ABSTRACT
Estradiol (E2) and progesterone (P4) are essential sex steroid hormones that play critical roles in the pituitary gland and uterus. Recently, nesfatin-1, a polypeptide hormone that regulates appetite and energy homeostasis in the hypothalamus, was found to be expressed in the pituitary gland and uterus. In this study, we aimed to investigate the relationship between these two steroid hormones and the expression and function of nesfatin-1 in the pituitary gland and uterus using GH3 cells, a lacto-somatotroph cell line, and THESC cells, an endometrial stromal cell line. First, we verified the presence of nesfatin-1 and nesfatin-1 binding sites in GH3 and THESC cells. E2 increased the mRNA expression of NUCB2, the gene encoding the nesfatin-1 protein, in GH3 cells, while P4 had no significant effect. In THESC cells, NUCB2 mRNA expression was decreased by E2 but increased by P4. In addition, nesfatin-1 significantly increased growth hormone (GH) and prolactin (PRL) mRNA expression in GH3 cells, and E2 enhanced this effect. In THESC cells, nesfatin-1 significantly increased the mRNA expression of insulin-like growth factor binding protein 1 (IGFBP1) and PRL, which are decidualization marker genes, and P4 further enhanced this effect. These results suggest that nesfatin-1 may act as a local regulator of GH and PRL production in the pituitary gland and decidualization in the uterus, modulating its effects in response to E2 and P4.
Introduction
Sex steroid hormones, including estradiol (E2) and progesterone (P4), play a crucial role in the development and regulation of the reproductive system, sexual characteristics, and overall sexual function in both males and females. In women, E2 and P4 are essential sex hormones that play a pivotal role in the menstrual cycle, pregnancy, and overall reproductive health (Jabbour et al. Citation2006; De Vos et al. Citation2010; Noyola-Martínez et al. Citation2019). During the menstrual cycle, E2 stimulates the growth and development of ovarian follicles, triggers the release of eggs from ovarian follicles, and maintains the endometrium after ovulation (Mihm et al. Citation2011). P4, another vital female sex hormone, is primarily produced by the corpus luteum in the ovary after ovulation, and it induces decidualization of the endometrium for implantation of a fertilized egg (Gellersen and Brosens Citation2014; Taraborrelli Citation2015). In addition to their roles in the reproductive system, E2 and P4 have significant effects on the brain and central nervous system (Boulware and Mermelstein Citation2005; Guennoun Citation2020). They are involved in multiple brain functions, including cognitive function, mood regulation, neuroprotection, and neural development (Bakker and Baum Citation2008; Hornung et al. Citation2020). Furthermore, E2 and P4 influence the secretion of hormones from the pituitary gland, including growth hormone (GH) and prolactin (PRL) (Muldoon Citation1986).
Nucleobindin 2 (NUCB2) is a precursor of nesfatin-1. NUCB2 generates nesfatin-1, -2, and -3 through post-translational processing in the presence of the prohormone convertase-1/3. Of the nesfatins, only nesfatin-1, an 82-amino acid product of the NUCB2 gene, has been shown to have physiological activity (Elmquist et al. Citation2005). Nesfatin-1 is primarily expressed in the hypothalamic nucleus and has been shown to play an essential role in maintaining energy homeostasis and regulating food intake (Oh-I et al. Citation2006; Schalla et al. Citation2020). Recent studies have shown that NUCB2/nesfatin-1 is also expressed in various human peripheral tissues, including the stomach, pancreas, and adipose tissue (Foo et al. Citation2010; Ramanjaneya et al. Citation2010; Schalla et al. Citation2020).
NUCB2/nesfatin-1 is also highly expressed in the pituitary gland. NUCB2/nesfatin-1 expression has been identified in the hypothalamus-pituitary-adrenal interrenal tissues of goldfish, with the highest expression in the pituitary gland (Pham et al. Citation2021). NUCB2/nesfatin-1-like immunoreactivity has also been identified in the pineal and pituitary glands of frogs (Senejani et al. Citation2014). In mice, NUCB2 mRNA and nesfatin-1 protein localize to cells in the anterior pituitary gland (Kim et al. Citation2014; Chung et al. Citation2015a). Similarly, autoradiography showed that nesfatin-1 binds to the pituitary gland in rats (Prinz et al. Citation2016). In addition to its expression in the pituitary gland, recent studies have shown that nesfatin-1 is highly expressed in the reproductive system of mice (Schalla and Stengel Citation2021). In our previous study, we demonstrated that NUCB2 mRNA and nesfatin-1 protein are detected in the mouse ovary and uterus, and their expression in both organs is highest during the estrus period of the estrus cycle (Kim et al. Citation2019).
