seed extract in a monosodium iodoacetate (MIA)-induced OA animal model.
Materials and methods
Preparation of RHP and GTSEs
R. canina L. was collected from Atlas cultivation in Chile. The dried rose hip fruit was crushed into a powder (RHP). Green tea seeds were collected from Boseong, Jeollanamdo in South Korea in November 2013 and authenticated by Dr Kim at the Jeollanamdo Institute of Natural Resources Research (JINR), Jangheung, Jeollanamdo. The green tea seeds were ground and sifted through a mesh to produce powder and then extracted with 20 volumes of water at 100°C for 3 h. The extracted solution was filtered, concentrated with an evaporator under vacuum conditions, and spray-dried. This process resulted in a water extract of green tea seed (GTSE). The extracts were stored at −20°C until future use.
Reagents
The reagents used included MIA, eosin Y solution, and hematoxylin solution from Sigma Chemicals (St Louis, MO, USA), anti-interleukin-6 (IL-6) antibody, and anti-COX-2 antibodies from Santa Cruz Biotechnology (Santa Cruz, CA, USA), and an anti-TNF-α antibody from Cell Signaling (New England Biolabs, USA). An antibody was purchased from Invitrogen (Carlsbad, CA, USA). All other reagents were of analytical grade.
Animals
Male Wistar rats (weight: 190–270 g) were purchased from the Central Lab Animal Inc. (Seoul, Korea) and housed 14 per cage in a light-controlled room (lights on from 8:00 am to 8:00 pm) at a temperature of 22 ± 2°C and a humidity of 50 ± 5% with food and water available ad libitum. All experimental procedures were conducted in accordance with the relevant guidelines for the care of experimental animals and were approved by the Jeollanamdo Institute of Natural Resources Research (JINR).
Grouping and administration
After the adaption period (1 week), rats were randomly assigned into one of six groups: (1) control group (1 ml saline/day); (2) negative control group (MIA injection + 1 ml saline/day); (3) positive control group (MIA injection + 1 ml water + 2 mg methotrexate per kg bw/day); (4) single RHP group (MIA injection + 1 ml water + 500 mg RHP per kg bw/day); (5) single GTSE group (MIA injection + 1 ml water + 50 mg GTSE per kg bw/day); (6) Mix-L group (MIA injection + 1 ml water + 500 mg RHP and 25 mg GTSE per kg bw/day); (6) Mix-H group (MIA injection + 1 ml water + 500 mg RHP and 50 mg GTSE per kg bw/day). All samples were orally administered for 4 weeks after MIA injection.
MIA-induced OA rat model
The rats were anesthetized with diethyl ether and given a single intra-articular injection of 3 mg MIA into the left knee. MIA was dissolved in saline and administered in a 50 μl volume.
Gait measurement using TreadScan
To measure the mechanical sensitivity of the rats in MIA-induced OA, gait measurements were carried out using the TreadScanTM system. The instruments were housed in a designated light- and temperature-controlled room. TreadScanTM data on each rat were measured for approximately 20 seconds of fast walking at 15 cm/sec. The recorded walking activity was analyzed using TreadScanTM version 3.0 software, which calculates the mean value for 37 locomotion parameters for each rat from approximately 1500 high-quality video frames. For the present study, data analysis was restricted to the hind feet. The stride cycle represents the sum of the stance and swing times. The stance time for a hind foot is the elapsed period between the first contact and the last contact with the surface and is the sum of brake and propulsion times. This position represents the normal still stance position for mice. The propulsion time is the stance time minus the brake time.
Western blot
After protein quantification, 10 μg of whole protein was separated via 8–12% SDS-polyacrylamide gel electrophoresis and transferred onto nitrocellulose membranes (Bio-rad) for measurement of TNF-α and IL-6. Primary antibodies against the following were used: TNF-α (1:1000) and IL-6 (1:1000). The signals were normalized to that of β-actin (1: 1000) or lamin B1 (1:2500). ImageQuantTM software (Amersham Biosciences/GE Healthcare, Piscataway, NJ, USA) was used for the densitometry evaluation of the visualized immunoreactive bands.
