ABSTRACT
Post-traumatic stress disorder (PTSD) is a stress-related psychiatric/mental condition. Tangeretin (TAN), a major polymethoxylated flavone of citrus plants, exhibits anti-inflammatory and neuroprotective activities. However, whether TAN leads to cognitive improvement in PTSD patients remains unclear. In the present study, we explored whether TAN improved cognitive impairment induced in rats by single prolonged stress (SPS episode mimicking PTSD induction) and determined whether TAN reversed reductions in dopamine (DA) and serotonin (5-HT) levels. Rats were intraperitoneally injected with TAN for 14 consecutive days after the SPS, which had induced cognitive deficits evident in the object recognition task and the Morris water maze test; the impairments were improved by TAN (100 mg/kg). TAN rescued the neurochemical abnormalities and the SPS-induced decreases in DA and 5-HT levels in the hippocampus and amygdala. These effects may be attributable in part to induction of hippocampal genes encoding tyrosine hydroxylase and tryptophan hydroxylase-1. Our results support the idea that rats with PTSD exhibit changes in DAergic and serotonergic transmission and in memory impairment. Thus, TAN mediated reversal of memory-related behavioral dysfunction associated with traumatic stress may be a useful therapeutic intervention in PTSD patients.
Introduction
Post-traumatic stress disorder (PTSD) is a stress-related psychiatric/mental condition associated with defined symptoms, including explicit memories, hyperarousal, and elusion (Lin et al. Citation2016). The disorder features re-experiencing of symptoms, avoidance behavior, and difficulties with memory recall (Lin et al. Citation2016). Cognitive deficits and memory dysfunction are frequently observed (Shafia et al. Citation2017). PTSD patients exhibit reduction in hippocampal volume that is evident on imaging and appears to be correlated with disease severity and the extent of cognitive impairment (Li et al. Citation2010). It is widely accepted that catecholamine dysfunction in general, and dopamine (DA) dysfunction in particular, play important roles in the pathophysiology of PTSD (Enman et al. Citation2015). DAergic neurotransmission in the mesolimbic pathway is important in terms of arousal and memory; changes in DA levels may contribute to the hyperarousal and re-experiencing symptoms associated with PTSD (Wilson et al. Citation2014). Also, dysregulation of serotonin (5-HT) production is evident in patients with PTSD (Lin et al. Citation2016).
Tangeretin (5,6,7,8,4’-pentamethoxyflavone; TAN) is a polymethoxylated flavones (PMFs) abundant in citrus peel (Sundaram et al. Citation2014). PMFs, including nobiletin, TAN, 5-demethylnobiletin, and sinensetin, are abundant in the peel tissues of certain types of citrus species such as Citrus tangerine, Citrus reticulata, and Citrus depressa (Nogata et al. Citation2005). TAN exhibits anti-cancer, anti-metastatic, apoptotic, anti-oxidative, anti-diabetic, anti-carcinogenic, anti-inflammatory, and neuroprotective activities (Yoon et al. Citation2011; Guo et al. Citation2017). Several studies have shown that TAN reduces dopaminergic neurotoxin-induced neuronal injury and prevents tunicamycin-induced cell death in mice by increasing glucose-regulated protein (GRP)78 expression in the renal tubular epithelium (Takano et al. Citation2007). A recent study showed that TAN increased lipopolysaccharide (LPS)-induced nitric oxide (NO) production in RAW 264.7 macrophages (Choi et al. Citation2007). Some studies have reported that the anti-oxidant and anti-inflammatory effects of TAN improved renal function and reduced cognitive and memory impairments in animals with chronic kidney disease (Wu et al. Citation2017). TAN exerted neuroprotective effects in the rat 6-hydroxydopamine (6-OHDA) lesional model of Parkinson's disease (Datla et al. Citation2001).
Few animal studies on the anti-oxidant, anti-inflammatory, and memory-restoring effects of TAN have appeared. The mechanisms underlying the effects of TAN in terms of ameliorating the impairments in recognition and spatial learning abilities associated with PTSD have not been fully explored. Here, we explored whether TAN prevented SPS-induced recognition and spatial memory deficits in a rat model of PTSD.