Although many studies have demonstrated that NUCB2/nesfatin-1 is highly expressed in the pituitary gland and uterus, the regulatory mechanisms and functions of this hormone in these organs have not been fully characterized. Therefore, we aimed to investigate whether E2 and P4 regulate nesfatin-1 expression in the pituitary gland and uterus, and whether nesfatin-1 influences hormone production and decidualization in these organs. In this study, we found that E2 and P4 increased NUCB2/nesfatin-1 expression in the pituitary gland and uterus. In addition, we discovered that nesfatin-1 increases mRNA expression of GH and PRL in GH3 cells, a lacto-somatotroph cell line, and mRNA expression of IGFBP1 and PRL in THESC cells, a human endometrial stromal cell line.
Materials and methods
Cell culture and hormone treatment
GH3 cells, a lacto-somatotroph cell line, were obtained from the Korean Cell Line Bank and cultured in DMEM (Gibco, USA) supplemented with FBS (Corning, USA) and penicillin-streptomycin (Gibco, USA). THESC cells, a telomerase-immortalized human endometrial stromal cell line, were purchased from ATCC (CRL-4003, USA) and cultured in DMEM/F12 medium (Gibco, USA) supplemented with FBS and penicillin-streptomycin (BD Biosciences, USA). Cells were incubated in a humidified incubator at 37°C with 5% CO2. The next day, fresh medium was added along with different concentrations of hormones and antagonists: 10−7, 10−6, and 10−5 μM estradiol (E2) (Sigma-Aldrich, USA), 10−6, 10−5, and 10−4 μM progesterone (P4) (Sigma-Aldrich, USA), 10−5 μM E2 combined with 10−5 μM E2 receptor antagonist fulvestrant (ICI 182780; Sigma-Aldrich, USA), or 10−4 μM P4 combined with 10−5 μM P4 receptor antagonist mifepristone (RU486; Sigma-Aldrich, USA). Additionally, GH cells and THESC cells were exposed to 0.1, 1, and 10 nM nesfatin-1, and 10 nM nesfatin-1 combined with 10−4 μM P4 or 10−5 μM E2.
Immunofluorescence staining
GH3 and THESC cells were washed and fixed with 100% ethanol. After washing, the cells were incubated with rabbit anti-rat nesfatin-1 polyclonal antibody (H-003-22, Phoenix Pharmaceuticals, USA) and rabbit anti-human nesfatin-1 polyclonal antibody (H-003-25, Phoenix Pharmaceuticals, USA), followed by incubation with Alexa Fluor 594-conjugated goat anti-rabbit IgG (111-585-003, Jackson ImmunoResearch Laboratory, USA). Cells were washed and counterstained with DAPI. For nesfatin-1 binding site localization, GH3 and THESC cells were washed and incubated with FITC-conjugated nesfatin-1 (FG-003-22B, Phoenix Pharmaceuticals, USA) and counterstained with DAPI. Pituitary glands were fixed in 4% paraformaldehyde buffered saline and embedded in paraffin blocks. Paraffin blocks were sectioned at 10 μm, deparaffinized, rehydrated, and washed with PBS. Sections were incubated with rabbit anti-rat nesfatin-1 polyclonal antibody (H-003-22, Phoenix Pharmaceuticals, USA), goat anti-rat GH polyclonal antibody (AF1566, Bio-Techne, USA), and goat anti-mouse/rat PRL polyclonal antibody (AF1445, Bio-Techne, USA), followed by incubation with Alexa Fluor 594-conjugated goat anti-rabbit IgG (111-585-003, Jackson ImmunoResearch Laboratory, USA) and Alexa Fluor 488-conjugated bovine anti-goat IgG (805-545-180, Jackson ImmunoResearch Laboratory, USA) and counterstaining with DAPI. A mounting solution (Vector Laboratories, USA) was applied to the glass slide, and the cells were observed under a fluorescence microscope (Axioskop2, Carl Zeiss, Germany).
RNA extraction and qRT-PCR
Total RNA was extracted from cells using the RNA isoplus (TaKaRa Bio, Japan) according to the manufacturer’s instructions. The total RNA was then used for cDNA synthesis using oligo dT primers, dNTPs (Bio Basic, Canada), and M-MLV reverse transcriptase (Invitrogen, USA). qRT-PCR was performed using a buffer solution containing template cDNA, SYBR Green (Dyne Bio, Korea), and specific primers in the LightCycler® 480 Real-time PCR System (Roche, Germany). The primer pairs used are listed in Supplementary Data 1.
Statistical analysis
Results were presented as a mean and the standard error of the mean (SEM). Data were analyzed by unpaired two-tailed Student’s t-test and one-way ANOVA followed by Bonferroni’s multiple comparison test. Values of p < 0.05 were considered significant.