Joint histology and immunohistochemistry
For joint histological analysis, the right tibias were removed and fixed in 4% paraformaldehyde (pH 7.4) and decalcified with decalcification solution (distilled water 90 ml, 40% formalin 10 ml, and 5.5 g of EDTA-2Na) for 3 weeks. Decalcified tissues were protected during the freezing process (Cryoprotection, 20% sucrose, 4°C, overnight), and then dehydration, cleaning, and paraffin embedding were performed. Tissue sections were cut at a thickness of 7 μm using a fine cutter (microtome, BRIGHT5040, USA) and stained with hematoxylin and eosin. Slides for immunohistochemistry were deparafinized and rehydrated using a graded ethanol series. The slides were depleted of endogenous peroxidase activity by adding methanolic H2O2 in PBS and then blocked with 10% bovine serum for 30 min. The samples were incubated overnight at 4°C with antibodies against COX-2 at a dilution of 1:50 and iNOS at 1:100, and then the samples were incubated with the respective secondary antibodies, biotinylated anti-mouse IgG, or anti-rabbit IgG, for 20 min. A streptavidine-peroxidase complex (Vector Laboratories, Burlingame, CA, USA) was added for 1 h, with final incubation with 3,30-diaminobenzidine (Dako, Glostrup, Denmark). The sections were counterstained with Mayer's hematoxylin and photographed using an Olympus photomicroscope (Olympus, Tokyo, Japan).
Photographing micro-CT of the OA joints
Recording three-dimensional micro-CT was performed for measuring changes in bone structure. Micro-CT (Skyscan 1076, SKYSCAN N.V., Belgium) was used to analyze the radiographed X-ray. The specimens were attached to a stage that rotated 180°, and the images were acquired every 0.4°. Three-dimensional analyses of joint tissue were determined using CTAn SkyScan software. After setting the region of interest, the subchondral bone volume, and thickness, the number of objects per slice were evaluated.
Data analysis
Each value for histological assessments and pain behaviors was expressed as mean ± standard error of the mean (SEM). The data were statistically evaluated using Student's t-tests or one-way analyses of variance (ANOVAs) followed by Duncan's multiple range test to compare groups. Differences were considered significant at P < 0.05, 0.01, and 0.001.
Results
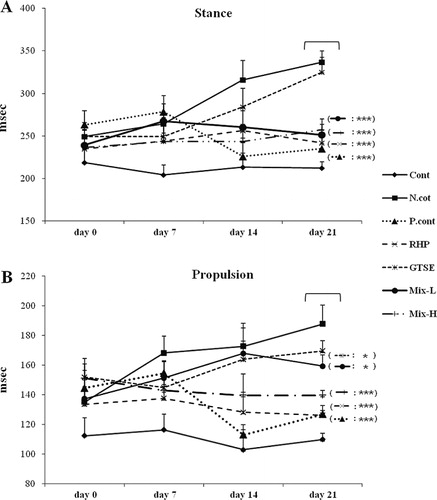
Effects of RHP and GTSE on stance and propulsion time in an OA rat model
The stance and propulsion time were measured for 3 weeks after the induction of OA using a TreadScanTM system. The gait data in six groups at days 0, 7, 14, and 21 are shown in . These data showed that stance time significantly increased 1.58-fold in the negative control group compared to the control group at 21 days. In contrast, the positive control group, RHP group, GTSE group, Mix-L group (500 mg/kg bw RHP + 25 mg/kg bw low dose GTSE), and Mix-H group (500 mg/kg bw RHP + 50 mg/kg bw high dose GTSE) showed a significant reduction in stance time compared to the negative control group (1.42-, 1.39-, 1.33-, and 1.3-fold change on day 21, respectively). Regarding propulsion time, we found an increased time in the negative control group and a significantly decreased time in the control group, positive control group, RHP group, and Mix-H groups (1.81-, 1.48-, 1.48-, and 1.34-fold change on day 21). The GTSE group was not significantly different than the negative control group for either measurement.