Materials and methods
Animals
Seven-weeks-old adult male Sprague–Dawley rats (Samtako Animal Co., Seoul, Korea), weighing 200–220 g, were used in all experiments. The rats were housed under a 12-h light/dark cycle (lights on at 8:00 am, lights off at 8:00 pm) under a controlled temperature at 21 ± 2°C and relative humidity of 55 ± 10%. All rats were allowed to adapt to these condition for 7 days after they arrived. All methods and procedures were approved by the Animal Care and Use Committee of Kyung Hee University [KHUASP(SE)-15-115]. All procedures were executed according to the Guide for the Care and Use of Laboratory Animals.
TAN administration
Different groups of rats, six or seven animals per group, were used for drugs treatment and tests in a randomized controlled animal experiments. All the experimental animals including control and drug-treatment groups were administration. TAN (20, 50 and 100 mg/kg, body weight; Sigma-Aldrich Chemical Co., St. Louise, MO, USA) and the positive drug fluoxetine (10 mg/kg, FLX, fluoxetine hydrochloride; Sigma) were injected by intraperitoneally (i.p.) after exposure to SPS. TAN and FLX were dissolved in 0.9% physiological saline before use. The entire experimental schedules of all drug administration and behavioral examinations are shown in .
Single prolonged stress
Rat were subjected to SPS for 14 consecutive days as described by Patki's group, with a slight modification (Patki et al. Citation2014). Brief, rats were held for 2 h in Plexiglas cylinders, then promptly entering a forced swimming condition for 20 min. The rats were dried and allowed to recuperate for 15 min, and then were exposed to ether vapor until they lost consciousness. Following the SPS stressor, the rats were housed one per cage and left undisturbed for 14 days to allow PTSD-like symptoms to manifest (Patki et al. Citation2014).
Object recognition task (ORT)
We used a novel ORT to assess cognitive capacity. We employed a hollow cube 45 × 45 × 45 cm in size made of black painted wood. The objects to be discriminated were two similar wooden toys (familiar objects, A1 and A2); they were sufficiently heavy that they could not be moved by the rats. A novel wooden block (object) differed in color and shape from A1 to A2. The test featured three phases; habituation, training, and testing. On day 1 (habituation), rat were placed in the test chamber for 10 min. On the next day (24 h after habituation), the rats were placed in the box containing two similar objects (A1 and A2), and they explored the objects for 5 min (training). During the test phase, the rats were exposed to one new object (B) and one old object for 3 min. The sniffing times for the novel and familiar objects were measured. The discrimination index was calculated as: (time spent sniffing novel object – time spent on familiar object)/(time spent sniffing novel object + time spent sniffing familiar object).
Morris water maze test (MWM)
After the ORT trial, the MWM test was used to measure the time and distance spent swimming to reach a submerged platform. MWM testing included a place navigation test and a spatial probe test. The place navigation test was conducted in a dark circular pool 2.0 m in diameter. The rats underwent three training trials per day for 5 consecutive days, during which they located a hidden platform using visual cues presented around the test room. The water temperature was 22 ± 2°C. Each trial was terminated after 180 s or when the rat found the platform. The spatial probe was performed on day 6, after removal of the escape platform; each trial was 60 s in duration. The frequency of platform crossings, swimming speed, swimming path length, and time spent in the target quadrant were measured. All training and probe trails were recorded by a ceiling-mounted video camera, and the data were analyzed with the aid of a tracking program (S-MART; PanLab Co., Barcelona, Spain).
Open field test (OFT)
Before completing the MWM trial, the rats were subjected to the OFT. The OFT was carried out according to a method described previously (Lee et al., Citation2014). Each rats was housed individually in a rectangular container (60 × 60 × 30 cm) in a dimly lit room. This provided the best contrast for white rats in a dimly lit room equipped with a video camera above the center of the room. Locomotor activities were indicated by the speed and distance of movements and monitored by a computerized video-tracking system using the S-MART program (PanLab Co., Barcelona, Spain). The number of rearing events of each rat was also recorded to analyze locomotor activity in the OFT.
