ABSTRACT
Vector-borne diseases are responsible for more than 20% of the infectious diseases worldwide. The prevalence of arboviruses transmit diseases to humans in Sudan has not been investigated. Mosquito-borne viral diseases increase globally incidence, including the Sudan. Frequent unknown fever outbreaks have been reported in eastern region, Sudan. However, diagnosis was based exclusively on clinical signs and symptoms without confirmatory laboratory investigations. However, for accurate detection of these viruses in outbreaks, molecular technique is considered. The objective of this study was to determine the prevalence of six arboviruses in the Kassala state of east Sudan during unknown fever outbreak. A cross sectional hospital-based study was conducted in the Kassala, Teaching Hospital. Blood samples from 119 patients suffering from unknown fever were used for screening of six arboviruses, hepatitis E virus and malarial using molecular techniques and serology. The overall arboviruses seroprevelance was 61.3% (73/119). The highest positivity rate was 73.1% (52/73) chikungunya virus; 29 males and 20 females patients were chikungunya positive. Other arboviruses were circulating in low rate 20.5% (15/73), and 6.8% (5/73) for sindbis and rift valley fever viruses respectively. Hepatitis E virus was negative in all cases and malaria positivity rate 13.4% (16/119). The prevalence of arboviruses among unknown fever patients present to Kassala teaching hospital of eastern region in Sudan is significantly high (61.3%). The chikungunya virus is the predominant causative agent of arboviruses. Molecular techniques such as PCR are important for accurate and rapid diagnosis of this viral outbreak.
1. Introduction
Arthropod-borne viruses (arboviruses) are transmitted via vectors, can affect both animals and humans [Citation1]. Globally, vector-borne diseases are responsible for more than 20% of the infectious diseases affecting all humanity worldwide, most commonly in the developing countries in Africa and Asia; many African communities suffer from vector-borne disease burden and its socioeconomic consequences [Citation2–Citation5]. Mosquito-borne viruses affecting humans, these viruses include: chikungunya (CHIKV), dengue fever (DFV), yellow fever (YFV), Rift Valley fever virus (RVFV), Sindbis virus (SINV), and alkhurma viruses [Citation2,Citation6–Citation9].
Dengue, yellow fever, chikungunya, Rift Valley fever, alkhurma haemorrhagic fever, and Sindbis viruses have been reported in more than 34 countries in sub-Saharan Africa and Middle East [Citation10–Citation14]. YFV originated in Africa and spread with the slave trade dating back to at least 1650 [Citation15,Citation16]. Majority of populations infected with arboviruses have mild disease and recover rapidly, except dengue and Rift Valley fever differ in severity; based on several studies in different locations in Sudan such as northern Khartoum and New Halfa Hospital in eastern region [Citation17–Citation19]. The clinical characteristics and symptoms of most of arboviruses are divided into two subgroups: neuroinvasive and non-neuroinvasive. Mostly are asymptomatic and often resolve after 1–2 weeks. The clinical symptoms usually were common include sudden onset of fever, headache, muscle pain, backache, general weakness, red eyes, nausea, and vomiting, lasting 2–4 days usually followed by uneventful recovery. However, the most clinical signs and symptoms of arbovirus infections are non-specific in nature and could be interfere with other parasitic or non-parasitic infections such as malaria or bacterial meningitis. Most of arbovirus infections could be seriously severe, resulting in haemorrhage, high fever, encephalitis, meningitis, or even death [Citation20].
Chikungunya fever is viral infection often characterized by acute febrile illness that may occur at any age. The infection is divided into acute and chronic phases. In 2016, several CHIKV outbreaks have been reported from different locations in Sudan, although the prevalence in the general population is unknown [Citation9,Citation21,Citation22].
The Sindbis virus (SINV) transmitted to humans from its enzootic hosts by mosquitoes, and causes polyarthritis, fever, and rash [Citation23,Citation24]
Hantaviruses have almost entirely been associated with human contact with rodent excrement, although recent human-to-human transmission has been reported for the Andes virus in South America. Hantavirus’s types cause potentially fatal diseases in humans, such as haemorrhagic fever with renal syndrome and hantavirus pulmonary syndrome; however, others have not been associated with human disease [Citation25–Citation30].