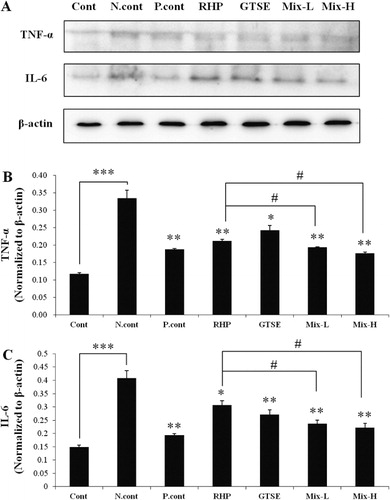
The reductive effects of RHP, GTSE, and Mix on pro-inflammatory cytokines in an OA rat model
Pro-inflammatory cytokines such as TNF-α and IL-6 are the main cytokines involved in OA that lead to an increase in COX-2 and iNOS (Kapoor et al. Citation2011). As shown in , the TNF-α expression level was increased 3.75-fold (P < 0.001) in the negative control group compared to the control group ( and ). However, TNF-α expression levels were significantly suppressed 1.73 (P < 0.01)-, 1.58 (P < 0.01)-, 1.37 (P < 0.05)-, 1.82 (P < 0.01)-, and 1.86 (P < 0.01)-fold in the positive control, RHP, GTSE, Mix-L and Mix-H groups, respectively, compared to the negative control group. Interestingly, TNF-α expression in the Mix-L and Mix-H groups was significantly decreased to 1.10 (P < 0.05)- and 1.16 (P < 0.05)-fold, respectively, compared to the RHP group. The expression level of IL-6 was increased 2.75-fold (P < 0.001) times in the negative control group compared to the control group ( and ), and IL-6 expression levels were significantly suppressed 1.81 (P < 0.01)-, 1.65 (P < 0.05)-, 1.33 (P < 0.05)-, 1.7 (P < 0.01),- and 1.9 (P < 0.01)-fold in the positive control, RHP, GTSE, Mix-L, and Mix-H groups compared to the negative control group. Furthermore, administration of Mix-L and Mix-H resulted in the significant suppression of MIA-induced IL-6 expression of 1.29 (P < 0.05)- and 1.37 (P < 0.05)-fold, respectively, compared to the group treated with the single treatment of RHP.
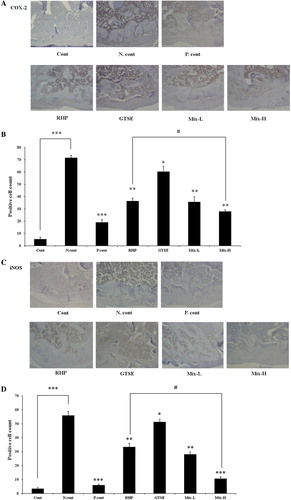
Combined effects of RHP and GTSE on COX-2 and iNOS expression in an OA rat model
To evaluate the chondroprotective effects of RHP and GTSE, the expressions of iNOS and COX-2 were measured by immunohistochemistry (). iNOS expression in chondrocytes was significantly increased in the negative control group (56.00 ± 2.89, P < 0.001) compared to the control group (3.33 ± 0.88). However, the positive control group (treatment with 2 mg methotrexate) exhibited a significantly reduced expression level of 6.00 ± 0.58 (P < 0.001). In the RHP group, GTSE group, Mix-L group, and Mix-H group, there were significantly reduced expression levels of 33.33 ± 4.51 (P < 0.01), 51.34 ± 2.45 (P < 0.05), 28.00 ± 1.73 (P < 0.01), and 10.67 ± 1.20 (P < 0.01), respectively, compared to the negative control group ( and ). The expression of COX-2 in chondrocytes was significantly reduced in the positive control, RHP, GTSE, Mix-L, and Mix-H groups to 19.00 ± 2.31 (P < 0.001), 36.33 ± 2.40 (P < 0.01), 60.53 ± 4.23 (P < 0.05), 35.67 ± 4.10 (P < 0.001), and 28.00 ± 1.15 (P < 0.001), respectively, compared to the negative control group (71.67 ± 1.76, and ). Notably, the Mix-H group was more significantly reduced than the RHP group with regard to iNOS and COX-2 expressions (P < 0.05).