Results
Localization of nesfatin-1 and nesfatin-1 binding sites in GH3 and THESC cells
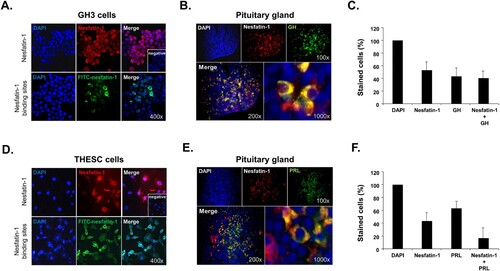
Immunocytochemical staining with nesfatin-1 antibody and fluorescence labeling with FITC-conjugated recombinant nesfatin-1 revealed the presence of nesfatin-1 protein and nesfatin-1 binding sites in both GH3 cells, a lacto-somatotroph cell line, and THESC cells, a telomerase-immortalized human endometrial stromal cell line. Notably, endogenous nesfatin-1 protein appeared as a distinct red stain in the cytoplasm of most GH3 cells. In addition, nesfatin-1 binding sites were visualized as green staining on the membrane of some GH3 cells after staining with FITC-conjugated nesfatin-1 (A). In THESC cells, nesfatin-1 protein exhibited intense staining in the cytoplasm, similar to that observed in GH3 cells. Nesfatin-1 binding sites were also detected as green staining on the membrane of THESC cells (D). No staining was observed in the negative control (A and D). Taken together, these results demonstrate that nesfatin-1 protein is expressed in both pituitary gland and endometrial cells, and that there are direct binding sites for nesfatin-1 on the surface of these cells. In addition, we stained the pituitary sections with antibodies against nesfatin-1, GH, and PRL to identify any co-localization. Immunohistochemical staining results showed that nesfatin-1 protein was co-localized with GH in specific pituitary cells (B). In the total pituitary cell population, 37% of the stained cells exhibited co-localization of nesfatin-1 and GH (C). Nesfatin-1 protein was also co-localized with PRL in specific pituitary cells (E). In the total pituitary cell population, 18% of the stained cells exhibited co-localization of nesfatin-1 and PRL (F).
Figure 1. Localization of nesfatin-1 and nesfatin-1 binding sites in GH3 and THESC cells. (A) Immunocytochemical staining shows the presence of nesfatin-1 protein (red) in the cytoplasm of GH3 cells. Nesfatin-1 binding sites appear in green on the membranes of these cells. (B) Co-localization of NUCB2/nesfatin-1 with growth hormone (GH) in the mouse pituitary gland. Nesfatin-1 protein co-stains with GH in lacto-somatotrophs within the pituitary glands. Insets display negative control images stained without nesfatin-1 primary antibody. (C) The percentage of cells stained with nesfatin-1 and GH. In the total pituitary cell population, 37% of the stained cells exhibit co-localization of nesfatin-1 and GH. (D) Nesfatin-1 protein is present in the cytoplasm of THESC cells by immunocytochemical staining. Nesfatin-1 binding sites are also observed on the membranes of these cells in green. (E) Co-localization of NUCB2/nesfatin-1 with prolactin (PRL) in the mouse pituitary gland. Nesfatin-1 protein is observed to co-staining with PRL in lacto-somatotrophs within the pituitary glands. (F) The percentage of cells stained with nesfatin-1 and PRL. In the total pituitary cell population, 18% of the stained cells exhibit co-localization of nesfatin-1 and PRL.
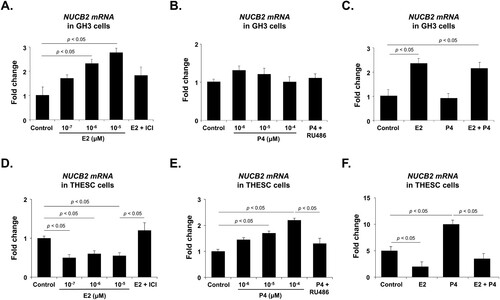
Effect of E2 and P4 on NUCB2 mRNA expression in GH3 and THESC cells
To determine whether E2 or P4 affects NUCB2 mRNA expression in GH3 and THESC cells, we assessed NUCB2 mRNA expression using qRT-PCR in the cells treated with either E2 or P4. E2 treatment dose-dependently increased NUCB2 mRNA expression in GH3 cells, whereas P4 treatment had no significant effect (A and B). Furthermore, the E2 inhibitor ICI 182780 effectively suppressed the E2-induced increase in NUCB2 mRNA expression in GH3 cells (A). P4 treatment did not change the NUCB2 mRNA level, but co-treatment with E2 and P4 increased it to the level of E2 treatment (C). In THESC cells, E2 treatment decreased NUCB2 mRNA expression, and this effect was attenuated by co-treatment with the ICI 182780 (D). Conversely, the P4 treatment dose-dependently increased NUCB2 mRNA expression in THESC cells, and this effect was attenuated by co-treatment with the P4 antagonist RU486 (E). Furthermore, the increase in NUCB2 mRNA expression induced by P4 treatment was attenuated by co-treatment with E2 (F). These findings suggest that both E2 and P4 act through their respective receptors, but that these receptors may interact and potentially interfere with each other's actions. Specifically, it may be that the upregulation of NUCB2 mRNA expression facilitated by the P4 receptor is somehow hindered when E2 receptors are simultaneously engaged.
