ABSTRACT
The purpose of this study was to investigate the possible role of PON-1, an antioxidant lipophilic enzyme linked to HDL-C (high-density lipoprotein cholesterol), on the pathophysiology and clinical follow-up of acute pancreatitis. Biochemical tests, PON-1 and oxidative stress parameters (malonyl dialdehyde, MDA; superoxide dismutase, SOD; total antioxidant capacity, TAC) were evaluated in the sera of patients with acute pancreatitis at admission (day 0), day 3 and day 10 of follow-up, between June and September 2017. SPSS 13.0 statistical software package programme was used for statistical analyses.Mean age was 51.4 of the total 25 patients. Ranson scores were 0–1 points (60%), 3–4 points (24%) and 5–6 points (16%). CTSI (computed tomography severity index) scores were calculated, and most of the patients were seen to have mild or average pancreatitis (96%). While total cholesterol, triacylglycerol and LDL-C (low-density lipoprotein) levels stayed in their normal limits, there was a significant decrement tendency. HDL-C level was seen to rise significantly above its upper limit at day 10 (p < 0.001). Mean PON-1 levels were measured as 69.23, 76.72 vs. 113.15 U/mL at days 0, 3 and 10, respectively; and it was positively correlated with HDL-C (p < 0.001). Serum SOD increased also in parallel with PON-1 (20.49 vs. 39.46 U/mL) while MDA level decreased significantly (3.9 vs. 2.28 μM, p < 0.001). TAC was seen to rise significantly after treatment (0.52 vs. 1.22 mM). In conclusion, decreased PON-1 and HDL-C together with antioxidants SOD and TAC at the early period of acute pancreatitis were seen to rise after treatment, while the previously higher MDA level decreased in parallel. This reveals the importance of the balance between oxidative stress and antioxidant defense mechanisms in clinical progression of the disease, and the potential of PON-1 as a promising clinical marker.
1. Introduction
There is a lack of robust estimates of the worldwide incidence and mortality of acute pancreatitis (AP) in the general population. For the past 30 years, there have been sudden increases in the incidence of AP worldwide [Citation1]. In Western countries, the incidence of AP varies from 4.9 to 45 per 100,000 populations per year [Citation2,Citation3]. To date, there are very few reports in the literature about the incidence, etiology and clinical outcome of AP from Middle East countries in general [Citation3]. However, the incidence seems to be similar [Citation4]. It has also become the most common inpatient gastrointestinal diagnosis and costs an estimated 2.6 billion dollars per year in the USA [Citation5], which is still increasing [Citation6].
AP is characterized by local and systemic inflammation, which is observed clinically from no systemic sign through the local and systemic inflammatory response, organ failure, pancreatic necrosis and even death [Citation7]. The underlying pathophysiology through which local pancreatic injury drives the systemic inflammatory response has not been fully elucidated. However, cumulative data including our previous studies suggest that oxidative stress plays the major role [Citation8–Citation10]. It has also been shown that impaired lipid metabolism plays an important role in the pathogenesis of numerous diseases, including cardiovascular conditions, infectious diseases, diabetes, carcinoma and many inflammatory diseases including acute pancreatitis [Citation8,Citation11,Citation12].
In AP, a final common pathway mediated by reactive oxygen species (ROS) appears to play a role in the associated tissue destruction both in the initiation and progression [Citation13]. Excessive ROS produced by the mitochondria are considered to play a central role in cellular dysfunction as observed in several pathophysiological states including diabetes and pancreatitis. In diabetes, these dual effects of ROS are well illustrated by their long-term deleterious effects on insulin-secreting and insulin target cells, as well as on endothelial cells [Citation9]. Augmented production of ROS in a self-perpetuating manner, in excess of antioxidant defenses, occurs predominantly in activated neutrophils [Citation8]. They can directly attack the lipoid matrix of biological membranes, stimulate arachidonic acid metabolism, thereby enhancing the accumulation and adherence of neutrophils and platelets to the capillary wall. Thus, ROS could impair the microcirculation and disturb the microvascular integrity, resulting in decreased perfusion, increased capillary permeability and fluid transudation. Neutrophils infiltrating the pancreas have also been demonstrated to contribute to the pathologic activation of digestive enzymes in acinar cells [Citation8,Citation13].
