ABSTRACT
Levosimendan is a calcium sensitizer used for managing heart failure (HF) because of its inotropic and vasodilatory effects. As many patients do not respond to levosimendan as a monotherapy, it may be necessary to combine it with other diuretic agents such as recombinant human brain natriuretic peptide (rhBNc P). The aim of this study was to investigate efficacy of levosimendan when combined with rhBNP in patients with diuretic resistance and low ejection fraction (EF) rate.
The study included HF patients with diuretic resistance and low EF. Before grouping, patients with a 24-hour urine volume of <0.5 mL/kg/h were administered with furosemide injection. Treatment group was administered levosimendan injection based on the original diuretic and rhBNP.
One hundred twenty-eight patients were included, with 64 patients each in the control and treatment arms. 24-hour urine volume of the treatment group was significantly higher than that of the control group. Moreover, dyspnea score of the treatment group significantly improved compared with control group. In the treatment group, 12.5% of patients had no significant changes in the urine volume, weight, and dyspnea score before and after the treatment, indicating poor curative effect of the treatment, whereas in the control group, 23.4% of patients had poor curative effect (P < .05). No significant change was observed in the systolic blood pressure, heart rate, and serum creatinine level before and after treatment in both groups.
Levosimendan in combination with rhBNP can effectively relieve diuretic resistance, reduce body weight, improve dyspnea, and ensure safety in the treatment process.
1. Introduction
Heart failure (HF) is a progressive disorder with an estimated prevalence of 64.3 million people worldwide[Citation1]. An important pathophysiologic process of HF is volume overload, which is conventionally treated with diuretics. However, their prolonged use can lead to diuretic resistance, which affects approximately 20% to 30% of patients with HF, increasing short- and long-term mortalities [Citation2–5]. Inotropic drugs improve cardiac output by enhancing cardiac contractility, and has been considered as an attractive approach to provide improvements in HF symptoms [Citation6]. Compared with other inotropes, levosimendan promotes sensitization of cardiomyocyte to calcium ions without increasing intracellular calcium levels, which may prevent an increased risk of cardiac arrythmia [Citation7]. Levosimendan also acts as a vasodilator by regulating ATP-dependent potassium channels. By acting via both inotropic and vasodilatory approaches, the drug enhances cardiac output without increasing myocardial oxygen demand [Citation8]. In most of the reported studies, the application of levosimendan on refractory HF has been mainly focused on the treatment of diuretic resistance [Citation9,Citation10]. However, despite advances in HF therapy, many patients do not respond to a levosimendan as a monotherapy and experience clinical deterioration [Citation11]. To accelerate and improve its effects to lower diuretic resistance, levosimendan can be used in combination with other diuretic agents such as natriuretic peptides.
Natriuretic peptides have been widely known to enhance diuresis by increasing cardiac output, inhibiting renin-angiotensin-aldosterone system (RASS), and improving diastolic function [Citation12]. The levels of plasma brain natriuretic peptide (BNP) are significantly enhanced in patients with more severe HF and are presently listed as an established biomarker for heart disease in international guidelines [Citation13,Citation14]. Although high levels of BNP are already present in patients with HF, they have a lower protective efficacy when compared with the immunoreactive effects of exogeneous recombinant human brain natriuretic peptide (rhBNP). Thus, the administration of rhBNP has shown to improve symptoms and hemodynamic conditions [Citation15]. The combination of rhBNP with levosimendan has been reported to improve clinical outcomes in terms of pulmonary congestion, edema, and New York Health Association (NYHA) functional class for the treatment of acute decompensated HF [Citation16]. However, the effect of these two agents to specifically relieve diuretic resistance and decrease volume load in patients with HF is currently unknown.
Levosimendan and rhBNP act via different mechanisms to improve diuretic resistance, where the former is a positive inotropic agent and the latter inhibits the RASS. In theory, their combination may offer synergistic effects and better improved outcomes when compared with those of a single drug. Hence, the aim of this study was to investigate the effects of levosimendan administered in conjunction with rhBNP in patients with diuretic resistance and low ejection fraction (EF) rate.
