ABSTRACT
Bile acids (BAs) are steroid acids found predominantly in the bile of mammals and other vertebrates. Though BAs have been known as digestive juice, recent studies have revealed that BAs act as signaling molecules to control metabolism and inflammation. Today, BAs are considered as potential therapeutic molecules for treatment of complex metabolic liver disease. However, the detergent properties of BAs lead to hepatic injury and intrahepatic cholestasis when BAs are accumulated in the liver with impaired bile flow into gall bladder. Cholestasis is a pathological condition of hepatic retention of cytotoxic bile acids. To date, hydrophilic ursodeoxycholic acid has been currently used to treat cholestasis, but the efficacy of UDCA for cholestasis is still limited. Given that BAs are endogenous ligands of several nuclear receptors, including Farnesoid X receptor and Pregnane X receptor, novel synthetic ligands for those nuclear receptors are promising for the treatment of cholestatic liver diseases.
Introduction
Cholestatic liver disease is an impaired bile formation condition in which bile flows into gall bladder, resulting in hepatic accumulation of bile acids (BAs), cholesterol and bilirubin (Trauner et al. Citation1998; Hofmann Citation2002; Zollner & Trauner Citation2009; Jonker et al. Citation2012). Accumulation of hydrophobic BAs results in the production of reactive oxidative species (ROS), leading to progressive liver disease with eventually cirrhosis (Copple et al. Citation2010). Increased levels of ROS have been observed in obstructive cholestasis in rodent model and human patients. And restoration of redox balance to reduce cellular ROS levels can prevent further oxidative liver injury.
In general, cholestasis results from (1) hepatocellular and/or cholangiocellular secretory defects and (2) obstruction of bile ducts. Pathological conditions including primary biliary cirrhosis (PBC) or primary sclerosing cholangitis (PSC) may also result in the cholestatic liver diseases (Maillette de Buy Wenniger et al. Citation2012). For the treatment of cholestatic liver diseases, treatment with ursodeoxycholic acid (UDCA) has been clinically approved to slow the progression of chronic cholangiopathies, but still has limitations to various chronic cholestatic liver diseases. UDCA, the only drug approved by Food and Drug Administration for the treatment of primary bilary cirrhosis, has antioxidant properties (Poupon et al. Citation1987; Pares et al. Citation2000; Guo et al. Citation2015). UDCA can scavenge hydroxyl radicals and induce gene expressions involved in antioxidant defenses, including glutathione (GSH) synthesis (Guo et al. Citation2015). However, the efficacy of UDCA is limited to the early stages PBC, novel therapeutic strategies for the treatment of cholestatic liver diseases are required to be developed.
Clinical effects of UDCA
After discovery of natural bile acid (BA) in 1970, oral treatment of chenodeoxycholic acid (CDCA) induces the dissolution of cholesterol gall stone (Marin et al. Citation2015). However, the hepatotoxic properties of CDCA induced biliary cirrhosis and dose-dependent diarrhea in human (Saeki et al. Citation1995). UDCA has been introduced for the treatment of gallstone disease without side effects by CDCA and been accepted a potential therapeutic approach for the cholestatic liver diseases (Pares et al. Citation2000; Marin et al. Citation2015). The rationale of UDCA treatment is that replacement of endogenous BAs by nontoxic BA (UDCA) can protect liver to slow down the progression of cholestatic liver disease. UDCA therapy is widely accepted for all patients with PBC.
The biliary secretion helps to maintain an alkaline pH in the hepatocytes and cholangiocytes to protect against protonated glycine-conjugated BAs (Beuers et al. Citation2010; Hohenester et al. Citation2012). Thus, biliary
serves to protect hepatocytes and cholangiocytes against the toxic effect of endogenous bile acids in bile. Functional-impaired biliary
secretion leads to an increase in vulnerability of hepatocytes and cholangiocytes to the hydrophobic BAs (Hohenester et al. Citation2012). Notably, UDCA stimulates biliary
secretion in PBC patients, suggesting that UDCA treatment would prevent hepatocytes and cholangiocytes from the hydrophobic endogenous BAs. Thus, the physiological effects of UDCA are crucial to protect against hepatocytes and cholangiocytes from the cholestatic liver disease.
Nuclear receptors
Nuclear receptors (NRs) are ligand-activated transcriptional factors able to bind to response element of target gene promoters (Evans Citation1988; McKenna et al. Citation2014). In human, total 48 NRs have been isolated, and categorized into 6 subfamily (1∼6) and extra subfamily (0) by the Nuclear Receptors Nomenclature Committee based on the categorization of cytochrome P450.