Figure 2. NUCB2 mRNA expression after E2 and P4 treatment in GH3 and THESC cells. (A) NUCB2 mRNA expression in GH3 cells is dose-dependently increased by E2 treatment but significantly decreased when cells are co-treated with an E2 inhibitor (ICI 182780). (B, C) P4 treatment does not affect NUCB2 mRNA expression, but its expression is enhanced when cells are co-treated with E2. (D) In THESC cells, E2 treatment decreases NUCB2 mRNA expression, which is attenuated by co-treatment with ICI 182780. (E) Conversely, P4 treatment dose-dependently increases NUCB2 mRNA expression, which is attenuated by co-treatment with the P4 antagonist RU486. (F) The elevation of NUCB2 mRNA expression induced by P4 treatment is attenuated by co-treatment with E2. All data are represented as mean ± SEM (n = 6). Differences between values were considered statistically significant when p < 0.05.
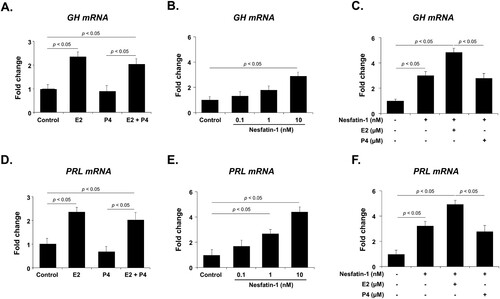
Effects of E2, P4, and nesfatin-1 treatment on GH and PRL mRNA expression in GH3 cells
Before investigating the effect of nesfatin-1 on GH and PRL mRNA expression in GH3 cells, we first evaluated their mRNA expression in cells treated with E2 and P4, which significantly regulate GH and PRL production in lacto-somatotrophs of the pituitary gland. GH3 cells were treated with E2 and P4, and the mRNA levels of GH and PRL were measured by qRT-PCR. GH and PRL mRNA expression was significantly increased by E2 treatment but was not affected by P4 treatment. The E2-induced increase in GH and PRL mRNA expression was not affected by co-treatment with P4 (A and D). Next, to determine whether nesfatin-1 regulates GH and PRL mRNA expression in GH3 cells, we measured the GH and PRL mRNA levels using qRT-PCR after nesfatin-1 treatment. A low concentration of nesfatin-1 did not affect GH or PRL mRNA expression, but a high concentration of nesfatin-1 significantly increased the expression of both genes (B and E). Furthermore, when GH3 cells were co-treated with nesfatin-1 and E2 or P4, E2 increased GH or PRL mRNA expression, whereas P4 had no effect (C and F).
Figure 3. GH and PRL mRNA expression after E2 and P4 treatment in GH3 cells. (A, D) E2 treatment significantly increases GH and PRL mRNA expression, but P4 has no effect. (B, E) GH and PRL mRNA expression is dose-dependently increased by nesfatin-1 treatment. (C, F) When cells are co-treated with nesfatin-1 and E2 or P4, E2 enhances GH and PRL mRNA expression, while P4 has no effect. All data are represented as mean ± SEM (n = 6). Differences between values were considered statistically significant when p < 0.05.
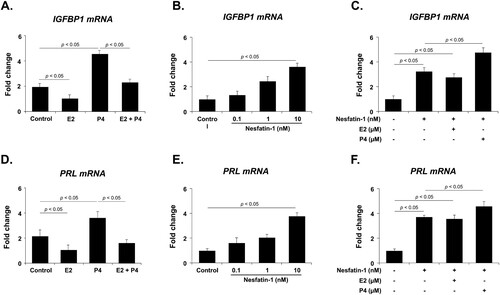
Effects of E2, P4, and nesfatin-1 treatment on IGFBP1 and PRL mRNA expression in THESC cells
It is well known that IGFBP1 and PRL are decidualization marker genes expressed in endometrial cells, and their expression is regulated by E2 and P4. Therefore, before investigating the effect of nesfatin-1 on IGFBP1 and PRL mRNA expression in THESC cells, we first evaluated their mRNA expression in cells treated with E2 and P4 using qRT-PCR. IGFBP1 and PRL mRNA expression was significantly decreased by E2 treatment, but increased by P4 treatment. Furthermore, the increase in IGFBP1 and PRL mRNA expression induced by P4 treatment was attenuated by co-treatment with E2 (A and D). We then examined the effect of nesfatin-1 on the expression of IGFBP1 and PRL mRNA in THESC cells by treating the cells with nesfatin-1. Nesfatin-1 treatment dose-dependently increased the expression of IGFBP1 and PRL mRNA in THESC cells (B and E). Furthermore, when nesfatin-1 was co-treated with E2 or P4, P4 increased the expression of IGFBP1 and PRL mRNA, whereas E2 had no effect (C and F).