2. Methods
2.1. Study population
This single-center, interventional study included patients with HF with diuretic resistance and low EF from cardiac care unit of Xinjiang Uygur Autonomous Region Hospital of traditional Chinese medicine, from June 2017 to June 2019. Diuretic resistance was defined as in need of more than two loop diuretic units per day. The diagnostic criteria of HF were those of the Chinese guidelines for diagnosis and treatment of HF 2018 [Citation17]. The study protocol was approved by Xinjiang Uygur Autonomous Region Hospital of traditional Chinese medicine ethical committee (ethical approval number: XUATM2015Y034) and was conducted in accordance with the Declaration of Helsinki. All patients provided written informed consent before participation in the study.
2.2. Inclusion and non-inclusion criteria
The inclusion criteria were HF patients with a EF <40%, and those who have one or more of the following: dyspnea score ≤4 points (dyspnea scale developed according to the recommendations of the European Society of Cardiology International Working Group on acute HF [Citation14], ), lung moist rales, paroxysmal nocturnal dyspnea, orthopnea, hepatomegaly or ascites, jugular vein distension, pulmonary edema, or pleural effusion. All patients were classified according to their symptoms based on NYHA classification system [Citation18] into class I–IV. Non-inclusion criteria were patients with acute coronary syndrome, estimated glomerular filtration rate (eGFR) ≤30 mL/min/1.73 m2, systolic blood pressure ≤90 mmHg, systemic infection, and blood potassium <3.5 mmol/L and those who participated in other clinical trials. At the time of study, the average BNP level of patients was 3000 pg/mL.
Box 1. Scoring criteria of dyspnea
2.3. Grouping and treatment
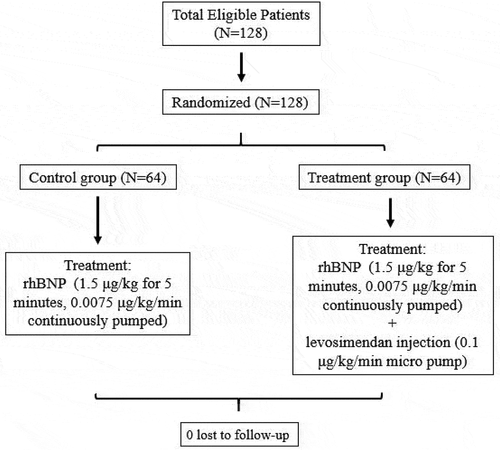
Eligible patients were randomized using a computer-generated random number table in a 1:1 ratio and allocated into either control group or treatment group. According to the guidelines for diagnosis and treatment of HF, both groups were given comprehensive drug treatment for HF, including thiazide diuretics, cardiotonic drugs, and vasodilators, to actively control the primary disease and eliminate incentives. During admission, all patients had a 24-hour urine volume of <0.5 mL/kg/h and were administered with furosemide injection at a dose of 80 mg/d. All patients rendered a urine output of more than 0.5 ml/kg/h. The patients in control group were administered with rhBNP (1.5 μg/kg for 5 minutes, 0.0075 μg/kg/min was continuously pumped), maintaining a 24-hour urine volume of <0.5 mL/kg/h. The dosage of natriuretic peptide was the same as before. The patients in the treatment group were given levosimendan injection (0.1 μg/kg/min using micropump) based on the original diuretic and rhBNP and were observed for 24 hours.
2.4. Study outcomes
The efficacy was assessed within the group, and also between the groups in terms of 24-hour urine volume (mL), changes in body weight (kg), and changes in dyspnea score before and after 24 hours of treatment in both groups also between the two groups. Safety outcomes included changes in the systolic blood pressure (mmHg), heart rate (beats/min), and serum creatinine levels (μmol/L) before and after the treatment.