Paraoxonase (PON-1) is an esterase associated with high-density lipoprotein cholesterol (HDL-C), which clinical interest resides in its ability to prevent or limit the lipid oxidation [Citation14]. PON-1, with its antioxidant activity, reduces the susceptipility of low-density lipoprotein (LDL-C) to lipid peroxidation by hydrolyzing specific lipid peroxides [Citation15]. Several recent studies have suggested that PON-1 concentration decreases in diabetes, cardiovascular diseases and AP, which are associated with an increase in oxidative stress [Citation14,Citation16].
Although the role of lipid peroxidation in AP has been extensively studied, the studies on changes in lipid profile and PON-1 were found to be very limited [Citation8,Citation16]. In the present prospective clinical study, because PON-1 is known as an antioxidant enzyme that inhibits the oxidative modification of LDL-C, and oxidative stress plays a pivot role in the pathogenesis of AP, we investigated the changes in PON-1 activity with particular emphasis on oxidative stress markers and lipid profile.
2. Materials and methods
The study was conducted with the approval of our institution’s Ethics Committee (23.05.2017/11430).
2.1. Patients’ information
Demographics, etiology, comorbidity and clinical outcome were recorded. Radiologic screening scans were noted to calculate clinical scores, and correlated with biochemical values.
A total of 25 patients were included; 14 were women (56%) and 11 were men (44%). The patients ranged in age from 24 to 81 years, with a median age of 51.4 years. The most frequent AP etiology was biliary (n = 19, 76%), followed by idiopathic (n = 5, 20%) and alcoholic (n = 1, 4%). Hypertension (n = 13, 52%), diabetes (n = 11, 44%) and chronic obstructive pulmonary disease (COPD, n = 3, 12%) were the most common comorbidities. Magnetic resonance cholangiopancreatography (MRCP) was added to radiologic work-up in patients with suspected biliary obstruction (n = 6, 24%), and endoscopic retrograde cholangiopancreatography (ERCP) was performed in four of them (16%).
2.2. Assessment of disease severity (scoring)
Ranson’s scores were calculated using data from the first 48 h from admission and patients were classified as mild AP or severe AP, based on the presence of organ failure for more than 48 h. Organ failure included shock (systolic blood pressure < 90 mmHg), pulmonary insufficiency (arterial PO2 < 60 mmHg or the need for mechanical ventilation) or renal failure (serum creatinine level > 2 mg/dL). All patients underwent hepatobiliary ultrasonography (USG) and abdominal computerized tomography (CT) on admission. The sum of Balthazar scores (normal pancreas: 0, enlargement of pancreas: 1, inflammatory changes in pancreas and peripancreatic fat: 2, ill-defined single peripancreatic fluid collection: 3 and two or more poorly defined peripancreatic fluid collections: 4 points) and pancreatic necrosis percentages (none: 0, ≤30%: 2, 30–50%: 4, >50%: 6 ponits) was used to calculate the final CT severity index (CTSI), and the severity of AP was categorized as mild (0–3 points), moderate (4–6 points) or severe (7–10 points).
Ranson scores were 0–2 points (n = 15, 60%), 3–4 points (n = 6, 24%) and 5–6 points (n = 4, 16%), respectively. There was no patient with 7–11 points in our series. When CTSI scores were evaluated, it was seen that majority of our patients had mild AP (0–3 points, n = 18, 72%), followed by moderate (4–6 points, n = 6, 24%) and severe (7–10 points, n = 1, 4%). Average length of hospital stay was 6 days (range, 3–14) and there was no mortality.
2.3. Treatment protocol
Patients with acute edematous pancreatitis were managed in general ward, while those with severe disease were admitted to intensive care unit (ICU). All patients were managed according to standard management protocols and monitored till discharge for outcome.
Most of our patients had mild (72%) and moderate (24%) AP, and all were managed in general surgical ward. One patient (4%) with severe disease was admitted to ICU. All patients were started on fluid therapy. Analgesics, antibiotics and enteral/parenteral nutrition were emloyed in case of clinical necessity. In our patients with diabetes (44%), insulin treatment was started and adjusted according to the results of their routine glucose tests (four times a day). All other patients with hypertension (52%) and COPD (12%) were allowed to take their additional medications. There was no hyperlipidemic patient in our series. Only two of our patients were taking statins, but their etiology of AP was biliary. These patients were allowed to take their statins, even they had normal lipid profiles.