Figure 4. IGFBP1 and PRL mRNA expression after E2 and P4 treatment in THESC cells. (A, D) IGFBP1 and PRL mRNA expression is significantly decreased by E2 treatment but increased by P4 treatment. The increase in IGFBP1 and PRL mRNA expression induced by P4 treatment is attenuated by co-treatment with E2. (B, E) Nesfatin-1 treatment dose-dependently increases the expression of IGFBP1 and PRL mRNA. (C, F) When THESC cells were co-treated with nesfatin-1 and E2 or P4, P4 treatment enhanced IGFBP1 and PRL mRNA expression, whereas E2 had no effect. All data are represented as mean ± SEM (n = 6). Differences between values were considered statistically significant when p < 0.05.
Discussion
E2 and P4 produced in the ovaries not only regulate endometrial cell functions during the menstrual cycle but also influence the secretion of various hormones by acting on the hypothalamus and pituitary cells (Muldoon Citation1986; Jabbour et al. Citation2006). Recent studies have reported that nesfatin-1, an appetite control hormone, is expressed in the uterus and pituitary gland and regulates the functions of these organs (Kim et al. Citation2019; Schalla and Stengel Citation2021). Based on these findings, we hypothesized that E2 and P4 might regulate uterine and pituitary functions by influencing the expression of nesfatin-1 in these organs. To investigate this hypothesis, we conducted experiments using GH3 cells, a lacto-somatotroph cell line, and THESC cells, an endometrial stromal cell line.
We first demonstrated that nesfatin-1 is expressed in GH3 cells using immunocytochemical staining. This finding is consistent with previous studies that demonstrated NUCB2 mRNA and nesfatin-1 protein expression in GH3 and RC-4B/C cells (Vélez and Unniappan Citation2020; Vélez et al. Citation2021). Furthermore, another study reported high NUCB2 mRNA expression in the anterior lobe using in situ hybridization, while the posterior lobe showed no expression (Könczöl et al. Citation2010). Our previous study also indicated that nesfatin-1 is primarily expressed in a subset of cells in the anterior pituitary gland (Chung et al. Citation2015a). In this study, immunohistochemical staining of mouse pituitary gland tissues revealed co-localization of nesfatin-1 with pituitary cells that produce GH and PRL. These findings suggest that nesfatin-1 may be involved in GH and PRL production in the pituitary gland.
We also demonstrated that nesfatin-1 is expressed in THESC cells, which is consistent with previous research reporting nesfatin-1 expression in human endometrium and mouse and rat uterus. Nesfatin-1 immunoreactivity has been detected in the endometrium, where it positively correlates with cell proliferation (Takagi et al. Citation2016). Another study found strong nesfatin-1 immunoreactivity in epithelial and glandular epithelial cells of the rat uterus during estrus (Tang et al. Citation2010). Our previous study also showed that NUCB2 mRNA and nesfatin-1 protein are expressed in mouse ovaries and uterus, and their expression is regulated by the hypothalamic-pituitary-ovarian axis (Kim et al. Citation2019). These findings suggest that nesfatin-1 may play a vital role in uterine processes, such as decidualization, implantation, and pregnancy.
Next, to investigate whether nesfatin-1 expression in GH3 cells is regulated by E2 and P4, we treated GH3 cells with E2 and P4 and examined NUCB2 mRNA expression. We found that E2 increased NUCB2 mRNA expression in GH3 cells, whereas P4 had no significant effect. Furthermore, the increase in NUCB2 mRNA expression induced by E2 was not affected by co-treatment with P4. These results are consistent with our previous in vivo study showing that NUCB2 mRNA expression in the pituitary gland is increased by E2 and decreased by P4 (Chung et al. Citation2015a). A related study showed that testosterone increased nesfatin-1 protein expression in LβT2 cells, a pituitary cell line (Hatef and Unniappan Citation2017). However, in goldfish, testosterone ingestion reduces NUCB2 mRNA expression in the pituitary gland, suggesting that it initially increases nesfatin-1 expression in the pituitary gland, but this is followed by a compensatory decrease (Bertucci et al. Citation2016). In mice, testosterone treatment increases NUCB2/nesfatin-1 expression in the pituitary gland, whereas castration decreases NUCB2 mRNA expression, which is restored by testosterone replacement (Seon et al. Citation2017). Consistent with these findings, NUCB2 mRNA expression in the pituitary gland is reduced by ovariectomy but increased by E2 and P4 treatment (Chung et al. Citation2015a). In addition, E2 increases NUCB2 mRNA and nesfatin-1 protein expression in LβT2 cells, as well as NUCB2 mRNA expression in cultured pituitary cells (Chung et al. Citation2015a; Hatef and Unniappan Citation2017). An in vivo study also revealed that E2 ingestion reduced NUCB2 mRNA expression in the pituitary gland of goldfish (Bertucci et al. Citation2016). Taken together, these results suggest that steroid hormones, including E2 and P4, produced in the ovary influence lacto-somatotrophs in the pituitary gland to regulate GH and PRL production.