2.5. Statistical analysis
The statistical analysis was performed using SPSS22.0 software. Chi-test or Fischer’s exact test was independent t test used for comparison between the two groups if the data were distributed normally and variance was homogenous. Mann-Whitney test was used if the data was not normally distributed. To compare the values within the individual treatment group, paired t-test was applied. P < .05 was considered to be statistically significant.
2.6. Sample size
A sample size calculation was done considering the effect size of the two groups. The effect size was calculated using the standardized difference – the absolute difference divided by the standard deviation. Using conventional values of α = 0.05 and 80% power, median effect size = 0.5 the required sample size was calculated as 64 per group [Citation19].
3. Results
3.1. Demographic and baseline characteristics
The study included 128 patients divided into two groups (treatment and control) of 64 patients each (). Of the total patients enrolled, 74 were males, with a mean age of 61.4 ± 10.8 years. The patients had an average BNP level of 3000 pg/mL, and based on the NYHA guidelines, 52 patients had NYHA class III and 76 had NYHA class IV. The average course of disease was 4.70 ± 1.40 years (). Statistically, no significant difference was observed in sex, age, weight, course of disease, dyspnea score, NYHA cardiac function classification, and combined diseases between the 2 groups (P > .05).
Table 1. Demographics and baseline characteristics of patients
3.2. Efficacy outcomes
All three measured outcomes (ie; urine volume, weight, and dyspnea score), rendered improvement in the treatment group when compared with the control group (P < .05; ).
Table 2. Comparison of efficacy outcomes between the 2 groups
Within the treatment group, a significant mean increase of 1168 mL±170 mL was observed after the administration of levosimendan, whereas an increase of 499 mL±42 mL was observed in the control group (P < .05; ). The average body weight of the patients significantly decreased by 3.2 ± 0.26 kg in the treatment group. However, no significant change was observed in the control group. The mean dyspnea score of the treatment group increased significantly by 4.2 ± 1.5 [Citation4,Citation5,Citation20] points (). The control group also showed a significant increase in dyspnea score by 1.45 ± 0.7 (P < .05).
There was no significant change in the urine volume, body weight and dyspnea scores in 23 patients before and after treatment in both groups. The mean eGFR was 38.4 ± 4.7 mL/min/1.73 m2, and the mean EF was 24.6 ± 4.9%, including 8 (12.5%) patients in the treatment group and 15 (23.4%) patients in the control group. The proportion of patients with poor efficacy in the treatment group (2%) was significantly lower than that in the control group (21%) (P < .05).
3.3. Safety outcomes
No significant differences were observed in the mean systolic blood pressure, heart rate or serum creatinine between the treatment and control groups (P > .05; ). Although a decrease in systolic blood pressure, heart rate, and serum creatinine levels was observed in the treatment group before and after treatment, these changes were not statistically significant (P > .05; ). Within the control group, the heart rate and serum creatinine levels were increased, and the systolic blood pressure was observed to decrease. Nevertheless, these changes were also not statistically significant (P > .05; )
Table 3. Comparison of safety indexes between the 2 groups before and after treatment
4. Discussion
In this study, we investigated the combined effects of levosimendan and rhBNP in patients with diuretic resistance. The results showed a significant increase in the 24-hour urine volume and dyspnea score and a significant reduction in the body weight, indicating better diuretic effects with the combination of levosimendan and rhBNP. Upon treatment with rhBNP, Colucci et al. [Citation21] reported an increase in the urine volume and dyspnea compared with placebo. Furthermore, in a study by He et al. [Citation22]., a significant increase in the 24-hour urine volume after treatment with rhBNP was reported when compared with that before treatment and also when compared with control. In another study, the effect of rhBNP in ST-segment elevation myocardial infarction and HF was evaluated [Citation23]. The study reported increased urine volume with rhBNP compared with nitroglycerine [Citation23].
Levosimendan has previously reported to demonstrate a significant increase in the 24-hour urine output compared with that before treatment [Citation24,Citation25]. In par with the previous studies, the present study also reported a significant increase in the 24-hour urine volume and dyspnea score in the treatment group compared with control, and also when compared within the group (before treatment and after treatment), a significant increase was observed after treatment in both groups.