DA and 5-HT measurement
Twenty-one day after inducing PTSD, DA and 5-HT concentrations was assayed in brain tissue using a method described previously (Lee et al. Citation2014). Four rats from each group were deeply anesthetized with isoflurane (1.2%), and were killed by sacrifice one day after the behavioral testing. The medial prefrontal cortex (mPFC), hippocampus (HPC), striatum (STR), and amygdala (AMYG) were quickly dissected from the rat brains in random order. The DA and 5-HT concentrations was assessed by a competitive enzyme-linked immunoassay (ELISA) using a mouse monoclonal DA and 5-HT antibody (DA and 5-HT ELISA Kit; Abcam, Cambridge, MA, USA).
Total RNA isolation and reverse transcription-polymerase chain reaction (RT–PCR) analysis
The levels of tyrosine hydroxylase (TH) and tryptophan hydroxylase-1 (Tph1) mRNA expression were measured by RT–PCR according to a method described previously (Yeom et al. Citation2015). In brief, total RNA was isolated from the HPC of each rat using TRIzol reagent according to the manufacturer's instructions. cDNA was synthesized from 2 μg total RNA using reverse transcriptase (Takara Bio, Otsu, Japan), and then amplified by PCR at 60°C for 30 cycles for TH, at 58°C for 30 cycles for Tph1 using Taq DNA polymerase (Takara, Kyoto, Japan) on a thermal cycler (MJ Research, Watertown, MA, USA). Data were normalized against glyceraldehyde 3-phosphate dehydrogenase (GADPH) expression in the corresponding sample.
Statistical analysis
Results are expressed as mean ± standard error. Differences within or between normally distributed data were analyzed using an analysis of variance (ANOVA) with SPSS (version 13.0; SPSS, Inc., Chicago, IL, USA) and Tukey's post hoc tests. A p-value < 0.05 was considered significant.
Results
Effects of TAN on SPS-induced memory impairment
Recognition memory was evaluated using the ORT in terms of the sniffing times of novel and familiar (old) objects and by calculation of discrimination indices ((A) and (B)). Analysis of old-object sniffing times by one-way ANOVA revealed no significant differences between the groups [F(5,38) = 0.998, p = 0.434]. However, the sniffing times for novel objects revealed significant among group differences [F(5,38) = 6.503, p < 0.001]. Post-hoc comparisons using Tukey's test indicated that these sniffing times were significantly reduced following SPS, compared with controls (p < 0.01). The PTSD + TAN100 group exhibited increased novel-object sniffing time compared with the PTSD group (p < 0.05). The discrimination indices differed significantly among the groups, decreasing after the SPS compared with controls (p < 0.001). The PTSD + TAN100 group exhibited a higher discrimination index than did the PTSD-only group (p < 0.01); recovery of recognition after induction of a stress-related memory deficit in the PTSD + TAN100 group was similar to that in the PTSD + FLX group. PTSD affected performance in the acquisition phase. Specifically, the latency of the PTSD group was significantly higher than that of the SAL group ((C)). ANOVA (6×5, treatment×time) revealed significant differences among groups [F(5,33) = 16.975, p < 0.001]. A significant effect was found for training day [F(4,132) = 169.719, p < 0.001]; however, no group×day interaction was evident [F(20,132) = 3.855, p = 0.012]. Tukey's post hoc test showed that the response latency of the PTSD + TAN100 group was marginally significantly lower than that of the PTSD group. When retention test data obtained on day 6 were compared, post-hoc testing revealed that the PTSD + TAN100 group spent more time around the platform than did the PTSD group (p < 0.05; (D) and (E)). The swimming latency of the PTSD + 100 mg/kg TAN rats was similar to that of rats receiving 10 mg/kg FLX. The PTSD group did not differ significantly from the other groups in terms of the mean swimming speed, calculated by dividing the total swimming distance by the latency ((F)).