We also investigated whether E2 and P4 regulate nesfatin-1 expression in the same manner in THESC cells. Our results demonstrated that NUCB2 mRNA expression in THESC cells was decreased by E2 treatment but increased by P4 treatment. Furthermore, the elevation of NUCB2 mRNA expression induced by P4 was attenuated when cells were co-treated with E2. In our previous research, we demonstrated that nesfatin-1 is expressed in endometrial epithelial cells, and its regulation is influenced by E2 and P4 administration in mice, which supports the findings of the current study (Kim et al. Citation2014; Kim et al. Citation2019). We also reported for the first time the expression of NUCB2 mRNA and nesfatin-1 protein in implantation sites during pregnancy. Nesfatin-1 promotes the accumulation of Th17 lymphocytes at implantation sites, maintaining pregnancy in a spontaneous abortion mouse model (Chung et al. Citation2015b). Although limited research has been conducted on the function of nesfatin-1 in the uterus, these studies suggest that nesfatin-1 may be an important local regulatory factor in the uterus.
In this study, we identified nesfatin-1 binding sites on the surface of GH3 cells. Consistent with this, recent research has shown that CF568-labeled nesfatin-1 binds to GH3 cell membranes (Vélez and Unniappan Citation2020). Consistently, a recent study demonstrated the intracellular signaling pathways of nesfatin-1, including the potential receptor responsible for mediating this peptide effect and downstream signaling (Rupp et al. Citation2021). These results suggest that nesfatin-1 interacts with receptors on GH3 cells, thereby influencing their functions. Based on these observations, we investigated the potential role of nesfatin-1 in modulating the production of GH and PRL in GH3 cells. Our results revealed that nesfatin-1 treatment dose-dependently increased GH and PRL mRNA expression in GH3 cells.
While the specific role of nesfatin-1 in the pituitary gland has not been thoroughly elucidated, several previous studies have shown that nesfatin-1 contributes to the regulation of several endocrine systems connected to the hypothalamus, particularly those associated with the stress response, metabolism, and reproduction (Gao et al. Citation2016; Weibert et al. Citation2019; Schalla et al. Citation2020; Schalla and Stengel Citation2021). Moreover, nesfatin-1 modulates the activity of the hypothalamic-pituitary-adrenal (HPA) axis by stimulating the release of corticotropin-releasing hormone (CRH) and adrenocorticotropic hormone (ACTH), which increases cortisol secretion by the adrenal glands (Yoshida et al. Citation2010). Nesfatin-1 also increases proopiomelanocortin (POMC) transcript abundance and protein expression and co-localizes with POMC in immortalized mouse tumor-derived corticotrophs (Nasri and Unniappan Citation2021). In accordance with our findings, recent research has shown that nesfatin-1 plays a role in regulating the secretion of pituitary hormones, such as GH, PRL, and luteinizing hormone (Vélez and Unniappan Citation2020; Vélez et al. Citation2021). Furthermore, our results demonstrated that when GH3 cells were co-treated with nesfatin-1 and E2 or P4, E2 enhanced the nesfatin-1-induced increase in GH and PRL mRNA expression, whereas P4 did not show a significant effect. This finding suggests that ovarian steroid hormones may modulate GH and PRL mRNA expression in the pituitary gland in conjunction with nesfatin-1.
In this study, we also identified nesfatin-1 binding sites on the membranes of THESC cells. This result suggests that nesfatin-1 may bind to these sites on THESC cells and influence their functions. Before investigating the effect of nesfatin-1 on IGFBP1 and PRL mRNA expression in THESC cells, we first confirmed that E2 and P4 regulate IGFBP1 and PRL mRNA expression because IGFBP1 and PRL are markers of decidualization that are regulated by E2 and P4 (Tang et al. Citation1993). We then examined whether nesfatin-1 regulates IGFBP1 and PRL mRNA expression in THESC cells. Our results showed that nesfatin-1 treatment dose-dependently increased IGFBP1 and PRL mRNA expression in these cells. Furthermore, when THESC cells were co-treated with nesfatin-1 and E2 or P4, P4 enhanced the nesfatin-1-induced increase in IGFBP1 and PRL mRNA expression, whereas E2 had no significant effect. Although it has been reported that nesfatin-1 is expressed in endometrial cells and its regulation is influenced by E2 and P4, the specific functions of nesfatin-1 in the uterus have not been fully elucidated.