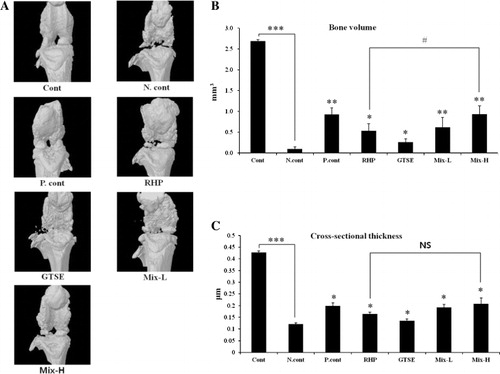
Combined effects of RHP and GTSE on bone volume and cross-sectional thickness in an OA rat model
The combined effects of RHP and GTSE on bone volume and cross-sectional thickness were measured using micro-computed tomography (micro-CT). As shown in , the negative group displayed widespread osteophyte formation around the outer layers after MIA injection. However, treatment with methotexate, RHP, GTSE, or mixed compounds showed gradual recovery. The negative control group (0.10 ± 0.05 mm3) exhibited a reduction of bone volume compared to the control group (2.69 ± 0.04 mm3). In contrast, the positive, RHP, GTSE, Mix-L, and Mix-H groups showed significantly restored bone volume to 0.93 ± .0.16 mm3 (P < 0.01), 0.53 ± 0.17 mm3 (P < 0.05), 0.26 ± 0.08 mm3 (P < 0.05), 0.62 ± 0.23 mm3 (P < 0.01), and 0.93 ± 0.20 mm3 (P < 0.01), respectively, compared to the negative control group (). Furthermore, the Mix-L and Mix-H groups exhibited significantly higher restoration than the RHP group (P < 0.05). As shown in , the cross-sectional thickness levels in the negative control group (0.11 ± 0.01 μm, P < 0.001) were significantly reduced after MIA injection compared to the control group (0.42 ± 0.01 μm). However, treatment with methotrexate (0.20 ± 0.03 μm, P < 0.05), RHP (0.16 ± 0.01 μm, P < 0.05), GTSE (0.13 ± 0.01 μm, P <0.05), and mixed compounds (0.19 ± 0.03 μm, P <0.05) significantly restored the cross-sectional thickness after MIA injection ().
Discussion
This study was designed and conducted to determine whether the combined administration of RHP and GTSE has an enhanced therapeutic effect on induced OA in an animal model. Our results showed, for the first time, that the oral consumption of RHP, green tae seed extract, and its mixture may suppress physical pain by reducing cartilage damage and pro-inflammatory cytokine expression. OA is the most common disease involving cartilage degradation. The synovial membrane, periosteum, and subchondral bone are also involved in the progression of the disease (Mach et al. Citation2002; Berenbaum Citation2013). To effectively induce OA in an animal model, we injected MIA into the rat knee. MIA consistently induces rapid chondral erosion and osteophytes and it is a good background measure of joint impairment during the course of experimental joint disease (Guingamp et al. Citation1997; Jiang et al. Citation2013).
We herein present four principal findings regarding the actions of RHP, GTSE, and its mixed compounds through in vivo studies aimed at explaining the anti-osteoarthritic effects of RHP against MIA-induced OA.
