ABSTRACT
Chronic hepatitis C virus (HCV) infection is associated with altered metabolism, including dyslipidemia and insulin resistance. These contribute to disease progression and influences the response to therapy. To investigate the relationships of new direct-acting antiviral drugs, simeprevir/sofosbuvir, with lipid profile and insulin resistance (IR). Eighty chronic hepatitis C genotype 4 patients were included; they were divided into four groups according to the severity of fibrosis as detected by fibroscan. Forty healthy persons volunteered as a control group. Lipid profile changes and IR were analyzed at baseline and after the end of treatment, and any effect of these changes on the response to treatment was studied. Before treatment, the levels of serum triglycerides were significantly higher in patients than in the control, and the levels of fasting insulin showed a progressive increase with advancing stage of fibrosis. At the end of treatment, there were a significant reduction in serum triglycerides, FBS, fasting insulin, and homeostasis model for the assessment of IR (P < 0.001), and a significant elevation of serum cholesterol and low-density lipoprotein (LDL)-c, high-density lipoprotein (HDL)-c, and LDL/HDL ratio (P = 0.001). An end-of-treatment response (week 12) was achieved in (99%) of the treated cases with 99% sustained viral response for 12 weeks post-treatment (week 24). Significant lipid profile changes were detected at the end of treatment. Serum lipid levels and IR are no longer predictors of response to DAAs. Follow-up of the lipid profile is warranted to avoid any possible remote effect of atherosclerotic heart disease.
Responsible Editor Amin Bredan, Previously with Inflammation Research Center & VIB, Ghent University, Belgium Alsemberg, Belgium
1. Introduction
Hepatitis C virus (HCV) represents the major causative agent of chronic liver disease affecting more than 170 million patients worldwide [Citation1]. Chronic hepatitis C (CHC) could be considered a special type of metabolic disease involving insulin resistance (IR), hepatic steatosis, and modulation of lipid-cholesterol biosynthesis that may lead to fatty liver, hypo-betalipoproteinemia, hypercholesterolemia, and increased risk for ischemic heart diseases (IHD) [Citation2,Citation3]. The association between chronic HCV infection and increased prevalence of IR and type 2 diabetes mellitus (DM) was extensively reported [Citation4]. IR was reported to accelerate fibrosis in chronic HCV-infected patients [Citation5,Citation6], which may lead to increased risk of cirrhosis and hepatocellular carcinoma [Citation7] and has been associated with reduced rate of sustained virological response (SVR) in response to pegylated interferon (IFN)-α and ribavirin therapy [Citation8].
In most countries, treatment of chronic HCV infection was shifted from IFN-based to IFN-free regimens using direct-acting antiviral drugs (DAAs). Although the association of baseline metabolic characteristics with treatment outcome has not been fully assessed for DAAs, this group was reported to result in improved rates of SVR and to reduce the predictive ability of these factors except for the baseline low-density lipoprotein (LDL) [Citation9]. The highest prevalence of HCV was reported in Egypt, where genotype 4 is responsible for 91% of infections [Citation10] and DAAs represented the main line of treatment in most centers [Citation11]. Although the changes in lipid metabolism after treatment with DAAs were reported for other genotypes [Citation12–Citation14], it was not studied in genotype 4 infected patients. The aim of this study was to evaluate the outcome of using SIM/SOF in treating genotype 4 infected patients and to study the relationship of these agents with lipid profile and IR.
2. Patients and methods
Of 600 CHC patients who attended the liver unit at our hospital in the period between February 2015 and January 2016, 80 patients met the inclusion criteria for this case-control study. The inclusion criteria were age ≥18 years, nondiabetic naïve patients with compensated CHC after HCV-4 infection, based on the presence of anti-HCV and detectable serum HCV-RNA for 6 months or more, who had different grades of fibrosis (F) as estimated by fibroscan, and will be treated with (SIM/SOF). The exclusion criteria were DM, patients who used lipid-lowering agents, as it could affect lipid metabolism or IR, and patients suffering coexisting liver disease as hepatitis B virus, human immunodeficiency virus, schistsoma infection, autoimmune hepatitis, etc. DM was defined with a fasting glucose of ≥126 mg/dL according to the American Diabetes Association criterion. Forty healthy volunteers, 20 males, and 20 females were randomly selected from the medical and paramedical staff working in the same hospital after their agreement to participate in the study as control subjects. Their age ranged from 20 to 60 years.