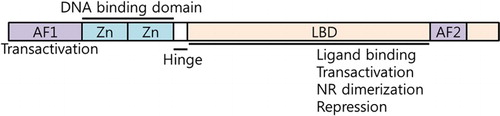
Depending on their endogenous ligands and physiological functions, NRs can be sorted into three groups. First group of NRs is ‘endocrine receptors’ with high affinity with endogenous ligands, including vitamin A (Retinoic acid receptors; RAR), vitamin D (Vitamin D receptor; VDR), thyroid (Thyroid hormone receptor; TR), and glucocorticoid (Glucocorticoid receptor; GR). Also, steroid hormone receptors including estrogen receptor (ER), progesterone receptor (Kir et al.) and androgen receptor are also categorized in the endocrine receptors. Based on the structures of endocrine receptors, other nuclear receptor genes were also cloned. Orphan nuclear receptors belong to the nuclear receptor superfamily and lack identified endogenous ligands when they were initially cloned. Recent studies have revealed that NRs and orphan nuclear receptors play various biological roles in the regulation of cell proliferation, cellular metabolism, circadian rhythm, energy metabolism, lipid and glucose metabolism, and drug metabolism. NRs are deoxyribonucleic acid (DNA)-binding transcriptional factors. In general, NRs consist of six domains (A–F) based on regions of conserved sequence and function. They typically have a highly conserved DNA-binding domain (DBD) in the N-terminus region and a ligand-binding domain (LBD) in the C-terminus region. Ligand-independent activation function-1 (AF1) and ligand-dependent activation function-2 (AF2) are located in the N-terminus and C-terminus, respectively. In DBD, two cysteine-Zn2+ finger motifs are critical for DNA binding and NR dimerization. Upon the binding of ligands, the LBDs change the three-dimensional structures to directly interact with the ligands. The structural changes of LBD lead to the cofactor exchanges between coactivators and corepressors (). Upon activation of ligands, NRs directly interact with the response elements of DNA to activate their target gene expressions.
Clinical effects of Farnesoid X receptor (FXR)
Like other nuclear receptors, FXR contains both DNA and ligand-binding domains. With retinoid X receptor (RXR), FXR forms heterodimer to bind to the FXR response elements, which is located in the FXR target gene promoters. The expression level of FXR is pretty abundant in the gastrointestinal tract, such as liver, intestine, and kidney (Chiang Citation2002).
FXR functions as an intracellular BA sensor to control hepatic BA synthesis (Chiang Citation2002). The increased BA binding to FXR enhances FXR transcriptional activity to promote FXR target gene induction dependent on an increase in intracellular BA levels. Upon BA binding, FXR induces small heterodimer partner, an orphan nuclear receptor, which suppresses CYP7A1 gene expression to reduce the rate of hepatic BA conversion from cholesterol (Jacinto & Fang Citation2014). Furthermore, FXR activation leads to induction of gene expressions involved in BA export from the liver to the gall bladder. Bile salt export pump (BSEP) is a well-known FXR target gene to transport BA from liver to gall bladder. Thus, FXR activation induces BSEP expression to increase BA flow to gall bladder, leading to reduction of hepatic BA levels. Studies of FXR-deficient mice have demonstrated elevated plasma level of BAs and increased fecal BA excretion, implying that the absence of FXR-mediated negative feedback leads to impaired BA homeostasis (Lee et al. Citation2006).
Clinical effects of FXR-FGF19 signaling axis
FGF15/19 is a member of the fibroblast growth factor family. FGF 15/19 serves as inter-organ hormones between gut and liver. FGF 15/19 is a post-prandial molecule secreted by food intake (Kir et al. Citation2011). After a meal, the gall bladder releases BA into the small intestine where BAs are re-absorbed in the ileum. The absorbed BAs then activate FXR in the enterocytes before they enter the portal vein of entero-hepatic circulation. In the ileum, FXR activation induces FGF 15/19 expression, then FGF 15/19 enters the entero-hepatic circulation to bind to the fibroblast growth factor receptor 4 (FGFR4) in the hepatocytes. Subsequently FGF 15/19 binds to FGFR4 in hepatocytes to stimulates the c-Jun N-terminal Kinase signaling pathway to suppress CYP7A1 gene expression to reduce hepatic BA synthesis (Kliewer & Mangelsdorf Citation2015).
Besides BA synthesis regulation, FGF15/19 suppresses insulin signaling to repress lipogenic gene expressions, including fatty acid synthase and sterol regulatory element-binding protein 1c, and stimulates glycogen synthesis and reduces gluconeogenesis (Kliewer & Mangelsdorf Citation2015). As a potential therapeutic strategy for cholestatic liver disease, FGF15/19 has the disadvantage that it needs to be injected instead of oral treatment. However, FGF19 derivative NGM282 with UDCA has been tested for clinical trial phase II in PBC patients (Luo et al. Citation2014). Given that FGF15/19 is a mitogenic peptide, the carcinogenic properties of FGF15/19 should be carefully considered for the clinical purpose.