First, we investigated the combined effect of RHP and GTSE on pain-related behaviors. The gait analysis results, which measured recovery from pain in the MIA-induced OA model, showed that a mixture of RHP and GTSE significantly enhanced stance and propulsion times. These results suggest that RHP and GTSE administration may result in pain relief from OA. However, because the RHP treatment group and the Mix treatment group were not significantly different, these results did not confirm a synergistic effect of RHP and GTSE.
Second, we demonstrated that the expression of IL-6 and TNF-α increased in the OA vehicle- treatment group. TNF-α and IL-6 appear to be the main pro-inflammatory cytokines involved in the pathophysiology of OA (Kapoor et al. Citation2011). TNF-α induces the overproduction of reactive oxygen species (ROS), diminishes the activity of antioxidant enzymes, and contributes to cartilage impairment (Afonso et al. Citation2007). IL-6 is a regulator of inflammatory and immunological processes and is produced at low levels in chondrocytes under normal conditions. However, a number of cytokines are activated during OA (Guerne et al. Citation1990), and increased IL-6 activates COX-2, resulting in the production of prostaglandin E2 (PGE2; Wang et al. Citation2010). These increased cytokines were suppressed in the RHP and GTSE mix groups. Moreover, the Mix-H group showed significantly reduced cytokine expression compared to the RHP group.
Third, we clarified the cross-talk between iNOS and COX-2 in OA after administration of RHP, GTSE, and the Mix groups. Nitric oxide (NO) is a key inducer of chondrocyte apoptosis, a pathogenic feature of OA (Abramson Citation2008). NO is cytotoxic at high concentrations and has the potential to react with superoxide, leading to cell toxicity. It has been shown that the iNOS-derived overproduction of NO can lead to the activation of NF-κB, which in turn leads to the upregulation of COX-2 and iNOS (Tsai et al. Citation2012). Therefore, when COX-2 and iNOS are increased, NO production increases in the OA knee. Our result showed that COX-2 and iNOS productions during OA were suppressed by the RHP, GTSE, and Mix groups. Additionally, the Mix-H group exhibited more significant reductions in COX-2 and iNOS than the RHP group. These results suggest that RHP and GTSE have synergistic anti-inflammatory effects in the MIA-induced OA model.
Finally, micro-CT was used to quantify structural changes in the subchondral bone in the MIA rat model (Mohan et al. Citation2011). Micro-CT has the advantage of reducing the number of animals used while increasing the sensitivity of the experiment to detect subtle changes (Boyd et al. Citation2006). We utilized micro-CT to allow a tissue-level characterization of the MIA-induced OA model including bone volume (mm3) and cross-sectional thickness (μm). The observed bone volume and cross-sectional thickness decreases after MIA injection were improved by treatment in the RHP, GTSE, Mix-L, and Mix-H groups. Notably, the bone volume levels of Mix-H group were more significantly restored when compared to the RHP group. However, there was no significant difference between the RHP group and Mix groups with regard to cross-sectional thickness levels.
Based on these results, we confirmed the combined chondroprotective activities of RHP and GTSE against MIA-induced OA. The results demonstrated that treatment with RHP and GTSE reduced pain and inflammation, which is in accordance with several studies that found that the expression of antioxidant enzymes, including superoxide, catalase and glutathione peroxide, downregulated pro-inflammatory cytokines during OA (Mathy-Hartert et al. Citation2008; Scott et al. Citation2010). Antioxidant activity is a major therapeutic factor during OA (Canter et al. Citation2007; Nemirovskiy et al. Citation2009). R. canina L. has been shown to be rich in polyphenols, such as flavonoids and phenolic compounds (Bravo Citation1998; Hvattum Citation2002; Guo et al. Citation2009), and to act on essential fatty acids, such as linoleic acid, alpha-linolenic acid, and triterpene acid (Jäger et al. Citation2007; Jäger et al. Citation2008; Wenzing et al. Citation2008). Additionally, green tea seed has been found to have anti-inflammatory effects in vitro (Lee et al. Citation2011; Noh et al. Citation2011). Thus, RHP and GTSE could play an antioxidant-related role, resulting in chondroprotective activity. Although the gait analysis and micro-CT data did not show synergistic effects of RHP and GTSE on MIA-induced OA, the pro-inflammatory cytokine and chemokine data clearly demonstrated that the mixture of RHP and GTSE had synergistic effects against MIA-induced OA. Further experiments to determine the anti-inflammatory factors of RHP and GTSE, and the molecular mechanisms involved in their action, will be needed in the future.