3. Treatment protocol
Each patient consumed one SIM 150 mg capsule and one SOF 400 mg tablet once a day (Od) for 12 weeks. The dose of SOF was reduced to a 200 mg tablet Od, if the glomerular filtration rate dropped below 30 mL/min,
4. Ethics and informed consent
After approval by the local institutional ethics committee, and an individual agreement of each participant in the study, informed consent was obtained from each participant. The study was conducted in accordance with the ethical guidelines of the Declaration of Helsinki and International Conference on Harmonization Guidelines for Good Clinical Practice.
5. Clinical and laboratory assessment
All participants underwent full history taking and thorough clinical examination with special stress on age, gender, daily alcohol intake (g/day) in the past 6 months, route of HCV transmission, body mass index (BMI), and waist circumference. BMI was calculated as weight divided by the square of the height (kg/m2). The waist circumference was measured 1 inch above the navel or midpoint between the lower margin of the least palpable rib and the top of the iliac crest parallel to the floor. Venous blood was drawn after 8 hours of overnight fasting to determine the serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin, bilirubin, platelet count, international normalized ratio (INR), serum glucose, and insulin. Then, the patients continued fasting to complete 12 hours for assessment of total cholesterol (TC), high-density lipoprotein (HDL) cholesterol (HDLc), LDL cholesterol (LDLc), and triglycerides by automated procedures. Serum insulin was determined by electro-chemiluminescence immunoassay (Elecsys 2010; Roche Diagnostics, Indianapolis, IN). Insulin resistance was investigated by the homeostasis model for the assessment of IR (HOMA-IR) using the standard formula: HOMA-IR = fasting insulin (uU/mL) × fasting glucose (mmol/L)/22.5. HCV genotype was detected using a line probe assay or reverse hybridization (InnoLipa; Innogenetics, Genetics, Gent, Belgium).
6. Assessment of HCV-RNA load and viral kinetics
HCV-RNA levels were assessed at baseline, at end of treatment (week 12), and at 12 weeks after completion of treatment (week 24). Assessment was done using the Roche COBAS TaqMan HCV assay V.2.0 (lower limit of detection 15 IU/m). Undetectable HCV-RNA at end-of-treatment response (EOTR) was defined as an undetectable HCV-RNA at the completion of HCV therapy. Undetectable HCV-RNA 12 weeks after completion of HCV therapy (week 24) was defined as SVR 12.
7. Statistical analyses
Symmetrically distributed continuous variables were summarized as a mean ± standard deviation (SD). The median and interquartile ranges were used for skewed continuous variables. The variables were compared between the groups by the Mann–Whitney U test and the Student t-test for continuous variables, and the χ2 or Fisher exact probability test was used for categorical data. The two-tailed paired Student t-test was used to test the significance of differences between baseline and post-treatment lipid profile, metabolic factors, and HOMA-IR. The Pearson correlation coefficients were used for the correlation between different parametric variables. Spearman rank correlation was used to quantify the association between continuous or ordered categorical variables. Logistic regression analysis was used to model the association between baseline lipid profile, HOMA-IR, and other covariates to determine the factors associated with hepatic fibrosis and changes in LDL-c. P ≤ 0.05 was considered statistically significant. SPSS software for Windows version 20 (SPSS Inc., Chicago, IL) was used to perform all analyses.
8. Results
8.1. Demographic and baseline characteristics
We studied 80 patients with CHC (genotype 4), treated with SIM+SOF. Their demographic, biochemical, metabolic, and fibroscan data are detailed in . Their mean age was 47.5 ± 12.3 years (range 20–67 years). Forty-eight patients were males, and 32 were females. The mean BMI was 22.2 ± 1.8 kg/m2 (range 16–26 kg/m2), and the mean waist/hip ratio was 0.93 ± 0.019 (range 0.9–0.97). In 45% of patients, HOMA-IR was >3. Fibroscan examination revealed 36 patients with mild fibrosis (15 cases were F1, and 21 cases were F2), while 44 patients had moderate to severe fibrosis (16 cases were F3, and 28 cases were F4).
Table 1. Demographic and baseline characteristics of chronic hepatitis C patients.