Clinical effects of Pregnane X receptor (PXR)
PXR has been a well-known orphan nuclear receptor to regulate gene expressions involved in drug metabolism. Previous reports have shown that PXR is activated by a broad spectrum of xenobiotics and endobiotics, including lithocholic acid (Jonker et al. Citation2012). Bile acids are toxic at high concentrations, and nuclear receptors including PXR also participate to regulate BA catabolism with FXR.
In PXR-deficient mice, the experimental cholestasis has been aggravated to induce more hepatic damage than wild-type animals (Stedman et al. Citation2005). However, the treatment of potent PXR ligand 5-pregrene-3β-ol-20-one-16α-carbonitrile (PCN) markedly reduced cholic acid feeding-induced liver injury (Teng & Piquette-Miller Citation2007). The beneficial effects of PCN to reduce hepatic injury have not been observed in PXR knockout mice, suggesting that PCN-mediated beneficial effects worked via PXR. PCN directly induces the expression of basolateral BA efflux transporter MRP3 in the hepatocytes to increase BA secretion from the hepatocytes (Teng & Piquette-Miller Citation2007).
Besides PCN, other PXR agonists, such as rifampicin, have been documented with an evidence to improve serum liver tests in PBC patients (Bachs et al. Citation1992; Khurana & Singh Citation2006). In healthy gallstone patients, rifampicin induced the expression of UGC1A1 and MRP2 that facilitate bilirubin elimination and increased CYP3A4 gene expression to detoxify endogenous BAs. Rifampicin has been treated to cholestatic liver disease patients for two weeks. Long-term treatment of rifampicin (more than four weeks) has been shown to induce hepatotoxicity within the rifampicin-treated patients (Bachs et al. Citation1992). Strikingly, rifampicin has been very effective to persistent hepatocellular secretory failure. Thus, the PXR agonists may be promising for the treatment of severe persistent hepatocellular secretory failure.
Conclusion
Numerous therapeutic reagents have been developed to treat patients with chronic cholestatic liver diseases. While UDCA has been approved with its anticholestatic effects, it is required to develop novel therapeutic agents to regulate gene expressions of bile secretion and cell protection. Several agonists for FXR and PXR in combination with UDCA have been advanced to phase III trials in chronic cholestatic liver diseases (). In addition to FXR and PXR, other nuclear receptors, such as GR, peroxisome proliferator-activated receptor α (PPARα), and VDR are well known to regulate BA detoxification. Thus, development of therapeutic reagents using synthetic agonists for GR, PPARα, and VDR is a promising treatment for the chronic cholestatic liver disease. Though no effective therapeutic strategy for the treatment of cholestatic liver disease was available 30 years ago, development of therapeutic reagents for cholestatic disorders is now optimistic for patients and their caring physicians.
Table 1. Potential therapeutic agents targeting FXR and PXR to regulate bile formation and secretion.
ORCiD
Sungsoon Fang http://orcid.org/0000-0003-0201-5567
Additional information
Funding
References
- Bachs L, Pares A, Elena M, Piera C, Rodes J. 1992. Effects of long-term rifampicin administration in primary biliary cirrhosis. Gastroenterology. 102:2077–2080. doi: 10.1016/0016-5085(92)90335-V
- Beuers U, Hohenester S, de Buy Wenniger LJ, Kremer AE, Jansen PL, Elferink RP. 2010. The biliary HCO(3)(-) umbrella: a unifying hypothesis on pathogenetic and therapeutic aspects of fibrosing cholangiopathies. Hepatology. 52:1489–1496. doi: 10.1002/hep.23810
- Chiang JY. 2002. Bile acid regulation of gene expression: roles of nuclear hormone receptors. Endocr Rev. 23:443–463. doi: 10.1210/er.2000-0035
- Copple BL, Jaeschke H, Klaassen CD. 2010. Oxidative stress and the pathogenesis of cholestasis. Semin Liver Dis. 30:195–204. doi: 10.1055/s-0030-1253228
- Evans RM. 1988. The steroid and thyroid hormone receptor superfamily. Science. 240:889–895. doi: 10.1126/science.3283939