Conclusion
In the present study, we demonstrated that RHP and GTSE treatments have combined chondroprotective effects in the MIA-induced OA model in rats. The Mix-H treatment reduced the expression of pro-inflammatory cytokines and chemokines in MIA-induced OA more effectively and significantly than the other treatments. Moreover, our data showed that MIA-induced pain and cartilage loss were attenuated by the administration of Mix. GTSE administration was enhanced by RHP supplementation to include anti-inflammatory effects, offering a useful therapeutic choice in the treatment of OA. In conclusion, these findings scientifically support the value of RHP and GTSE as therapeutics for OA.
Acknowledgement
This research was supported by the growth of establishment technology development program (S2125632) funded by the Small and Medium Business Administration (SMBA, Korea) and by the Jeollanamdo Institute of Natural Resources Research (JINR) Core-Competence Program.
References
- Abramson SB. 2008. Osteoarthritis and nitric oxide. Osteoarthritis Cartilage. 16:S15–S20. 10.1016/S1063-4584(08)60008-4
- Afonso V, Champy R, Mitrovic D, Collin P, Lomri A. 2007. Reactive oxygen species and superoxide dismutases: role in joint diseases. Joint Bone Spine. 74:324–329. 10.1016/j.jbspin.2007.02.002
- Berenbaum F. 2013. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthritis Cartilage. 21:16–21. 10.1016/j.joca.2012.11.012
- Bravo L. 1998. Polyphenols: chemistry, dietary sources, metabolism, and nutritional significance. Nutr Rev. 56:317–333. 10.1111/j.1753-4887.1998.tb01670.x
- Boyd SK, Davison P, Müller R, Gasser JA. 2006. Monitoring individual morphological changes over time in ovariectomized rats by in vivo micro-computed tomography. Bone. 39:854–862. 10.1016/j.bone.2006.04.017
- Canter PH, Wider B, Ernst E. 2007. The antioxidant vitamins A, C, E and selenium in the treatment of arthritis: a systematic review of randomized clinical trials. Rheumatology. 46:1223–1233. 10.1093/rheumatology/kem116
- Chrubasik C, Roufogalis BD, Muller-Ladner U, Chrubasik S. 2008. A systematic review on the Rosa canina effect and efficacy profiles. Phytother Res. 22:725–33.
- Chrubasik C, Wiesner L, Black A, Muller-Ladner U, Chrubasik S. 2008. A one-year survey on the use of a powder from Rosa canina Lito. in acute exacerbations of chronic pain. Phytother Res. 22:1141–1148. 10.1002/ptr.2352
- Deliorman OD, Hartevioglu A, Kupeli E, Yesilada E. 2007. In vivo anti-inflammatory and antinociceptive activity of the crude extract and fractions from Rosa canina L. fruits. J Ethnopharmacol. 112:394–400. 10.1016/j.jep.2007.03.029
- Francesca L, Emanuela G. 2011. In vivo anti-inflammatory effect of Rosa canina L. extract. J Ethnopharmacol. 137:880–885. 10.1016/j.jep.2011.07.006
- Goldring MB, Goldring SR. 2007. Osteoarthritis. J Cell Physiol. 213:626–634. 10.1002/jcp.21258
- Guerne PA, Carson DA, Lotz M. 1990. IL-6 production by human articular chondrocytes. Modulation of its synthesis by cytokines, growth factors, and hormones in vitro. J Immunol. 144:499–505.