Figure 2. Effect of TAN on recognition memory was assessed using a novel ORT in which we measured the times taken to sniff familiar and novel objects during a 3-min choice trial (A) and the ability to discriminate between familiar and novel objects (B). The MWM test was used to assess the effect of TAN on spatial learning and memory; we measured the time taken to escape from water (latency) during acquisition trials using a submerged platform (C), the percentages of time spent in the target quadrant (D), the proportion of the total distance traversed in the target quadrant (E), and swimming speed (F). The OFT was used to assess the effect of TAN on locomotor activity (counts) and total number of rearings (G). *p < 0.05, **p < 0.01, ***p < 0.001 vs. the SAL group, #p < 0.05, ##p < 0.05 vs. the PTSD group.
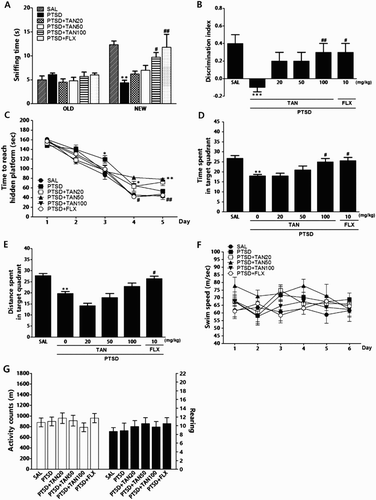
Results of the parametric one-way ANOVA, which are shown in (G), indicated that no PTSD-related effects were evident in terms of locomotor activity (motor function) or total number of rearing behaviors (exploration) in the OFT. We found no significant difference among control, saline-treated, and TAN-treated rats in terms of locomotor activity [F(5,38) = 0.561, p = 0.729] or the total number of rearing behaviors [F(5,38) = 0.334, p = 0.889].
Effects of TAN on SPS-induced changes in DA in the HPC
shows that the brain region levels of DA were significantly different when the group were compared. Tissue levels of DA were measured in the mPFC, STR, HPC, and AMYG after SPS and again 3 week later (Day 21). The post-hoc test results indicated a significant decrease in the levels of DA in the HPC of the PTSD groups compared with that in the untreated PTSD group (p < 0.01). Daily administration of TAN significantly increased the SPS-induced decrease in DA concentration in the HPC compared with that in the PTSD group (p < 0.05). After TAN treatment, the levels of DA in the AMYG also increased significantly to 226.97% of that in the PTSD group (p < 0.05). The DA concentrations in the brain regions of rats receiving 10 mg/kg FLX were similar to those in rats receiving 100 mg/kg TAN.
Figure 3. Effect of TAN on DA concentration in the brains of rats exposed to SPS for 14 consecutive days. *p < 0.05, **p < 0.01 vs. the SAL group; #p < 0.05, ##p< 0.01 vs. the PTSD group.
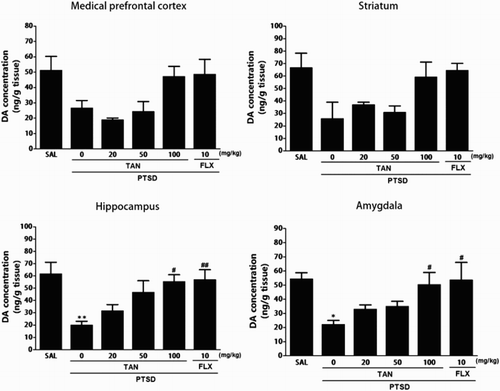
The ELISA showed that SPS exposure for 21 days decreased the DA concentration in the mPFC and STR of rats by 52.20% and 38.96% compared with rats in the saline-treated group, respectively, although this result was only marginally significant. However, administration of TAN inhibited the SPS-induced decrease in DA levels in the mPFC and STR, respectively, although this result was only marginally statistically.