On one hand, levosimendan is able to inhibit the reabsorption of NaCl in the thick wall segment of renal tubular medullary loop, whereas rhBNP helps in antagonizing the RASS [Citation26]. These stimulatory effects of both levosimendan and rhBNP synergistically help in reducing the diuretic resistance, and such combined treatment strategies seem promising in future HF therapies.
Although patients with eGFR <30 mL/min/1.73 m2 were excluded from the study, there were still a certain proportion (17.9%) of patients with poor curative effect. These patients showed poor EF and/or renal function even with other diuretics, which may be due to individual patient’s sensitivity to drugs but the exact underlying reason for poor curative effect is unknown.
The mean systolic blood pressure, heart rate, and serum creatinine level decreased after treatment with levosimendan and rhBNP, but not statistically significant. The results from the present study are in accordance with reported studies. A study by Pan et al. [Citation27] reported no significant change in the heart rate and serum creatinine levels during the treatment with rhBNP. Similarly, no significant decrease in the mean systolic blood pressure was reported with levosimendan and rhBNP in a study by Bocchi et al. [Citation28]. In another study, it was reported that the application of levosimendan in the patients with advanced HF was safe with no significant difference in the systolic blood pressure, heart rate, and serum creatinine values between the treatment and control groups [Citation29, Citation30]. In addition, the present study reported no all-cause deaths in the two groups during the treatment process. Hence, from the results, combined use of levosimendan and rhBNP is not only effective in treating patients with HF with diuretic resistance, but it is also safe.
4.1. Study limitations
Major limitations of the study include short end point analysis of the 24-hour urine volume, and single-center study, implicating that the data may not be generalized for a larger population. Nevertheless, to the best of the authors’ knowledge, this is the first study that investigates the combination of levosimendan and rhBNP in treating patients with HF with diuretic resistance and low EF, and provides a rationale to develop more in-depth studies with multicenter, randomized, controlled clinical trials with larger patient cohorts and follow-up timelines.
5. Conclusion
Levosimendan when combined with rhBNP can effectively relieve diuretic resistance, increase urine volume, reduce body weight, improve dyspnea, and ensure safety in the treatment process.
Ethics approval and consent to participate
The study protocol was approved by Xinjiang Uygur Autonomous Region Hospital of traditional Chinese medicine ethical committee (ethical approval number: XUATM2015Y034) and was conducted in accordance with the Declaration of Helsinki. All patients provided written informed consent before participation in the study.
Data availability statement
All data used in this study will be available from the corresponding author upon reasonable request.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the global burden of disease study 2017 - pubmed [Internet]. [ cited 2021 Aug 9]. Available from: https://pubmed.ncbi.nlm.nih.gov/30496104/
- Cox ZL, Lenihan DJ. Loop diuretic resistance in heart failure: resistance etiology-based strategies to restoring diuretic efficacy. J Card Fail. 2014 Aug;20(8):611–6.
- Iqbal J, Javaid MM. Br J Hosp Med Lond Engl. 2005 Diuretic resistance and its management. Br J Hosp Med (Lond). 2014 Jul;75(7):C103–107. .
- Shchekochikhin D, Al Ammary F, Lindenfeld JA, et al. Role of diuretics and ultrafiltration in congestive heart failure. Pharm Basel Switz. 2013 Jul 4;6(7):851–866.
- Mecklai A, Subacius H, Katz S. Diuretic resistance and clinical outcomes in patients hospitalized for worsening heart failure: insights from the EVEREST (Efficacy of Vasopressin Antagonism in Heart Failure: outcome Study with Tolvaptan) trial. J Card Fail. 2013 Aug;1(19):S33–4.
- Tariq S, Aronow WS. Use of inotropic agents in treatment of systolic heart failure. Int J Mol Sci. 2015 Dec 4;16(12):29060–29068.