- Guingamp C, Gegout-Pottie P, Philippe L, Terlain B, Netter P, Gillet P. 1997. Mono-iodoacetate-induced experimental osteoarthritis: a dose-response study of loss of mobility, morphology, and biochemistry. Arthritis Rheum. 40:1670–1679. 10.1002/art.1780400917
- Guo W, Kong E, Meydani M. 2009. Dietary polyphenols, inflammation, and cancer. Nutr Cancer. 61:807–810. 10.1080/01635580903285098
- Haas LF. 1995. Rosa canina (dog rose). J Neurol Neurosurg Psychiatry. 59:470.
- Hvattum E. 2002. Determination of phenolic compounds in rose hip (Rosa canina) using liquid chromatography coupled to electrospray ionisation tandem mass spectrometry and diode-array detection. Rapid Commun Mass Spectrom. 16:655–662. 10.1002/rcm.622
- Jäger AK, Eldeen IM, van Staden J. 2007. COX-1 and COX-2 activity of rose hip. Phytother Res. 21:1251–1252.
- Jäger AK, Petersen KN, Thomasen G, Christensen SB. 2008. Isolation of linoleic and alpha-linolenic acids as COX-1 and -2 inhibitors in rose hip. Phytother Res. 22:982–984.
- Jiang L, Li L, Geng C, Gong D, Jiang L, Ishikawa N, Kajima K, Zhong L. 2013. Monosodium iodoacetate induces apoptosis via the mitochondrial pathway involving ROS production and caspase activation in rat chondrocytes in vitro. J Orthop Res. 31:364–369. 10.1002/jor.22250
- Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. 2011. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 7:33–42. 10.1038/nrrheum.2010.196
- Kharazmi A, Winther K. 1999. Rose hip inhibits chemotaxis and chemiluminescence of human peripheral blood neutrophils in vitro and reduces certain inflammatory parameters in vivo. Inflammopharmacology. 7:377–386. 10.1007/s10787-999-0031-y
- Kumarasamy Y, Cox PJ, Jaspars M, Rashid MA, Sarker DS. 2003. Bioactive flavonoid glycosides from the seeds of Rosa canina. Pharm Biol. 41:237–242. 10.1076/phbi.41.4.237.15663
- Larsen E, Kharazmi A, Christensen LP, Christensen SB. 2003. An anti-inflammatory galactolipid from rose hip (Rosa canina) that inhibits chemotaxis of human peripheral blood neutrophils in vitro. J Nat Prod. 66:994–995. 10.1021/np0300636
- Lattanzio F, Greco E, Carretta D, Cervellati R, Govoni P, Speroni E. 2011. In vivo anti-inflammatory effect of Rosa canina L. extract. J Ethnopharmacol. 137:880–885. 10.1016/j.jep.2011.07.006
- Lee HB, Kim EK, Park SJ, Bang SG, Kim TG, Chung DW. 2011. Isolation and anti-inflammatory effect of astragalin synthesized by enzymatic hydrolysis of tea seed extract. J Sci Food Agric. 91:2315–2321. 10.1002/jsfa.4457
- Mach DB, Rogers SD, Sabino MC, Luger NM, Schwei MJ, Pomonis JD, Keyser CP, Clohisy DR, Adams DJ, O'Leary P, Mantyh PW. 2002. Origins of skeletal pain: sensory and sympathetic innervation of the mouse femur. Neuroscience. 113:155–166. 10.1016/S0306-4522(02)00165-3
- Mathy-Hartert M, Hogge L, Sanchez C, Deby-Dupont G, Crielaard JM, Henrotin Y. 2008. Interleukin-1β and interleukin-6 disturb the antioxidant enzyme system in bovine chondrocytes: a possible explanation for oxidative stress generation. Osteoarthritis Cartilage. 16:756–763. 10.1016/j.joca.2007.10.009