Effects of TAN on SPS-induced changes in 5-HT in the HPC
shows that the brain region levels of 5-HT were significantly different when the group were compared. Tissue levels of 5-HT were measured in the mPFC, STR, HPC, and AMYG after SPS and again 3 week later (Day 21). The post-hoc test results indicated a significant decrease in the levels of 5-HT in the HPC of the PTSD groups compared with that in the untreated PTSD group (p < 0.01). Daily administration of TAN significantly increased the SPS-induced decrease in 5-HT concentration in the HPC compared with that in the PTSD group (p < 0.01). After TAN treatment, the levels of 5-HT in the AMYG also increased significantly to 223.08% of that in the PTSD group (p < 0.05). The 5-HT concentrations in the brain regions of rats receiving 10 mg/kg FLX were similar to those in rats receiving 100 mg/kg TAN.
Figure 4. Effect of TAN on 5-HT concentration in the brains of rats exposed to SPS for 14 consecutive days. *p < 0.05, **p < 0.01 vs. the SAL group; #p < 0.05,##p< 0.01 vs. the PTSD group.
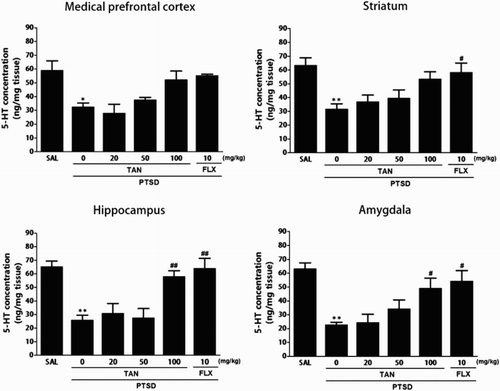
The ELISA showed that SPS exposure for 21 days significantly decreased the 5-HT concentration in the mPFC and STR of rats by 55.09% and 50.0% compared with rats in the saline-treated group (p < 0.05 and p < 0.01), respectively. However, administration of TAN inhibited the SPS-induced decrease in 5-HT levels in the mPFC and STR, respectively, although this result was only marginally statistically.
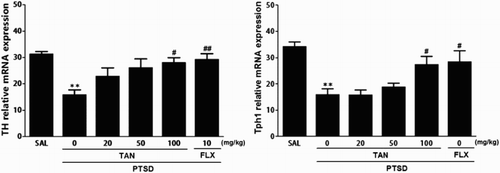
Effects of TAN on SPS-induced expression of TH and Tph1 mRNA in the HPC
TH and Tph1 mRNA expression was analyzed to examine the effect of TAN on the expression levels of TH and Tph1 mRNA in the HPC of rats exposed to SPS (). The mRNA level of TH in the PTSD group decreased significantly compared with that in the SAL group (p < 0.01). The decreased expression levels of TH mRNA in the PTSD group were significantly re-established to levels similar to those in the SAL group after administration of 100 mg/kg TAN (p < 0.05). Tph1 mRNA levels in the PTSD group were decreased significantly compared with those in the SAL group (p < 0.01). The decreased expression levels of Tph1 mRNA in the PTSD group was significantly re-established to a level similar to that seen in the SAL group after receiving 100 mg/kg TAN (p < 0.05). These results indicate that expression of TH and Tph1 mRNA in the HPC of rats receiving 100 mg/kg of TAN was similar to that of rats receiving 10 mg/kg FLX.
Figure 5. Effect of TAN on expression of mRNA encoding TH and Tph1 in rats subjected to SPS-induced memory impairment. The expression levels were normalized to that of glyceraldehyde 3-phosphate dehydrogenase (GAPDH)(internal control). *p < 0.05 vs. the SAL group, #p < 0.05, ##p < 0.01 vs. the PTSD group.
Discussion
The SPS rats in this study exhibited impaired recognition/location memory and lower brain DA and 5-HT levels. TAN improved recognition and spatial memory function, and modulated hippocampal TH and Tph1 expression. Our results encourage the development of novel PTSD therapies.