2.4. Blood collection protocol
Complete blood count, blood biochemistry with amylase and lipase, glucose, C-reactive protein (CRP), lipid profile, PON-1 and oxidative stress parameters (malonyl dialdehyde, MDA; superoxide dismutase, SOD; total antioxidant capacity, TAC) were evaluated in the sera of patients with acute pancreatitis at admission (day 0), day 3 and day 10 of follow-up, between June and September 2017. Blood biochemistry and lipid profile were determined by routine laboratory methods using Architect C16000 and Cell-Dyn 3700 autoanalyzer (Abbott Diagnostics, US). Blood samples collected in vacutainer tubes were transferred to the laboratory to measure serum parameters [Citation17]. On arrival, serum was separated by centrifugation (+4°C, 4000 rpm, 10 min) and stored at −80°C until analyzed.
3. Assay of PON-1 activity
PON-1 activity was assayed using synthetic paraoxon (diethyl-p-nitrophenol phosphate, Sigma Co, London, UK) as a substrate. PON-1 activity was determined by measuring the initial rate of substrate hydrolysis to p-nitrophenol; absorbance was monitored at 412 nm in the assay mixture containing 2 mM paraoxon, 2 mM CaCl2 and 20 μL of plasma in 100 mM tris-HCl buffer (pH 8.0). The blank sample containing incubation mixture without plasma was run simultaneously to correct for spontaneous substrate breakdown. The enzyme activity was calculated from E412 of p-nitrophenol (18.290 M−1 cm−1) and was expressed as U/mL.
4. Assay of MDA activity
Lipoperoxidation was ascertained by the formation of MDA, which was estimated by the modified thiobarbituric acid method, described by Buege and Aust [Citation18]. Thiobarbituric acid-reactive substance concentration was calculated using 1.56 × 105 M−1 cm−1 as molar extinction coefficient. The results were expressed as μM.
5. Assay of SOD activity
It was determined following the nitro blue tetrazolium (NBT) method, with xanthine-xanthine oxidase used as a superoxide generator, adapted from Sun et al [Citation19]. The results were expressed as U/mL.
6. Assay of TAC
Serum TAC levels were measured with spectrophotometric commercial kits (CAYMAN Chemical, antioxidant assay kit, Michigan, USA), and recorded as mM.
7. Statistical analyses
All values were saved in Excel (Microsoft 2010, US) and expressed as mean ± SD. Statistical analyses were conducted with Kolmogorov-Smirnov, Friedman and Wilcoxon tests by using SPSS 13.0 statistical software package programme and correlations were done with Spearman’ s test. p < 0.05 was regarded as statistically significant.
8. Results
Improvement in blood biochemistry after the start of treatment was remarkable, and summarized in . Statistically significant regression in levels of amylase, lipase, liver function tests and CRP was noted (each, p < 0.001). Increased LDH (mean 452.5 ± 174.9 U/L) and AST (mean 417 ± 249.6 U/L) levels were seen to be the most important criteria contributing to Ranson scores, for our series.
Table 1. Biochemical values in patients with AP
The most significant change in lipid profile was shown to be the outstanding rise in HDL-C level, exceeding its upper limit of normal (from 32.5 ± 10.1 to 86 ± 24.3 mg/dL, p < 0.001, ). Even total cholesterol, triacylglycerol (TAG) and LDL-C levels stayed in their normal limits from day 0 to day 10, there was a significant decrement tendency (200.2 ± 40.4 vs. 184.4 ± 44.1, 107 ± 38.8 vs. 96.5 ± 34.5 and 122.5 ± 30.2 vs. 106.4 ± 28 mg/dL, respectively). There was no significant change in VLDL-C value (p > 0.05).