During spring of 2003 in US suspected cases of haemorrhagic illness were reported, YFV was identified as the causative agent of the outbreak [Citation31]. During the period from November 2010 to March 2011 an outbreak of hepatitis E virus (HEV) among pregnant women presented at Port Sudan Hospital, as well Other outbreaks occur in eastern region in Sudan were reported [Citation32,Citation33].
In June 2015, in eastern region at Kassala state in Sudan, the Ministry of Health was notified an outbreak of unknown fever cases and many of the patients had correlated to hemorrhagic illness. Since it has collaboration between the Sudanese Ministry of Higher Education and Swedish research projects in the different epidemics in many African countries specifically ‘arthropods viruses’. All the patients presented with illness characterized by fever, severe headache, and backache. Approximately all the patients had hemorrhagic signs, including myalgia, arthralgia, nausea, vomiting, and few cases had gingival bleeding melena and nearly 15% had jaundice. Since few years back dengue and YFV outbreaks occur in eastern region in Africa especially in Sudan.
Depending on this data we hypothesized that some of febrile patients who frequently diagnosed as malaria patients may harbour one or more of the viral hemorrhagic fever (VHF). Therefore, the objective of the current study was to determine the prevalence and identification the types of arboviruses and VHFs suspected cases from eastern region were admitted in Kassala state hospital using molecular techniques.
2. Methods
2.1. Participants and setting
A cross-sectional hospital-based study was conducted in Kassala state, Teaching Hospital of Kassala, eastern Sudan, during 1 June–30 November 2015. Since this study was a part of the collaboration between the Sudanese Ministry of Higher Education and Swedish research projects in the different epidemics in Sudan [Citation34,Citation35].
All the patients’ records retrieved and socio-demographic characteristics (age, gender), various clinical symptoms and signs (fever, headache, vomiting, nausea, backache, abdominal pain, dizziness, diarrhoea, and jaundice), haematological and biochemical characteristics (complete blood count, alanine aminotransferase, and aspartate aminotransferase [Citation36]) were recorded.
Five millilitres blood samples were collected using sterile syringes from all the admitted patients (119) who exhibited suspected signs and symptoms of vector-borne viral diseases. The blood samples divided in two blood containers: 2.5 mL blood was collected into anticoagulants free containers to obtain serum used for enzyme-linked immunosorbent assay (ELISA); where the remaining 2 mL blood was divided into: 1.5 mL anticoagulant EDTA containers for performing malaria rapid diagnostic test and the rest whole blood sample (0.5 mL) spotted on filter papers (Whatman FTA classic card (GE Healthcare, Little Chalfont, UK)); dried overnight at room temperature before being processed for PCR (polymerase chain reaction) to assess the stability of viral RNA; cDNA was reverse transcribed from RNA and used for quantitative polymerase chain reaction (qPCR).
2.1.1. Serology
2.5 mL blood was collected in anticoagulants free containers to obtain serum for ELISA (PanBio®, Brisbane, Australia) were used for detecting dengue IgM antibodies. All the patients’ serum samples were screened for DFV [Citation35] and YFV using rapid strip tests, IgM antibodies against DENV and YFV were negative in all suspected cases.
2.2. Elution and RNA extraction
After drying and storing the filter papers, the absorption zones of the filter papers were treated with 500 μL of RNAse-free water for 1 h at room temperature with gentle rocking, to elute the sample. Total RNA was extracted from whole blood spotted on filter papers using the Qiagen RNeasy mini kit and columns (Qiagen, Hilden, Germany) according to manufacturer’s instructions and recovered in 40 µL nuclease-free water.