We used the ORT and MWM tests to explore the effects of TAN on recognition and on spatial learning and memory, respectively. As reported previously (Li et al. Citation2010), cognitive memory was impaired after SPS, as evidenced by a significant increase in the time spent exploring familiar objects, a significant decrease in the time spent exploring novel objects, and a decrease in the discrimination index. This suggests that brain degeneration is intense following exposure to a memory impairing event, compromising episodic memory and recognition ability (Haider et al. Citation2016). We found that SPS significantly decreased the time spent sniffing novel objects and reduced the discrimination index. By contrast, TAN significantly increased the sniffing time for novel objects and improved recognition memory following SPS. TAN rats exhibited faster learning and shorter escape latencies compared with SPS-alone animals. Furthermore, the latter rats performed poorly relative to controls on successive probe trials conducted 24 h after SPS, indicating that both recall and retrieval were impaired. TAN improved such behavioral abnormalities, restoring spatial learning and memory. Chronic fluoxetine administration was associated with similar effects. Thus, we confirmed that TAN ameliorated the spatial memory impairment induced by traumatic stress.
No significant among-group difference in locomotor activity or the total number of rearing behaviors was observed, suggesting that TAN did not affect sensorimotor and motor performance, motor impairment or psychomotor function in the OFT. Consequently, the changes in behavioral performance evident in the MWM task were likely attributable to memory improvement, not to a difference in active responding or psychomotor function.
We found that memory impairment following SPS was consistent with the DAergic system impairment evident in patients with PTSD. DA levels were reduced in the AMYG and HPC of SPS rats; the DAergic tone of the mesolimbic pathway, was thus reduced. PTSD symptoms are re-experienced because of DA dysfunction or imbalance within the memory impairment circuits of the mRFC, STR, AMYG and HPC (Lin et al. Citation2016). Studies on severe stressors support the notion that neurobiological events trigger dysregulation of the DA system (Enman et al. Citation2015). We hypothesize that TAN-mediated modulation of SPS-induced memory impairment-like symptoms reflects improvement in central DA and 5-HT status. We found that SPS animals given TAN exhibited significant increases in the DA and 5-HT levels of the AMYG and HPC, inhibiting PTSD pathophysiology. The effects of TAN may be reversed by manipulating DA and 5-HT levels (Lin et al. Citation2016). Thus, TAN, like FLX, restores the behavioral and neurochemical alterations associated with memory impairment by modulating the brain DAergic and serotonergic systems (Aykac et al. Citation2012).
The SPS reduced hippocampal DA efflux and the level of mRNA encoding TH, indicating the possible involvement of presynaptic adaptation/plasticity (Haider et al. Citation2016). TH is involved in DA synthesis, and is important in terms of both stress-induced activation of the DAergic system and the associated psychopathological symptoms, including memory impairment (Wilson et al. Citation2014). The SPS reduced 5-HT synthesis, with concomitant downregulation of Tph1 expression, thus elevating the brain 5-HT level. Tph1 is the rate-limiting enzyme of 5-HT biosynthesis and is involved in various aspects of memory impairment disorders (Chen et al. Citation2011). We found that the SPS reduced the levels of mRNAs encoding TH and Tph1 in the HPC and triggered memory impairment-like behavior. TAN restored hippocampal TH and Tph1 expression, suggesting that modulation of the DA and 5-HT systems played a role in the improvement of learning and memory afforded by TAN.
Recent, some study reported that nobiletin is an active ingredient in PMFs, ameliorate isoflurane-induced cognitive impairment through antioxidant, anti-inflammatory and anti-apoptotic effects via modulation of Art, Bax, p-cAMP response element binding protein (CREB) and brain-derived neurotrophic factor (BDNF) in aging rats (Bi et al. Citation2016). It inhibited microglial activation and preserved the expression of the glial cell line-derived neurotrophic factor, which is a therapeutic agent against Parkinson's disease in the substantia nigra (Jeong et al. Citation2015). Therefore, our results suggest that PMFs including TAN may improve learning and memory ability in rats.