Table 2. Lipid profile
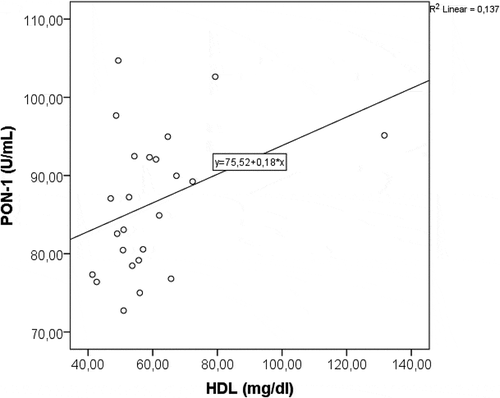
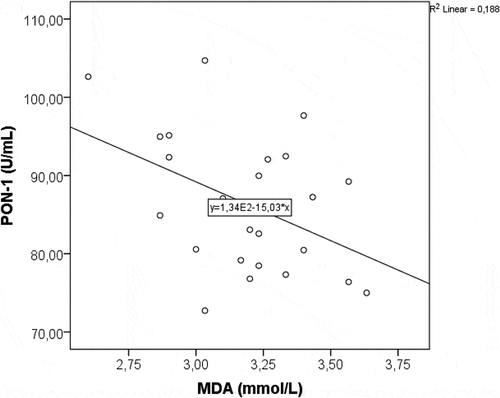
Changes in the serum levels of PON-1 and oxidative stress parameters (MDA, SOD and TAC) were summarized in . While mean PON-1 value was 69.2 ± 6.8 U/mL on admission, it gradually increased to 76.7 ± 7.3 U/mL at day 3 (p < 0.001), and reached to 113.1 ± 18.9 U/mL at day 10 (p < 0.001). Serum SOD and TAC levels showed a statistically significant increment similar to that of PON-1 (each, p < 0.001). However, MDA value decreased significantly (p < 0.001). Positive correlation between PON-1 and HDL-C, and negative correlation between PON-1 and MDA were shown in and .
Table 3. PON-1 and oxidative stress parameters in AP
9. Discussion
AP is an inflammatory disease, with episodes ranging in severity from mild and self-limiting to severe fulminant clinic with extensive necrosis, systemic inflammation and multiorgan failure [Citation7]. Despite the great advances in critical care medicine, the mortality rate of acute pancreatitis has remained at about 10–20% [Citation20]. The exact pathogenesis of pancreatitis still remains unknown, but several mechanisms related to oxidative and inflammatory stress are implicated. Oxidative stress is caused by a combination of increased production of ROS and impaired antioxidant capacity [Citation8]. ROS have been implicated as an important factor in both the initiation and progression of AP in many experimental models. Augmented ROS production could cause lipid peroxidation which induces either apoptosis or necrosis depending on its extent, and the overproduction of ROS induced by an inflammatory process is injurious to pancreatic acinar cells [Citation8]. At this critical point, the antioxidant capacity becomes a critical factor in the progression of this disease.
MDA is the most frequently used biomarker of oxidative stress, while SOD and TAC are the main markers of antioxidant defense mechanisms of the body [Citation8–Citation10]. In our previous experimental studies, the increased MDA and the decreased SOD that we observed in the circulation of rats 24 h after establishment of alcohol-induced AP reflected oxidative stress on whole body basis [Citation8,Citation9]. In this prospective clinical study, changes in the serum levels of oxidative stress parameters (MDA, SOD and TAC) confirmed our previous findings. In our patients with AP, improvement in blood biochemistry after the start of treatment was remarkable, and it was in parallel with the rise in SOD and TAC levels which are the components of antioxidant defense system.
PON-1 is an HDL-C-associated antioxidant enzyme that inhibits LDL-C oxidation in human serum [Citation12,Citation14]. It confers protection against free radicals by limiting the oxidation of phospholipids and is known to lose its activity in the oxidative environment. Several recent studies have suggested that PON-1 concentration decreases in some inflammatory and ischemic diseases, such as atherosclerosis, diabetes and coronary artery disease (CAD), which are associated with an increase in oxidative stress [Citation21–Citation23]. In these studies, Mackness et al have shown that low PON-1 activity may contribute to the increased atherosclerosis found in type 1 diabetes by reducing the ability of HDL-C to retard LDL-C oxidation [Citation21]. Graner et al have indicated that PON-1 activity and concentration are lower in subjects with significant CAD and that there is a significant relationship between PON-1 activity and CAD assessed by coronary angiograpy [Citation22]. Furthermore, Manresa et al have established the nonclassical risk factors of coronary heart disease as lipid status, inflammation, PON1 and oxidative stress [Citation23].