2.3. Complement DNA synthesis and PCR
Preparation of cDNA was performed as described previously [Citation34]. qRT-PCR was performed using the ABI Prism 7900HT Sequence Detection System 2.0 (Applied Biosystems) and Mastercycler® ep realplex, real-time thermal cycler (Eppendorf AG, Germany). Two frequently used in-house PCR kits, Power SYBR Green One-step RT-PCR master mix (ThermoFisher) and KAPA SYBR® FAST qPCR kit (KAPA Biosystems, Boston, MA, USA), were purchased from DNA Technology (Denmark). The primers sequences for RVFV were: forward primer, 5′-AAGGCAAAGCAACTGTGGAG-3′ (Tm = 54.3–56.4) and reverse primer, 5′-TGAGTGGCTTCCTGTCACTG-3′ (Tm = 53–59.5); for pan-Hanta: forward, 5′-TGCWGATGCIACRAAATGGTC-3′ (Tm = 57.5–61.2) and reverse, 5′-GCATCATCWGARTGATGIGCAA-3′ (Tm = 58.4–62.1); dengue virus: mD1 forward primer, 5′-TCAATATGCTGAAACGCGAGAGAAACCG-3′ (Tm = 65.6–70.9) and reverse primer, 5′-TTGCACCAACAGTCAATGTCTTCAGGTTC-3′ (Tm = 59.9–67.2); CHIKV (CHIK PnsP1): forward primer, 5´- TAGAGCAGGAAATTGACCCC-3′ (Tm = 59.9–67.2) and reverse primer 5′-CTTTAATCGCCTGGTGGTAT-3′(Tm = 59.9–67.2); for ALKHV: S1, 5′-GTG AGT GGC GCT TTG TTTG TA-3′ and R, 5′-CCC CCT TTC CTT TAA GGA CG-3′; for SINV: forward primer, 5´-GGTTCCTACCACAGCGACGAT-3′ and reverse primer 5´-TGATACTGGTGCTCGGAAAACA-3′; for HEV: JVHEVFw, 5′-GGTGGTTTCT GGGGTGAC-3′ and JVHEVRv; 5′AGGG GTTGGTTGGATGAA-3′. All primers were purchased from DNA Technology A/S (Risskov, Denmark). For evaluation, plasmids harbouring the synthetic genes were isolated using standard procedures, and used at indicated dilutions as templates for the above primer pairs [Citation37]. The primer–primer interactions were determined using Oligo Analyser 3.0 (http://207.32.43.70/biotools/oligocalc/oligocalc.asp) and (http://www.idtdna.com/analyzer/Applications/OligoAnalyzer/).
Reaction volume was 20 µL, which contained 5 µg RNA template, 25 µM of each primer, and 10 µL KAPA master mixtures (KAPA SYBR® FAST qPCR kit). The samples were initially incubated at 42°C for 30 min for reverse transcription, followed by incubation at 95°C for 3 min for denaturation. In total, 40 cycles were performed, each consisting of a denaturation step at 95°C for 3 s and an annealing at 60°C for RVFV and CHIKV and at 65°C for hantavirus. Negative and positive controls were included for each test to confirm the specificity of the selected primer pairs.
2.4. Statistical analysis
The SPSS version 22 software was used for statistical analysis. P value ˂ 0.05 was considered significant.
2.4.1. Ethics
A cross-sectional hospital-based study was conducted in Kassala Teaching Hospital; the patient’s data were analysed anonymously and no new personal data were required. The study received ethical clearance from the Health Research Board at Ministry of Health, Kassala state, Sudan with reference number: KS/MOH/50/A/1.
All participants were provided with a written consent for the purpose of this study.
3. Results
The participants were categorized according to age into adults and children, with those aged less ≤18 years being considered as children. Accordingly, 16.8% patients (20/119) were children and 83.2% (99) were adults representing the majority of cases. The mean age of the all patients was 31.98 years, whereas the mean ages for male and female patients were 33.22 and 30.34 years, respectively. Male gender constituted 57.1%, with male:female ratio 1.4:1. There was no significant difference in age on the basis of gender (P value = 0.34, STD 14.7 and mean = 31.4). All differences were significant (P ˂ 0.05)
One hundred and nineteen patients with clinical features suggestive of viral infections were referred to Kassala hospital. Out of these, 73 (61.3%) patients were positive PCR for RVFV, CHIKV, and SINV infections.
Most samples were positive for chikungunya and Sindbis (73.1% and 19.4%, respectively). Out of the 73 arboviruses-positive samples, 49 were positive for chikungunya (29 males and 20 females). In contrast to this, other arboviruses were circulating in low rate such as RVFV (7.4%). Results also revealed that both SINV and malaria parasite shared the same positive proportions among the detected samples (10.9%) with significant differences (P ˂ 0.05).