9. Lipid and metabolic factors
The levels of serum triglycerides, fasting blood glucose (FBS), fasting insulin, and HOMA-IR were significantly higher in patients compared with the control (P = 0.006, 0.01, 0.001, and 0.001 respectively), . With increasing hepatic fibrosis, a significant increase was detected in fasting insulin level (19.2 ± 5.4 in F0–F2, 21.7 ± 4 in F3–F4, P = 0.01), but nonsignificant differences were detected in the lipid profile, fasting glucose, and HOMA-IR, .
Table 2. Baseline metabolic data and lipid profile in chronic hepatitis C (CHC) patients versus the control group.
Table 3. Metabolic factors, lipid profile, according to fibrosis stage in chronic hepatitis C patients.
10. Changes in laboratory data, imaging, metabolic, and lipid markers
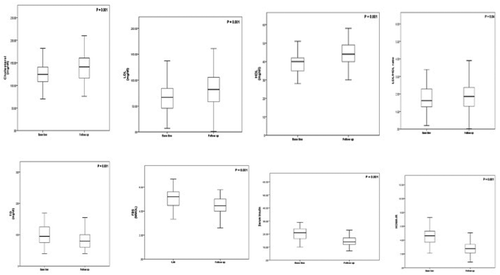
Patients who achieved EOTR showed significant reduction in hemoglobin, serum AST and ALT levels (P = 0.001), fibrosis stages, which were clearly shown in FEB4 (P = 0.03) and fibroscan (P = 0.001; ). Also, a significant reduction in serum triglycerides, FBS, fasting insulin and HOMA-IR (P < 0.001), and a significant elevation of serum cholesterol, LDLc and HDLc (P = 0.001), and LDL/HDL ratio (P = 0.04) were detected, , .
Table 4. Changes in laboratory data and imaging before and at the end of treatment with SOF/SIM (12 months) in CHC patients.
Table 5. Changes of metabolic factors, lipid profile before and after the end of treatment of SOF/SIM treatment (12 weeks) in CHC patients.
Figure 1. Changes in lipid profile, FBS, fasting insulin, and HOMA-IR in 80 chronic hepatitis C patients before and at the end of treatment with SOF/SIM. The charts show the changes in lipid profile, FBS, fasting insulin, and HOMA-IR in chronic hepatitis C (HCV) patients before and at the end of treatment (12 weeks) with SOF/SIM. LDL-c: low-density lipoprotein cholesterol; HDL: high-density lipoprotein cholesterol; TG: triglycerides; FBS: fasting blood sugar.
11. Virological outcomes
Measurements of HCV-RNA at completion of therapy (12 weeks) were undetectable (EOTR) in 79 cases (99%). The only one nonresponding case was a 66-year-old man with a high viral load (6.9), cirrhotic liver and splenomegaly, and his fibroscan score was 22–24. Measurements of HCV-RNA 12 weeks after completion of therapy (24 weeks) were undetectable (SVR 12) in 99% of the followed cases.
12. Factors associated with ΔLDLc values at the end of treatment (week 12)
Patients were stratified into two groups by their ΔLDLc levels. The high-ΔLDLc group was defined as having a median ΔLDL-c level ≥78.0 mg/dl and included 42 patients, while the low-ΔLDL-c group was defined as having a median ΔLDL-c level <78.0 mg/dl and included 38 cases.
We performed a multiple step-wise logistic regression analysis to identify the factors associated with ΔLDL-c elevation at 12 weeks from the start of therapy and the relation of increased LDLc with age, gender, BMI, AST, ALT, albumin, bilirubin, hemoglobin, platelets, white blood cell count, log viral load, FBS, fasting insulin, HOMA-IR, TC, triglycerides, and HDL. TC and stage of fibrosis were closely associated with the ΔLDLc values [OR (95% CI: 1.066 (1.031–1.102) and 1.162 (1.018–1.328) respectively] (P ≤ 0.001 and 0.026) respectively ().
Table 6. Factors associated with ΔLDL-C value with HCV administered SOF/SIM regimen, analyzed by multiple logistic-regression analysis.