Based on the above-mentioned pioneering information related to PON-1 and the well-known pathophysiological roles of inflammation and oxidative stress in the progression of AP, Unal et al have investigated serum PON-1 activity and lipid profile in experimental AP for the first time in world literature [Citation24]. In this previous study, we found that PON-1 activity significantly decreased in AP-induced rats with a positive correlation to the serum HDL-C level, while there was a significant increase in LDL-C and the oxidative stress agent, MDA. After our study, in an animal model of taurocholate-induced mild and severe AP, Franco-Pons et al have suggested that serum PON-1 undergoes inhibition and proteolysis during pancreatitis [Citation25]. In an another experimental study, Tvarijonaviciute et al have proposed that serum PON-1 activity is lower in dogs with AP and together with triglyceride and C-reactive protein concentrations is a potential marker of disease severity [Citation26]. Relying on the findings of these experimental studies, we investigated the changes in PON-1 activity with particular emphasis on oxidative stress markers and lipid profile in the present prospective clinical trial.
In the present study, CTSI scores showed that most of our patients had mild or average AP (96%). Antioxidants, SOD and TAC, showed a statistically significant increment similar to that of PON-1 during treatment (each, p < 0.001). However, oxidative stress marker, MDA, decreased significantly from admission to day 10 (p < 0.001). The most significant change in lipid profile was shown to be the outstanding rise in HDL-C level, exceeding its upper limit of normal (p < 0.001). A positive correlation between PON-1 and HDL-C, and negative correlation between PON-1 and MDA were shown, as well. These results are consistent with the findings of the previous studies. However, there were two limitations in our study that need to be considered. First, the study was designed as a preliminary trial and patient number recruited was relatively small. Second, we were unable to compare the results between mild and severe AP as there was only one patient with severe pancreatitis (4%). In spite of the limitations mentioned, our findings may suggest that the depletion of antioxidant defense system can be a compensatory mechanism against the increase in the oxidative stress created by the pathologic inflammatory changes seen in pancreatitis. Besides, it can be hypothesized that the pathogenesis of AP is associated with increased oxidative stress and impaired HDL-associated antioxidant defense, evidenced by decreased PON-1 activity.
Similarly, in vitro analysis of Franco-Pons et al revealed that incubation with oxidized lipids obtained from pancreatitis samples results in the inactivation of the enzyme in a concentration-dependent manner [Citation25]. In addition to oxidative inactivation, they observed by Western blot, an immunoreactive band suggestive of proteolytic degradation of the enzyme, altogether indicating that during severe AP, there is a significant decrease in serum PON-1 activity. This decrease is related with inactivation of the enzyme by oxidized lipids, probably followed by proteolytic degradation of the enzyme.
In conclusion, decreased PON-1 and HDL-C values together with antioxidants SOD and TAC concentrations at the early period of acute pancreatitis were seen to rise after treatment, while the previously higher MDA level, as an oxidative stress marker, decreased in parallel. This reveals the importance of the balance between oxidative stress and antioxidant defense mechanisms in clinical progression of the disease, and the potential of PON-1 as a promising clinical marker.
Acknowledgments
We would like to thank to our Residents in Surgery and Nursing Staff (especially to Isilay Yigitalp), for their help in selection of the patient population with acute pancreatitis and in obtaining blood samples from the patients included in the study, which were used in the analyses of biochemical and statistical data of the present study.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Roberts SE, Akbari A, Thorne K, et al. The incidence of acute pancreatitis: impact of social deprivation, alcohol consumption, seasonal and demographic factors. Aliment Pharmacol Ther. 2013;38:1–6.
- Vengadakrishnan K, Koushik AK. A study of the clinical profile of acute pancreatitis and its correlation with severity indices. Int J Health Sci. 2015;9:410–417.
- Albulushi A, Siddiqi A, Alqarshoubi I, et al. Pattern of acute pancreatitis in a tertiary care center in Oman. Oman Med J. 2014;29:358–361.
- Elzouki AN, Alsaed O, Saeed A, et al. Incidence and epidemiological features of acute pancreatitis among adult inhabitants in Qatar. Turk J Gastroenterol. 2019;30:95–100.