Detection of HEV and four VHF viruses (YFV, hanta, dengue, and ALKH) using molecular methods yielded negative results for all participants (). All the patients screened for IgM dengue antibodies; only 13 patients were serology positive for DFV (data not shown).
Figure 1. Distribution of all patients diagnosed with emerging arboviruses (adults and children) using real-time PCR P < 0.009**.
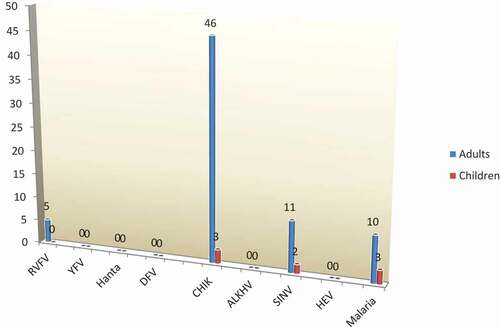
CHIKV was the dominant virus (39.5 %) causing unknown fever, followed by SINV 10.92% cases and RVFV (P ˂ 0.05). However, malaria was more dominant than RVFV, representing 10.92% cases (). The distribution of arboviruses, HEV, and malaria infection for all patients presented to the hospital with unknown fever have been summarized in .
Table 1. Distribution of arboviruses, hepatitis E virus and malaria for all the patients with unknown fever
4. Discussion
In the present study, we collected blood samples from patients admitted to Kassala Teaching Hospital (eastern Sudan) ().
The most common symptoms in all admitted patients were fever, followed by headache, nausea, vomiting, and abdominal pain. Cases with these symptoms were considered to be suspected for vector-borne viral diseases.
In the current study, from the whole patients we detected arboviruses 61.3% (73/119). The highest positivity rate was 73.1% (52/73) CHIKV; 29 male and 20 female patients were chikungunya positive. CHIKV, SINV, and RVFV in using qRT-PCR, whereas hantavirus, DFV, ALKHV, and HEV were not detected.
CHIK fever in children is accompanied by specific symptoms, whereas high prevalence was observed for dermatological manifestations and neurological complications [Citation32].
In the present study, we observed that the most common symptoms were fever, followed by headache, nausea, vomiting, and abdominal pain which are not restricted to only RVFV, CHIKV, and SINV infections, previous studies shown that infection with chikungunya and Sindbis viruses characterize by specific symptoms such as skin rash, myalgia, and arthralgia. The degree of the infection based on the severity of nausea and vomiting, skin rashes, and abdominal pain differed significantly between adults and children, which could be explained by the fact that most participants were adults. Considering the variations in symptoms, efficient and accurate diagnostic methods for these viral diseases are urgently required.
CHIKV infection had occurred in Africa in antiquity, there have been several recorded outbreaks in several African countries including Sudan [Citation9,Citation22]. CHIKV infection was the most dominant infection in the present study, with 67.1% of the all arboviruses positive patients; whereas 63% (26) infected adults. There was a significant difference between the number of adults and children infected with CHIKV (P value = 0.009, P ˂ 0.05) (); however, no significant difference was observed regarding gender (P value = 0.70, P ˂ 0.05).
In the current study, we detected 13 SINV infections (11 adults and 2 children) which was dominant in adults (11/13 infections); however, the positivity rate was low 17.8% in comparison of CHIKV in this study. The endemic areas of SINV reflect high SINV seroprevalence [Citation24,Citation38]. This virus was linked to outbreaks in South Africa [Citation39], although human viral diseases related to SINV are mostly restricted to northern Europe [Citation23].
RVFV causes epizootic outbreaks in tropical areas, mainly in Africa, including Sudan, which is the largest country in Africa [Citation12–Citation14,Citation40–Citation42]; however, we detected only five infections in adult patients in this study. Another study conducted on 290 patients in eastern Sudan revealed the presence of highly seropositive RVFV IgG [Citation18]. Although the IgG serology might be the result of previous outbreaks, such as the large epidemic of 2010, the previous study included more participants than the present study [Citation17,Citation18]. The less infection rate detected in the present study may due to improvements in health and education. In addition, the previous study was conducted during dry season (September and November 2007), when Sudan was severely affected with a RVFV outbreak [Citation31].