In conclusion, this study demonstrated the presence of nesfatin-1 and nesfatin-1 binding sites in GH3 and THESC cells. We also observed that E2 and P4 regulated NUCB2 mRNA expression in both cell types. Additionally, our results showed that nesfatin-1 upregulated GH and PRL mRNA expression in GH3 cells and IGFBP1 and PRL mRNA expression in THESC cells. Furthermore, the effect of nesfatin-1 on these mRNA expressions was enhanced when the cells were co-treated with E2 or P4. These findings suggest that E2 and P4, steroid hormones produced by the ovary, may play a role in modulating the expression and function of nesfatin-1 in the pituitary gland and uterus. To the best of our knowledge, this is the first report that proposes a connection of nesfatin-1 with uterine decidualization and pituitary hormone production, and that nesfatin-1 acts as a local regulator under the influence of ovarian steroid hormones. Further research is needed to fully understand the detailed roles of nesfatin-1 in the pituitary gland and uterus.
Supplemental Material
Download TIFF Image (1.6 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Bakker J, Baum MJ. 2008. Role for estradiol in female-typical brain and behavioral sexual differentiation. Front Neuroendocrinol. 29(1):1–16.
- Bertucci JI, Blanco AM, Canosa LF, Unniappan S. 2016. Estradiol and testosterone modulate the tissue-specific expression of ghrelin, ghs-r, goat and nucb2 in goldfish. Gen Comp Endocrinol. 228:17–23.
- Boulware MI, Mermelstein PG. 2005. The influence of estradiol on nervous system function. Drug News Perspect. 18(10):631–637.
- Chung Y, Kim H, Im E, Kim P, Yang H. 2015b. Th 17 cells and nesfatin-1 are associated with Spontaneous abortion in the CBA/j × DBA/2 mouse model. Dev Reprod. 19(4):243–252.
- Chung Y, Kim J, Im E, Kim H, Yang H. 2015a. Progesterone and 17β-estradiol regulate expression of nesfatin-1/ NUCB2 in mouse pituitary gland. Peptides. 63:4–9.
- De Vos M, Devroey P, Fauser BC. 2010. Primary ovarian insufficiency. Lancet. 376(9744):911–921.
- Elmquist JK, Coppari R, Balthasar N, Ichinose M, Lowell BB. 2005. Identifying hypothalamic pathways controlling food intake, body weight, and glucose homeostasis. J Comp Neurol. 493(1):63–71.
- Foo KS, Brauner H, Ostenson CG, Broberger C. 2010. Nucleobindin-2/nesfatin in the endocrine pancreas: distribution and relationship to glycaemic state. J Endocrinol. 204(3):255–263.
- Gao X, Zhang K, Song M, Li X, Luo L, Tian Y, Zhang Y, Li Y, Zhang X, Ling Y, et al. 2016. Role of nesfatin-1 in the reproductive axis of male rat. Sci Rep. 6:32877.
- Gellersen B, Brosens JJ. 2014. Cyclic decidualization of the human endometrium in reproductive health and failure. Endocr Rev. 35(6):851–905.
- Guennoun R. 2020. Progesterone in the brain: hormone, neurosteroid and neuroprotectant. Int J Mol Sci. 21(15):5271.
- Hatef A, Unniappan S. 2017. Gonadotropin-releasing hormone, kisspeptin, and gonadal steroids directly modulate nucleobindin-2/nesfatin-1 in murine hypothalamic gonadotropin-releasing hormone neurons and gonadotropes. Biol Reprod. 96(3):635–651.
- Hornung J, Lewis CA, Derntl B. 2020. Sex hormones and human brain function. Handb Clin Neurol. 175:195–207.
- Jabbour HN, Kelly RW, Fraser HM, Critchley HO. 2006. Endocrine regulation of menstruation. Endocr Rev. 27(1):17–46.
- Kim J, Chung Y, Kim H, Im E, Lee H, Yang H. 2014. The tissue distribution of nesfatin-1/NUCB2 in mouse. Dev Reprod. 18(4):301–309.
- Kim J, Sun S, Lee D, Youk H, Yang H. 2019. Gonadotropin regulates NUCB2/nesfatin-1 expression in the mouse ovary and uterus. Biochem Biophys Res Commun. 513(3):602–607.
- Könczöl K, Bodnár I, Zelena D, Pintér O, Papp RS, Palkovits M, Nagy GM, Tóth ZE. 2010. Nesfatin-1/NUCB2 may participate in the activation of the hypothalamic-pituitary-adrenal axis in rats. Neurochem Int. 57(3):189–197.
- Mihm M, Gangooly S, Muttukrishna S. 2011. The normal menstrual cycle in women. Anim Reprod Sci. 124(3–4):229–236.
- Muldoon TG. 1986. Steroid hormone receptor regulation by various hormonal factors during mammary development and growth in the normal mouse. Ann N Y Acad Sci. 464:17–36.