- Peery AF, Dellon ES, Lund J, et al. Burden of gastrointestinal disease in the USA: 2012 update. Gastroenterology. 2012;143:1179–1187.
- Wadhwa V, Patwardhan S, Garg SK, et al. Health care utilization and costs associated with acute pancreatitis. Pancreas. 2017;46:410–415.
- Zhang Y, Guo F, Li S, et al. Decreased high density lipoprotein cholesterol is an independent predictor for persistent organ failure, pancreatic necrosis and mortality in acute pancreatitis. Sci Rep. 2017;7:8064.
- Andican G, Gelisgen R, Unal E, et al. Oxidative stress and nitric oxide in rats with alcohol-induced acute pancreatitis. World J Gastroenterol. 2005;11:2340–2345.
- Kiziler AR, Aydemir B, Gulyasar T, et al. Relationships among iron, protein oxidation and lipid peroxidation levels in rats with alcohol-induced acute pancreatitis. Biol Trace Elem Res. 2008;124:135–143.
- Bopanna S, Nayak B, Prakash S, et al. Increased oxidative stress and deficient antioxidant levels may be involved in the pathogenesis of idiopathic recurrent acute pancreatitis. Pancreatology. 2017;17:529–533.
- Ishikawa T, Ayaori M, Uto-Kondo H, et al. High-density lipoprotein cholesterol efflux capacity as a relevant predictor of atherosclerotic coronary disease. Atherosclerosis. 2015;242:318–322.
- Craciun EC, Leucuta DC, Rusu RL, et al. Paraoxonase-1 activities in children and adolescents with type 1 diabetes mellitus. Acta Biochim Pol. 2016;63:511–515.
- Tsai K, Wang SS, Chen TS, et al. Oxidative stress: an important phenomenon with pathogenetic significance in the progression of acute pancreatitis. Gut. 1998;42:850–855.
- Tsuzura S, Ikeda Y, Suehiro T, et al. Correlation of plasma oxidized low-density lipoprotein levels to vascular complications and human serum paraoxonase in patients with type 2 diabetes. Metabolism. 2004;53:297–302.
- Unal E, Eris C, Kaya B, et al. Paraoxonase and arylesterase activities, lipid profile, and oxidative damage in experimental ischemic colitis model. Gastroenterol Res Pract. 2012;2012:979506.
- Kodydkova J, Vavrova L, Stankova B, et al. Antioxidant status and oxidative stress markers in pancreatic cancer and chronic pancreatitis. Pancreas. 2013;42:614–621.
- Tuck MK, Chan DW, Chia D, et al. Standard operating procedures for serum and plasma collection: early detection research network consensus statement standard operating procedure integration working group. J Proteome Res. 2009;8:113–117.
- Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310.
- Sun Y, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clin Chem. 1988;34:497–500.
- Werner J, Feuerbach S, Uhl W, et al. Management of acute pancreatitis: from surgery to interventional intensive care. Gut. 2005;54:426–436.
- Mackness B, Durrington PN, Boulton AJ, et al. Serum paraoxonase activity in patients with type 1 diabetes compared to healthy controls. Eur J Clin Invest. 2002;32:259–264.
- Graner M, James RW, Kahri J, et al. Association of paraoxonase-1 activity and concentration with angiographic severity and extent of coronary artery disease. J Am Coll Cardiol. 2006;47:2429–2435.
- Manresa JM, Zamora A, Tomas M, et al. Relationship of classical and non-classical risk factors with genetic variants relevant to coronary heart disease. Eur J Cardiovasc Prev Rehabil. 2006;13:738–744.
- Unal E, Uzun H, Kusaslan R, et al. Serum paraoxonase (a high-density lipoprotein-associated lipophilic antioxidant) activity and lipid profile in experimental acute pancreatitis. Pancreas. 2005;31:84–87.
- Franco-Pons N, Marsillach J, Joven J, et al. Serum paraoxonase undergoes inhibition and proteolysis during experimental acute pancreatitis. J Gastrointest Surg. 2008;12:891–899.
- Tvarijonaviciute A, Garcia-Martinez JD, Caldin M, et al. Serum paraoxonase 1 (PON1) activity in acute pancreatitis of dogs. J Small Anim Pract. 2015;56:67–71.