All participants were screened for hantavirus, DFV, ALKHV, and HEV infection using molecular techniques. The PCR reveals negative results. Although not all these viruses were responsible for VHF, we screening these viruses due to many studies were conducted in eastern Sudan revealed the presence of highly seropositive of dengue and HEV [Citation33,Citation43–Citation45]. Thirteen patients were positive for dengue IgM in ELISA, although the PCR results for all DFV types were negative, which may be explained by the presence of several genetically and antigenically different serotypes of the DFV worldwide [Citation6,Citation7,Citation43,Citation46–Citation48].
Mosquito-infested areas show high rates of malarial parasite infection. High prevalence of malaria is usually observed in children [Citation49]; however, in this study, most malaria patients were adults (10 patients); however, only three children had malaria positive. The African continent has witnessed a long-term decline in the prevalence of Plasmodium falciparum, malaria burden in the six endemic countries (Afghanistan, Djibouti, Pakistan, Somalia, Sudan, and Yemen) [Citation50]. PCR is used for identification and characterization of viral, bacterial, and parasitic agents. In particular, RT-PCR is useful for diagnosing the presence of RNA viruses [Citation51]. We used RT-PCR to accurately determine the presence of various VHFs, which will enable to get better awareness of outbreaks and timely management of VHF and prevention of new infections. These viruses and the malarial parasite are transmitted via mosquitoes and arthropods, and hence, infected patients should be quarantined. Finally, public health education and awareness campaigns should be initiated (for the public as well as health workers) and mosquito breeding areas should be eradicated to eliminate these pathogens.
5. Conclusion
The prevalence of arboviruses among all unknown fever patients present to Kassala Teaching Hospital of eastern region in Sudan is significantly high (61.3%, P ˂ 0.05). In conclusion, we observed that CHIKV was the predominant virus causing unknown fever outbreak in Kassala state. Further laboratory investigations and epidemiological studies required upon larger number of patients and different periods for better prediction and prevention outbreaks.
Acknowledgments
We would like to thank all patients involved in all study sites. We are grateful to the Medical staff of Kassala Teaching Hospital, Sudan for helping with blood samples collection, and the staff of Blue Nile Institution, Ministry of Health, Sudan for helping with laboratory analysis. We thank Prof. Evander, Umeå University, Sweden, for comments on an earlier version of the manuscript. The authors also extend their appreciation to Research Centre of the Science and Medical studies Departments at King Saud University for funding this study.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Verani P. [Ecology and epidemiology of arboviruses in Italy]. Parassitologia. 1981;23(1–3):1–7.
- Braack L, Gouveia de Almeida AP, Cornel AJ, et al. Mosquito-borne arboviruses of African origin: review of key viruses and vectors. Parasit Vectors. 2018;11(1):29.
- Hanson RP, Trainer DO. Significance of changing ecology on the epidemiology of arboviruses in the USA. Proc Annu Meet U S Anim Health Assoc. 1969;73:291–294.
- Worth CB, Paterson HE, de Meillon B. The incidence of arthropod-borne viruses in a population of culicine mosquitoes in Tongaland, Union of South Africa (January, 1956, through April, 1960). Am J Trop Med Hyg. 1961;10:583–592.
- Smithburn KC. Problems of the arthropod-borne viruses in Africa. Ann Soc Belg Med Trop (1920). 1958;38(2):347–358.
- Suleman M, Faryal R, Alam MM, et al. Dengue virus serotypes circulating in Khyber Pakhtunkhwa Province, Pakistan, 2013–2015. Ann Lab Med. 2017;37(2):151–154.
- Ali A, Ahmad H, Idrees M, et al. Circulating serotypes of dengue virus and their incursion into non-endemic areas of Pakistan; a serious threat. Virol J. 2016;13:144.
- Ahmed SS, Soghaier MA, Mohammed S, et al. Concomitant outbreaks of yellow fever and hepatitis E virus in Darfur States, Sudan, 2012. J Infect Dev Ctries. 2016;10(1):24–29.