13. Discussion
The recent development of DAAs for treatment of viral hepatitis continues to generate much interest. This study examined the changes in lipid profile and IR, as well as other parameters, associated with the use of such therapy for infection with hepatitis C type 4, which is responsible for almost all cases HCV infection in Egypt, a country with the highest rate of hepatitis. Though other similar studies exist, few or none of them are about type 4 HCV, on which this study sheds some light. With continuous research, the effects and relationships of these drugs continue to emerge, but many of these effects did not reach the level of the facts and are still controversial. In concordance with Buti et al. [Citation15], the present study reached an EOTR of 99% of the treated cases and 99% SVR-12 rate. Based on these results, the role of metabolic factors as predictors of the DAAs has decreased or even ameliorated. Also, the changes in lipid profile after therapy represented an interesting finding, as recently reported with the use DAAs [Citation12,Citation16]. We found a significant reduction in serum triglycerides and a significant elevation of serum cholesterol, LDL, HDL, and LDL/HDL ratio at 12 weeks post-treatment.
The direct link between HCV and host lipoproteins explicates the significant interrelationship between HCV and host lipid metabolism, as proved both in vitro [Citation17] and in clinical studies [Citation12]. Meissner et al. [Citation12] reported a concomitant decrease in triglycerides and VLDL particle size and a marked increase in serum LDLc after 24 weeks of treating HCV genotype 1 infected patients with SOF/RBV, irrespective of the treatment outcome [Citation12]. This might explain a direct effect of HCV clearance on lipid metabolism. Townsend et al. [Citation13] reported a rapid increase in LDLc and TC values that was also sustained after treating HIV/HCV-coinfected patients with ledipasvir and sofosbuvir (LDV/SOF) combination therapy for 12 weeks. Thus, the increment in serum LDLc and TC levels occurs in both HIV/HCV-coinfected and HCV mono-infected patients. Mauss et al. [Citation16] reported that the use of IFN-free DAAs regimens increased TC levels with no effect on triglycerides, while IFN-based therapy led to a reduction in TC levels with increased triglycerides, followed by a re-increase in TC levels after achieving SVR. Recently, Gitto et al. [Citation14] reported that the treatment of CHC patients genotype 1b led to an improvement in IR with worsening of the lipid profile in the form of increased HDL, LDL/HDL ratio, apolipoprotein (APO) A1, and APO B1 after 24 weeks. These findings might explore whether the changes in lipid profile may be genotype-specific.
The changes in the lipid profile may differ according to the type of DAAs. Upon treating CHC genotype 1 patients, Hashimoto et al. [Citation18] observed a rapid increase in LDLc and TC during the first 28 days of treatment that was stronger in patients who received LDV/SOF than in those who received daclatasvir/sunaprevir (DCV/ASV), while Endo et al. [Citation19] reported a significantly greater increase in TC and LDLc in the SOF/LDV group than in the DCV/ASV group during treatment, but at 4 and 12 weeks after therapy, the serum levels of TC and LDL-c were similar in both groups.
The factors that could predict changes in LDL levels were inferred in a logistic regression analysis. The main independent predictors for LDL changes in our study were advancing stage of fibrosis and lower cholesterol levels. This was in concordance with Khattab et al. [Citation20] who reported lower HDLc and cholesterol levels in CHC genotype 4 patients with severe hepatic fibrosis than those with mild fibrosis. Thus, increased serum cholesterol and LDL levels after treating patients with advanced fibrosis might represent an improvement in liver pathology or inflammation. In our study, the changes in lipid profile after DAAs were atherogenic, which might represent a predictor for cardiovascular risk. This may justify long-term monitoring of lipid parameters.
The HCV infection showed a complex relationship with IR. IR appears in the early stages of HCV infection and increases the rate and the progression of hepatic fibrosis through compensatory hyper-insulinemia, hepatic stellate cells increment, and type 1 collagen proliferation [Citation21]. Hsu et al. [Citation22] reported a correlation between HCV-RNA levels and HOMA-IR score. Even in nondiabetic patients, Moucari et al. [Citation23] reported IR in 32.4% of CHC patients. Also, despite being nonobese, nondiabetic, or prediabetic, 45% of our patients had IR >3, and the mean fasting insulin and HOMA-IR were significantly higher in the patients group in comparison with control subjects (20.15 ± 5.13 vs 13.15 ± 4.2, P = 0.001) and (4.49 ± 1.28 vs 2.72 ± 0.87, P = 0.001) respectively. Moreover, the fasting insulin showed a progressive increase, with advancing stage of hepatic fibrosis in agreement with Petit et al. [Citation6], who reported that IR in nondiabetic HCV-infected patients was related to liver fibrosis grading and may occur early in the course of HCV infection.