- Nasri A, Unniappan S. 2021. Nucleobindin-derived nesfatin-1 and nesfatin-1-like peptide stimulate pro-opiomelanocortin synthesis in murine AtT-20 corticotrophs through the cAMP/PKA/CREB signaling pathway. Mol Cell Endocrinol. 536:111401.
- Noyola-Martínez N, Halhali A, Barrera D. 2019. Steroid hormones and pregnancy. Gynecol Endocrinol. 35(5):376–384.
- Oh-I S, Shimizu H, Satoh T, Okada S, Adachi S, Inoue K, Eguchi H, Yamamoto M, Imaki T, Hashimoto K, et al. 2006. Identification of nesfatin-1 as a satiety molecule in the hypothalamus. Nature. 443(7112):709–712.
- Pham V, Pemberton JG, Chang JP, Blanco AM, Nasri A, Unniappan S. 2021. Nesfatin-1 stimulates the hypothalamus-pituitary-interrenal axis hormones in goldfish. Am J Physiol Regul Integr Comp Physiol. 321(4):R603–R613.
- Prinz P, Goebel-Stengel M, Teuffel P, Rose M, Klapp BF, Stengel A. 2016. Peripheral and central localization of the nesfatin-1 receptor using autoradiography in rats. Biochem Biophys Res Commun. 470(3):521–527.
- Ramanjaneya M, Chen J, Brown JE, Tripathi G, Hallschmid M, Patel S, Kern W, Hillhouse EW, Lehnert H, Tan BK, et al. 2010. Identification of nesfatin-1 in human and murine adipose tissue: a novel depot-specific adipokine with increased levels in obesity. Endocrinology. 151(7):3169–3180.
- Rupp SK, Wölk E, Stengel A. 2021. Nesfatin-1 Receptor: Distribution, Signaling and Increasing Evidence for a G Protein-Coupled Receptor - A Systematic Review. Front Endocrinol (Lausanne). 12:740174.
- Schalla MA, Stengel A. 2021. The role of the gastric hormones ghrelin and nesfatin-1 in reproduction. Int J Mol Sci. 22(20):11059.
- Schalla MA, Unniappan S, Lambrecht NWG, Mori M, Taché Y, Stengel A. 2020. NUCB2/nesfatin-1 - Inhibitory effects on food intake, body weight and metabolism. Peptides. 128:170308.
- Senejani AG, Gaupale TC, Unniappan S, Bhargava S. 2014. Nesfatin-1/nucleobindin-2 like immunoreactivity in the olfactory system, brain and pituitary of frog, Microhyla ornate. Gen Comp Endocrinol. 202:8–14.
- Seon S, Jeon D, Kim H, Chung Y, Choi N, Yang H. 2017. Testosterone regulates NUCB2 mRNA expression in male mouse hypothalamus and pituitary gland. Dev Reprod. 21(1):71–78.
- Takagi K, Miki Y, Tanaka S, Hashimoto C, Watanabe M, Sasano H, Ito K, Suzuki T. 2016. Nucleobindin 2 (NUCB2) in human endometrial carcinoma: a potent prognostic factor associated with cell proliferation and migration. Endocr J. 63(3):287–299.
- Tang B, Guller S, Gurpide E. 1993. Mechanisms involved in the decidualization of human endometrial stromal cells. Acta Eur Fertil. 24(5):221–223.
- Tang M, Naidu D, Hearing P, Handwerger S, Tabibzadeh S. 2010. LEFTY, a member of the transforming growth factor-beta superfamily, inhibits uterine stromal cell differentiation: a novel autocrine role. Endocrinology. 151(3):1320–1330.
- Taraborrelli S. 2015. Physiology, production and action of progesterone. Acta Obstet Gynecol Scand. 94(Suppl 161):8–16.
- Vélez EJ, Nasri A, Unniappan S. 2021. Nesfatin-1 and nesfatin-1-like peptide suppress basal and TRH-induced expression of prolactin and prolactin regulatory element-binding protein mRNAs in rat GH3 somatolactotrophs. Mol Cell Endocrinol. 529:111269.
- Vélez EJ, Unniappan S. 2020. Nesfatin-1 and nesfatin-1-like peptide suppress growth hormone synthesis via the AC/PKA/CREB pathway in mammalian somatotrophs. Sci Rep. 10(1):16686.
- Weibert E, Hofmann T, Stengel A. 2019. Role of nesfatin-1 in anxiety, depression and the response to stress. Psychoneuroendocrinology. 100:58–66.
- Yoshida N, Maejima Y, Sedbazar U, Ando A, Kurita H, Damdindorj B, Takano E, Gantulga D, Iwasaki Y, Kurashina T, et al. 2010. Stressor-responsive central nesfatin-1 activates corticotropin-releasing hormone, noradrenaline and serotonin neurons and evokes hypothalamic-pituitary-adrenal axis. Aging (Albany NY). 2(11):775–784.