- Weaver SC, Lecuit M. Chikungunya virus and the global spread of a mosquito-borne disease. N Engl J Med. 2015;372(13):1231–1239.
- Semenza JC, Sudre B, Miniota J, et al. International dispersal of dengue through air travel: importation risk for Europe. Plos Neglect Trop D. 2014;8(12).
- Baba M, Masiga DK, Sang R, et al. Has Rift Valley fever virus evolved with increasing severity in human populations in East Africa? Emerg Microbes Infect. 2016;5:e58.
- Arsevska E, Hellal J, Mejri S, et al. Identifying areas suitable for the occurrence of Rift Valley fever in North Africa: implications for surveillance. Transbound Emerg Dis. 2016;63(6):658–674.
- Metras R, Jewell C, Porphyre T, et al. Risk factors associated with Rift Valley fever epidemics in South Africa in 2008–11. Sci Rep. 2015;5:9492.
- Glancey MM, Anyamba A, Linthicum KJ. Epidemiologic and environmental risk factors of Rift Valley fever in Southern Africa from 2008 to 2011. Vector Borne Zoonotic Dis. 2015;15(8):502–511.
- Quichaud J. The epidemiology of yellow fever in Africa. Sem Med Prof Med Soc. 1955;31(38):1179–1181.
- Mahaffy AF. The epidemiology of yellow fever in Central Africa. Trans R Soc Trop Med Hyg. 1949;42(6):511–530.
- Aradaib IE, Erickson BR, Elageb RM, et al. Rift Valley fever, Sudan, 2007 and 2010. Emerg Infect Dis. 2013;19(2):246–253.
- Hassanain AM, Noureldien W, Karsany MS, et al. Rift Valley fever among febrile patients at New Halfa hospital, eastern Sudan. Virol J. 2010;7.
- Adam AA, Karsany MS, Adam I. Manifestations of severe Rift Valley fever in Sudan. Int J Infect Dis. 2010;14(2):e179–e180.
- Liang G, Gao X, Gould EA. Factors responsible for the emergence of arboviruses; strategies, challenges and limitations for their control. Emerg Microbes Infect. 2015;4(3):e18.
- Adam A, Seidahmed OM, Weber C, et al. Low seroprevalence indicates vulnerability of Eastern and Central Sudan to infection with Chikungunya virus. Vector Borne Zoonotic Dis. 2016;16(4):290–291.
- Gould LH, Osman MS, Farnon EC, et al. An outbreak of yellow fever with concurrent chikungunya virus transmission in South Kordofan, Sudan, 2005. Trans R Soc Trop Med Hyg. 2008;102(12):1247–1254.
- Adouchief S, Smura T, Sane J, et al. Sindbis virus as a human pathogen epidemiology, clinical picture and pathogenesis. Rev Med Virol. 2016;26(4):221–241.
- Symington J, Schlesinger MJ. Characterization of a Sinbis virus variant with altered host range. Arch Virol. 1978;58(2):127–136.
- Vrbovska V, Chalupa P, Strakova P, et al. [Human hantavirus diseases - still neglected zoonoses?]. Epidemiol Mikrobiol Imunol. 2015;64(4):188–196.
- Sane J, Reimerink J, Harms M, et al. Human hantavirus infections in the Netherlands. Emerg Infect Dis. 2014;20(12):2107–2110.
- Latus J, Tenner-Racz K, Racz P, et al. Detection of Puumala hantavirus antigen in human intestine during acute hantavirus infection. PLoS One. 2014;9(5):e98397.
- Tersago K, Verhagen R, Vapalahti O, et al. Hantavirus outbreak in Western Europe: reservoir host infection dynamics related to human disease patterns. Epidemiol Infect. 2011;139(3):381–390.
- Martinez VP, Bellomo C, San Juan J, et al. Person-to-person transmission of Andes virus. Emerg Infect Dis. 2005;11(12):1848–1853.
- Sandmann S, Meisel H, Razanskiene A, et al. Detection of human hantavirus infections in Lithuania. Infection. 2005;33(2):66–72.
- World Health Organization G: Outbreak news. Rift Valley fever, Sudan--update. Wkly Epidemiol Rec 2007, 82(48):417–418.