Although the relationship between the use of DAAs and IR was not extensively studied except in a limited number of studies [Citation14], the high rate of SVR to SOF/SIM-based therapy in our CHC-naïve patients may mean that IR is no longer having a predictor role for SVR, as that was reported for the peg-interferon/ribavirin regimens. This concept was also supported by other studies [Citation9].
Serfaty et al. [Citation24] treated CHC genotype 1 patients with telaprevir-based regimens. Their results revealed a minor role of metabolic factors in influencing antiviral response. Many studies reported that HOMA-IR did not show any relation to SVR [Citation9,Citation24,Citation25]. A significant improvement in IR was reported in patients who achieved SVR after treatment from HCV genotype 1 infection compared with those who did not [Citation9,Citation25]. Others [Citation26–Citation29] concluded that achieving viral clearance significantly reduces the risk for both type 2 DM and de novo IR in nondiabetic HCV patients. Similarly, in our study, patients who achieved SVR showed a significant improvement in fasting insulin and HOMA-IR.
This study had many limitations that need to be overcome in a further study, including the low number of cases, the failure to evaluate CVD predictors and risk factors as APO A1/APO B1 ratio and lipoprotein (a), the lack of analysis of dynamic changes in LDL and IR early during therapy, the failure to link the significant laboratory parameters to CVD risk because of the design/nature of this study, and the short-term follow-up that did not allow us to determine whether the changes in LDLc levels would continue after the completion of treatment.
14. Conclusions
The SOF/SIM-based therapy continues to show a high degree of effectiveness in the treatment of CHC GT4. High SVR ameliorates the predictive effect of metabolic factors as IR. The lipid changes in this study were interesting to the extent that long-term follow-up is strongly warranted for a better clarification of the possible role of CHC as a potential risk for CVD.
Author contributions
Ghada El Sagheer proposed the design of the study and revised the manuscript. Assmaa Ahmad and Elwy Soliman shared the tasks of patient recruitment, data management, reviewing the literature, and writing the manuscript. Lamiaa Hamdy performed the laboratory tests for the study.
Disclosure statement
No potential conflict of interest is reported by the authors.
References
- Armstrong GL, Wasley A, Simard EP, et al. The prevalence of hepatitis C virus infection in the USA, 1999 through 2002. Ann Intern Med. 2006;144:1–7.
- Felmlee DJ, Hafirassou ML, Lefevre M, et al. Hepatitis C virus, cholesterol and lipoproteins–impact for the viral life cycle and pathogenesis of liver disease. Viruses. 2013;5:1292–1324.
- Chew KW, Bhattacharya D, McGinnis KA, et al. Short communication: coronary heart disease risk by framingham risk score in hepatitis C and HIV/hepatitis C-coinfected persons. AIDS Res Hum Retroviruses. 2015;31:718–722.
- Imazeki F, Yokosuka O, Fukai K, et al. Prevalence of diabetes mellitus and insulin resistance in patients with chronic hepatitis C: comparison with hepatitis B virus-infected and hepatitis C virus-cleared patients. Liver Int. 2008;28:355–362.
- Svegliati-Baroni G, Ridolfi F, Di Sario A, et al. Insulin and insulin-like growth factor-1 stimulate proliferation and type I collagen accumulation by human hepatic stellate cells: differential effects on signal transduction pathways. Hepatology. 1999;29:1743–1751.
- Petit JM, Bour JB, Galland-Jos C, et al. Risk factors for diabetes mellitus and early insulin resistance in chronic hepatitis C. J Hepatol. 2001;35:279–283.
- Wang CS, Yao WJ, Chang TT, et al. The impact of type 2 diabetes on the development of hepatocellular carcinoma in different viral hepatitis statuses. Cancer Epidemiol Biomarkers Prev. 2009;18:2054–2060.
- Khattab M, Eslam M, Sharwae MA, et al. Insulin resistance predicts rapid virologic response to peginterferon/ribavirin combination therapy in hepatitis C genotype 4 patients. Am J Gastroenterol. 2010;105:1970–1977.