In conclusion, our findings support the hypothesis that TAN improves SPS-induced behavioral and memory impairments in the rat. TAN significantly attenuated the symptoms of SPS-induced damage, as indicated by improved cognitive memory during behavioral tests, maintenance of normal DA and 5-HT levels, and normalization of the DAergic and serotoninergic systems. TAN ameliorated the neurochemical aspects and the psychological symptoms of memory impairment. TAN may prevent the memory impairment-like behaviors associated with PTSD. TAN may be a useful food material for ameliorating memory loss, improving learning and memory, and preventing development of the neuronal abnormalities associated with PTSD.
Disclosure statement
The authors declare no potential conflicts of interests.
Additional information
Funding
References
- Aykaç A, Aydın B, Cabadak H, Gören MZ. 2012. The change in muscarinic receptor subtypes in different brain regions of rats treated with fluoxetine or propranolol in a model of post-traumatic stress disorder. Behav Brain Res. 232:124–129. doi: 10.1016/j.bbr.2012.04.002
- Bi J, Zhang H, Lu J, Lei W. 2016. Nobiletin ameliorates isoflurane-induced cognitive impairment via antioxidant, anti-inflammatory and anti-apoptotic effects in aging rats. Mol Med Rep. 14:5408–5414. doi: 10.3892/mmr.2016.5919
- Chen D, Liu F, Yang C, Liang X, Shang Q, He W, Wang Z. 2011. Association between the TPH1 A218C polymorphism and risk of mood disorders and alcohol dependence: evidence from the current studies. J Affect Disord. 138:27–33 doi: 10.1016/j.jad.2011.04.018
- Choi SY, Ko HC, Ko SY, Hwang JH, Park JG, Kang SH, Han SH, Yun SH, Kim SJ. 2007. Correlation between flavonoid contentand the NO production inhibitory activity of peel extracts from various citrusfruits. Biol Pharm Bull. 30:772–778. doi: 10.1248/bpb.30.772
- Datla KP, Christidou M, Widmer WW, Rooprai HK, Dexter DT. 2001. Tissue distribution and neuroprotective effects of citrus flavonoid tangeretin in a rat model of Parkinson's disease. Neuroreport. 12:3871–3875. doi: 10.1097/00001756-200112040-00053
- Enman NM, Arthur K, Ward SJ, Perrine SA, Unterwald EM. 2015. Anhedonia, reduced cocaine reward, and dopamine dysfunction in a Rat model of posttraumatic stress disorder. Biol Psychiatry. 78:871–879. doi: 10.1016/j.biopsych.2015.04.024
- Guo XQ, Cao YL, Hao F, Yan ZR, Wang ML, Liu XW. 2017. Tangeretin alters neuronal apoptosis and ameliorates the severity of seizures in experimental epilepsy-induced rats by modulating apoptotic protein expressions, regulating matrix metalloproteinases, and activating the PI3 K/Akt cell survival pathway. Adv Med Sci. 62:246–253. doi: 10.1016/j.advms.2016.11.011
- Haider S, Sadir S, Naqvi F, Batool Z, Tabassum S, Khaliq S, Anis L, Sajid I, Haleem DJ. 2016. Magnesium treatment palliates noise-induced behavioral deficits by normalizing DAergic and 5-HTergic metabolism in adult male rats. Metab Brain Dis. 31:815–825. doi: 10.1007/s11011-016-9811-4
- Jeong KH, Jeon MT, Kim HD, Jung UJ, Jang MC, Chu JW, Yang SJ, Choi IY, Choi MS, Kim SR. 2015. Nobiletin protects dopaminergic neurons in the 1-methyl-4-phenylpyridinium-treatemd rat model of Parkinson’s disease. J Med Food. 18:409–414. doi: 10.1089/jmf.2014.3241