- Rayis DA, Jumaa AM, Gasim GI, et al. An outbreak of hepatitis E and high maternal mortality at Port Sudan, Eastern Sudan. Pathog Glob Health. 2013;107(2):66–68.
- Elduma AH, Osman WM. Dengue and hepatitis E virus infection in pregnant women in Eastern Sudan, a challenge for diagnosis in an endemic area. Pan Afr Med J. 2014;19:391.
- Mohamed N, Nilsson E, Johansson P, et al. Development and evaluation of a broad reacting SYBR-green based quantitative real-time PCR for the detection of different hantaviruses. J Clin Virol. 2013;56(4):280–285.
- Gyarmati P, Mohammed N, Norder H, et al. Universal detection of hepatitis E virus by two real-time PCR assays: taqMan and primer-probe energy transfer. J Virol Methods. 2007;146(1–2):226–235.
- Suwandono A, Kosasih H, Nurhayati, et al. Four dengue virus serotypes found circulating during an outbreak of dengue fever and dengue haemorrhagic fever in Jakarta, Indonesia, during 2004. Trans R Soc Trop Med Hyg. 2006;100(9):855–862.
- Mohamed N, Belak S, Hedlund KO, et al. Experience from the development of a diagnostic single tube real-time PCR for human caliciviruses, Norovirus genogroups I and II. J Virol Methods. 2006;132(1–2):69–76.
- Keegstra K, Burke D. Comparison of the carbohydrate of Sinbis virus glycoproteins with the carbohydrate of host glycoproteins. J Supramol Struct. 1977;7(3–4):371–379.
- van Niekerk S, Human S, Williams J, et al. Sindbis and Middelburg old world alphaviruses associated with neurologic disease in horses, South Africa. Emerg Infect Dis. 2015;21(12):2225–2229.
- Jansen van Vuren P, Kgaladi J, Patharoo V, et al. Human cases of Rift Valley fever in South Africa, 2018. Vector Borne Zoonotic Dis. 2018.
- McMahon BH, Manore CA, Hyman JM, et al. Coupling vector-host dynamics with weather geography and mitigation measures to model Rift Valley fever in Africa. Math Model Nat Phenom. 2014;9(2):161–177.
- Himeidan YE, Kweka EJ, Mahgoub MM, et al. Recent outbreaks of Rift Valley fever in East Africa and the Middle East. Front Public Health. 2014;2:169.
- Eldigail MH, Adam GK, Babiker RA, et al. Prevalence of dengue fever virus antibodies and associated risk factors among residents of El-Gadarif state, Sudan. BMC Public Health. 2018;18(1):921.
- Hamid Z, Hamid T, Alsedig K, et al. Molecular investigation of dengue virus serotype 2 circulation in Kassala state, Sudan. Jpn J Infect Dis. 2018.
- Soghaier MA, Himatt S, Osman KE, et al. Cross-sectional community-based study of the socio-demographic factors associated with the prevalence of dengue in the eastern part of Sudan in 2011. BMC Public Health. 2015;15:558.
- Taslim M, Arsunan AA, Ishak H, et al. Diversity of dengue virus serotype in endemic region of South Sulawesi Province. J Trop Med. 2018;2018:9682784.
- Malik A, Earhart K, Mohareb E, et al. Dengue hemorrhagic fever outbreak in children in Port Sudan. J Infect Public Health. 2011;4(1):1–6.
- Muraduzzaman AKM, Alam AN, Sultana S, et al. Circulating dengue virus serotypes in Bangladesh from 2013 to 2016. Virusdisease. 2018;29(3):303–307.
- Kayentao K, Florey LS, Mihigo J, et al. Impact evaluation of malaria control interventions on morbidity and all-cause child mortality in Mali, 2000–2012. Malar J. 2018;17(1):424.
- Atta H, Barwa C, Zamani G, et al. Malaria and complex emergencies in the Eastern Mediterranean Region (Editorial). East Mediterr Health J. 2016;22(4):235–236.
- Mohamed N, Elfaitouri A, Fohlman J, et al. A sensitive and quantitative single-tube real-time reverse transcriptase-PCR for detection of enteroviral RNA. J Clin Virol. 2004;30(2):150–156.