- Guo T, Chang L, Xiao Y, Liu Q. 2015. S-adenosyl-L-methionine for the treatment of chronic liver disease: a systematic review and meta-analysis. PLoS One. 10:e0122124. doi: 10.1371/journal.pone.0122124
- Hofmann AF. 2002. Cholestatic liver disease: pathophysiology and therapeutic options. Liver Int. 22:14–19. doi: 10.1034/j.1600-0676.2002.00002.x
- Hohenester S, Wenniger LM, Paulusma CC, van Vliet SJ, Jefferson DM, Elferink RP, Beuers U. 2012. A biliary HCO3- umbrella constitutes a protective mechanism against bile acid-induced injury in human cholangiocytes. Hepatology. 55:173–183. doi: 10.1002/hep.24691
- Jacinto S, Fang S. 2014. Essential roles of bile acid receptors FXR and TGR5 as metabolic regulators. Anim Cells Syst. 18:359–364. doi: 10.1080/19768354.2014.987318
- Jonker JW, Liddle C, Downes M. 2012. FXR and PXR: potential therapeutic targets in cholestasis. J Steroid Biochem Mol Biol. 130:147–158. doi: 10.1016/j.jsbmb.2011.06.012
- Khurana S, Singh P. 2006. Rifampin is safe for treatment of pruritus due to chronic cholestasis: a meta-analysis of prospective randomized-controlled trials. Liver Int. 26:943–948. doi: 10.1111/j.1478-3231.2006.01326.x
- Kir S, Beddow SA, Samuel VT, Miller P, Previs SF, Suino-Powell K, Xu HE, Shulman GI, Kliewer SA, Mangelsdorf DJ. 2011. FGF19 as a postprandial, insulin-independent activator of hepatic protein and glycogen synthesis. Science. 331:1621–1624. doi: 10.1126/science.1198363
- Kliewer SA, Mangelsdorf DJ. 2015. Bile acids as hormones: the FXR-FGF15/19 pathway. Dig Dis. 33:327–331. doi: 10.1159/000371670
- Lee H, Zhang Y, Lee FY, Nelson SF, Gonzalez FJ, Edwards PA. 2006. FXR regulates organic solute transporters alpha and beta in the adrenal gland, kidney, and intestine. J Lipid Res. 47:201–214. doi: 10.1194/jlr.M500417-JLR200
- Luo J, Ko B, Elliott M, Zhou M, Lindhout DA, Phung V, To C, Learned RM, Tian H, DePaoli AM, et al. 2014. A nontumorigenic variant of FGF19 treats cholestatic liver diseases. Sci Transl Med. 6:247ra100. doi: 10.1126/scitranslmed.3009098
- Maillette de Buy Wenniger LJ, Oude Elferink RP, Beuers U. 2012. Molecular targets for the treatment of fibrosing cholangiopathies. Clin Pharmacol Ther. 92:381–387. doi: 10.1038/clpt.2012.111
- Marin JJ, Macias RI, Briz O, Banales JM, Monte MJ. 2015. Bile acids in physiology, pathology and pharmacology. Curr Drug Metab. 17:4–29. doi: 10.2174/1389200216666151103115454
- McKenna NJ, Evans RM, O’Malley BW. 2014. Nuclear receptor signaling: a home for nuclear receptor and coregulator signaling research. Nucl Recept Signal. 12:e006.
- Pares A, Caballeria L, Rodes J, Bruguera M, Rodrigo L, Garcia-Plaza A, Berenguer J, Rodriguez-Martinez D, Mercader J, Velicia R. 2000. Long-term effects of ursodeoxycholic acid in primary biliary cirrhosis: results of a double-blind controlled multicentric trial. UDCA-Cooperative Group from the Spanish Association for the Study of the Liver. J Hepatol. 32:561–566. doi: 10.1016/S0168-8278(00)80216-0
- Poupon R, Chretien Y, Poupon RE, Ballet F, Calmus Y, Darnis F. 1987. Is ursodeoxycholic acid an effective treatment for primary biliary cirrhosis? Lancet. 329:834–836. doi: 10.1016/S0140-6736(87)91610-2
- Saeki R, Ogino H, Kaneko S, Unoura M, Kobayashi K. 1995. Effects of chenodeoxycholic and ursodeoxycholic acids on interferon-gamma production by peripheral blood mononuclear cells from patients with primary biliary cirrhosis. J Gastroenterol. 30:739–744. doi: 10.1007/BF02349640
- Stedman CA, Liddle C, Coulter SA, Sonoda J, Alvarez JG, Moore DD, Evans RM, Downes M. 2005. Nuclear receptors constitutive androstane receptor and pregnane X receptor ameliorate cholestatic liver injury. Proc Natl Acad Sci U S A. 102:2063–2068. doi: 10.1073/pnas.0409794102
- Teng S, Piquette-Miller M. 2007. Hepatoprotective role of PXR activation and MRP3 in cholic acid-induced cholestasis. Br J Pharmacol. 151:367–376. doi: 10.1038/sj.bjp.0707235
- Trauner M, Meier PJ, Boyer JL. 1998. Molecular pathogenesis of cholestasis. N Engl J Med. 339:1217–1227. doi: 10.1056/NEJM199810223391707
- Zollner G, Trauner M. 2009. Nuclear receptors as therapeutic targets in cholestatic liver diseases. Br J Pharmacol. 156:7–27. doi: 10.1111/j.1476-5381.2008.00030.x