- Mohan G, Perilli E, Kuliwaba JS, Humphries JM, Parkinson IH, Fazzalari NL. 2011. Application of in vivo micro-computed tomography in the temporal characterisation of subchondral bone architecture in a rat model of low-dose monosodium iodoacetate-induced osteoarthritis. Arthritis Res Ther. 13:R210. 10.1016/j.joca.2007.03.014
- Nagatomo A, Nishida N, Matsuura Y, Shibata N. 2013. Rosehip extract inhibits lipid accumulation in white adipose tissue by suppressing the expression of peroxisome proliferator-activated receptor gamma. Prev Nutr Food Sci. 18:85–91. 10.3746/pnf.2013.18.2.085
- Nemirovskiy OV, Radabaugh MR, Aggarwal P, Funcke Shippy CL, Mnich SJ, Meyer DM, Sunyer T, Rodney Mathews W, Misko TP. 2009. Plasma 3-nitrotyrosine is a biomarker in animal models of arthritis: pharmacological dissection of iNOS' role in disease. Nitric Oxide. 20:150–156. 10.1016/j.niox.2008.12.005
- Noh KH, Kim JK, Song YS. 2011. Suppressive effects of ethyl acetate faction from green tea seed coats on mediators in human umbilical vein endothelial cells. J Korean Soc Food Sci Nutr. 40:635–641. 10.3746/jkfn.2011.40.5.635
- Orhan D, Hartevioğlu A, Küpeli E, Yesilada E. 2007. In vivo anti-inflammatory and antinociceptive activity of the crude extract and fractions from Rosa canina L. fruits. J Ethnopharmacol. 122:394–400. 10.1016/j.jep.2007.03.029
- Saklatvala J. 2007. Inflammatory signaling in cartilage: MAPK and NF-κB pathways in chondrocytes and the use of inhibitors for research into pathogenesis and therapy of osteoarthritis. Curr Drug Targets. 8:305–313.
- Scott JL, Gabrielides C, Davidson RK, Swingler TE, Clark IM, Wallis GA, Boot-Handford RP, Kirkwood TB, Taylor RW, Young DA. 2010. Superoxide dismutase down regulation in osteoarthritis progression and end-stage disease. Ann Rheum Dis. 69:1502–1510.
- Simon LS, Pincus T, Whelton A, Makuch R, Eisen G, Agrawal NM, Stenson WF, Burr AM, Zhao WW, Kent JD, et al. 2000. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: a randomized controlled trial. JAMA. 284:1247–1255.
- Stralsjö L, Alklint C, Olsson ME, Sjöholm I. 2003. Total folate content and retention in rosehips (Rosa ssp.) after drying. J Agric Food Chem. 51:4291–4295.
- Tsai KL, Huang YH, Kao CL, Yang DM, Lee HC, Chou HY, Chen YC, Chiou GY, Chen LH, Yang YP, et al. 2012. A novel mechanism of coenzyme Q10 protects against human endothelial cells from oxidative stress-induced injury by modulating NO-related pathways. J Nutr Biochem. 23: 458–468. 10.1016/j.jnutbio.2011.01.011
- Uggla M, Gao X, Werlemark G. 2003. Variation among and within dog rose taxa (Rosa sect. caninae) in fruit weight, percentages of fruit flesh and dry matter, and vitamin C content. Acta Agric Scand Sect B. 53:147–155.
- Wang P, Zhu F, Konstantopoulos K. 2010. Prostaglandin E2 induces interleukin-6 expression in human chondrocytes via cAMP/protein kinase A- and phosphatidylinositol 3-kinase-dependent NF-κB activation. Am J Physiol Cell Physiol. 298:C1445–C1456. 10.1152/ajpcell.00508.2009
- Wenzing EM, Widowitz U, Kunert O, Chrubasik S, Bucar F, Knauder E, Bauer R. 2008. Phytochemical composition and in vitro pharmacological activity of two rose hip (Rosa canina L.) preparations. Phytomedicine. 15:826–835. 10.1016/j.phymed.2008.06.012