- Lee B, Sur B, Yeom M, Shim I, Lee H, Hahm DH. 2014. L-tetrahydropalmatine ameliorates development of anxiety and depression-related symptoms induced by single prolonged stress in rats. Biomol Ther. 22:213–222. doi: 10.4062/biomolther.2014.032
- Li XM, Han F, Liu DJ, Shi YX. 2010. Single-prolonged stress induced mitochondrial-dependent apoptosis in hippocampus in the rat model of post-traumatic stress disorder. J Chem Neuroanat. 40:248–255. doi: 10.1016/j.jchemneu.2010.07.001
- Lin CC, Tung CS, Lin PH, Huang CL, Liu YP. 2016. Traumatic stress causes distinctive effects on fear circuit catecholamines and the fear extinction profile in a rodent model of posttraumatic stress disorder. Eur Neuropsychopharmacol. 26:1484–1495. doi: 10.1016/j.euroneuro.2016.06.004
- Nogata Y, Sakamoto K, Shiratsuchi H, Ishii T, Yano M, Ohta H. 2005. Nobiletin and its related flavonoids with CRE-mediated transcription-stimulating and neuritegenic activities. Biochem Biophys Res Commun. 337:1330–1336. doi: 10.1016/j.bbrc.2005.10.001
- Patki G, Li L, Allam F, Solanki N, Dao AT, Alkadhi K, Salim S. 2014. Moderate treadmill exercise rescues anxiety and depression-like behavior as well as memory impairment in a rat model of posttraumatic stress disorder. Physiol Behav. 130:47–53. doi: 10.1016/j.physbeh.2014.03.016
- Shafia S, Vafaei AA, Samaei SA, Bandegi AR, Rafiei A, Valadan R, Hosseini-Khah Z, Mohammadkhani R, Rashidy-Pour A. 2017. Effects of moderate treadmill exercise and fluoxetine on behavioural and cognitive deficits, hypothalamic-pituitary-adrenal axis dysfunction and alternations in hippocampal BDNF and mRNA expression of apoptosis-related proteins in a rat model of post-traumatic stress disorder. Neurobiol Learn Mem. 139:165–178. doi: 10.1016/j.nlm.2017.01.009
- Sundaram R, Shanthi P, Sachdanandam P. 2014. Effect of tangeretin, a polymethoxylated flavone on glucose metabolism in streptozotocin-induced diabetic rats. Phytomedicine. 21:793–799. doi: 10.1016/j.phymed.2014.01.007
- Takano K, Tabata Y, Kitao Y, Murakami R, Suzuki H, Yamada M, Iinuma M, Yoneda Y, Ogawa S, Hori O. 2007. Methoxyflavones protect cells against endoplasmic reticulum stress and neurotoxin. Am J Physiol Cell Physiol. 292:C353–C361. doi: 10.1152/ajpcell.00388.2006
- Wilson CB, Ebenezer PJ, McLaughlin LD, Francis J. 2014. Predator exposure/psychosocial stress animal model of post-traumatic stress disorder modulates neurotransmitters in the rat hippocampus and prefrontal cortex. PLoS One. 9:e89104. doi: 10.1371/journal.pone.0089104
- Wu J, Zhao YM, Deng ZK. 2017. Tangeretin ameliorates renal failure via regulating oxidative stress, NF-κB-TNF-α/iNOS signalling and improves memory and cognitive deficits in 5/6 nephrectomized rats. Inflammopharmacology. 4:1–14.
- Yeom M, Sur BJ, Park J, Cho SG, Lee B, Kim ST, Kim KS, Lee H, Hahm DH. 2015. Oral administration of lactobacillus casei variety rhamnosus partially alleviates TMA-induced atopic dermatitis in mice through improving intestinal microbiota. J Appl Microbiol. 119:560–570. doi: 10.1111/jam.12844
- Yoon JH, Lim TG, Lee KM, Jeon AJ, Kim SY, Lee KW. 2011. Tangeretin reduces ultraviolet B (UVB)-induced cyclooxygenase-2 expression in mouse epidermal cells by blocking mitogen-activated protein kinase (MAPK) activation and reactive oxygen species (ROS) generation. J Agric Food Chem. 59:222–228. doi: 10.1021/jf103204x