- Grasso A, Malfatti F, Testa R. Are metabolic factors still important in the era of direct antiviral agents in patients with chronic hepatitis C? World J Gastroenterol. 2013;19:6947–6956.
- Wantuck JM, Ahmed A, Nguyen MH. Review article: the epidemiology and therapy of chronic hepatitis C genotypes 4, 5 and 6. Aliment Pharmacol Ther. 2014;39:137–147.
- El Kassas M, Elbaz T, Hafez E, et al. Safety of direct antiviral agents in the management of hepatitis C. Expert Opin Drug Saf. 2016;15:1643–1652.
- Meissner EG, Lee YJ, Osinusi A, et al. Effect of sofosbuvir and ribavirin treatment on peripheral and hepatic lipid metabolism in chronic hepatitis C virus, genotype 1-infected patients. Hepatology. 2015;61:790–801.
- Townsend K, Meissner EG, Sidharthan S, et al. Interferon-free treatment of hepatitis C virus in HIV/hepatitis C virus-coinfected subjects results in increased serum low-density lipoprotein concentration. AIDS Res Hum Retroviruses. 2015;32:456–462.
- Gitto S, Cicero AFG, Loggi E, et al. Worsening of serum lipid profile after direct acting antiviral treatment. J Hepatol. 2017;66:S740.
- Buti M, Calleja JL, Lens S, et al. Simeprevir in combination with sofosbuvir in treatment-naive and experienced patients with hepatitis C virus genotype 4 infection: a Phase III, open-label, single-arm study (PLUTO). Aliment Pharmacol Ther. 2016;45:468–475.
- Mauss S, Berger F, Wehmeyer MH, et al. Effect of antiviral therapy for HCV on lipid levels. Antivir Ther. 2016;21:81–88.
- Kapadia SB, Chisari FV. Hepatitis C virus RNA replication is regulated by host geranylgeranylation and fatty acids. Proc Natl Acad Sci U S A. 2005;102:2561–2566.
- Hashimoto S, Yatsuhashi H, Abiru S, et al. Rapid increase in serum low-density lipoprotein cholesterol concentration during hepatitis C interferon-free. Treatment PLoS One. 2016;11:e0163644.
- Endo D, Satoh K, Shimada N, et al. Impact of interferon-free antivirus therapy on lipid profiles in patients with chronic hepatitis C genotype 1b. World J Gastroenterol. 2017;23:2355–2364.
- Khattab MA, Eslam M, Aly MM, et al. Serum lipids and chronic hepatitis C genotype 4: interaction and significance. Ann Hepatol. 2011;11:37–46.
- El-Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 2004;126:460–468.
- Hsu CS, Liu CJ, Liu CH, et al. High hepatitis C viral load is associated with insulin resistance in patients with chronic hepatitis C. Liver Int. 2008;28:271–277.
- Moucari R, Asselah T, Cazals-Hatem D, et al. Insulin resistance in chronic hepatitis C: association with genotypes 1 and 4, serum HCV RNA level, and liver fibrosis. Gastroenterology. 2008;134:416–423.
- Serfaty L, Forns X, Goeser T, et al. Insulin resistance and response to telaprevir plus peginterferon alpha and ribavirin in treatment-naive patients infected with HCV genotype 1. Gut. 2012;61:1473–1480.
- Fattovich G, Covolo L, Pasino M, et al. The homeostasis model assessment of the insulin resistance score is not predictive of a sustained virological response in chronic hepatitis C patients. Liver Int. 2011;31:66–74.
- Thompson AJ, Patel K, Chuang WL, et al. Viral clearance is associated with improved insulin resistance in genotype 1 chronic hepatitis C but not genotype 2/3. Gut. 2009;61:128–134.
- Delgado-Borrego A, Jordan SH, Negre B, et al. Reduction of insulin resistance with effective clearance of hepatitis C infection: results from the HALT-C trial. Clin Gastroenterol Hepatol. 2010;8:458–462.
- Arase Y, Suzuki F, Suzuki Y, et al. Sustained virological response reduces incidence of onset of type 2 diabetes in chronic hepatitis C. Hepatology. 2009;49:739–744.
- Aghemo A, Prati GM, Rumi MG, et al. Sustained virological response prevents the development of insulin resistance in patients with chronic hepatitis C. Hepatology. 2012;56:1681–1687.