- Tariq S, Aronow WS. Use of inotropic agents in treatment of systolic heart failure. Int J Mol Sci. 2015 Dec 4;16(12):29060–29068.
- Kyrzopoulos S, Adamopoulos S, Parissis JT, et al. Levosimendan reduces plasma B-type natriuretic peptide and interleukin 6, and improves central hemodynamics in severe heart failure patients. Int J Cardiol [Internet]. 2005 Mar [cited 2021 Jul 12];99(3):409–413. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0167527304002785
- Lannemyr L, Ricksten S-E, Rundqvist B, et al. Differential effects of levosimendan and dobutamine on glomerular filtration rate in patients with heart failure and renal impairment: a randomized double-blind controlled trial. J Am Heart Assoc. 2018;7(16):e008455. 21.
- Fedele F, Bruno N, Brasolin B, et al. Levosimendan improves renal function in acute decompensated heart failure: possible underlying mechanisms. Eur J Heart Fail. 2014 Mar;16(3):281–288.
- Cavusoglu Y. The use of levosimendan in comparison and in combination with dobutamine in the treatment of decompensated heart failure. Expert Opin Pharmacother [Internet]. 2007 Apr [cited 2021 Jul 12];8(5):665–677. Available from: http://www.tandfonline.com/doi/full/10.1517/14656566.8.5.665
- Zhang S, Wang Z. Effect of recombinant human brain natriuretic peptide (rhBNP) versus nitroglycerin in patients with heart failure: a systematic review and meta-analysis. Medicine (Baltimore). 2016 Nov;95(44):e4757.
- Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American college of cardiology/american heart association task force on clinical practice guidelines and the heart failure society of America. Circulation. 2017 Aug 8;136(6):e137–61.
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016 Aug;18(8):891–975.
- Kuwahara K, Nakagawa Y, Nishikimi T. Cutting edge of brain natriuretic peptide (BNP) research ― The diversity of BNP immunoreactivity and its clinical relevance. Circ J Internet]. 2018 Sep 25 [cited 2021 Jul 12];82(10):2455–2461. Available from. [];():. : https://www.jstage.jst.go.jp/article/circj/82/10/82_CJ-18-0824/_article
- Jia Z, Guo M, Zhang L-Y, et al. Levosimendan and nesiritide as a combination therapy in patients with acute heart failure. Am J Med Sci. 2015 May;349(5):398–405.
- Heart failure group of Chinese society of cardiology of Chinese medical association. Chinese heart failure association of Chinese medical doctor association, editorial board of Chinese journal of cardiology. [Chinese guidelines for the diagnosis and treatment of heart failure 2018]. Zhonghua Xin Xue Guan Bing Za Zhi. 2018 Oct 24;46(10):760–789.
- Classes of Heart Failure [Internet]. www.heart.org. [cited 2021 Jul 16]. Available from: https://www.heart.org/en/health-topics/heart-failure/what-is-heart-failure/classes-of-heart-failure
- Allen JC. Sample size calculation for two independent groups: a useful rule of thumb. Proc Singap Healthc. 2011 Jun 1;20(2):138–140.
- Diuretic Resistance - PubMed [Internet]. [cited 2021 Aug 9]. Available from: https://pubmed.ncbi.nlm.nih.gov/27814935/
- Colucci WS, Elkayam U, Horton DP, et al. Intravenous nesiritide, a natriuretic peptide, in the treatment of decompensated congestive heart failure. N Engl J Med. 2000 Jul 27;343(4):246–253.
- He X-M, Chen L, Luo J-B, et al. Effects of rhBNP after PCI on non-invasive hemodynamic in acute myocardial infarction patients with left heart failure. Asian Pac J Trop Med. 2016 Aug;9(8):791–795.
- Xing K, Fu X, Wang Y, et al. Effect of rhBNP on renal function in STEMI-HF patients with mild renal insufficiency undergoing primary PCI. Heart Vessels. 2016 Apr;31(4):490–498.
- Yilmaz MB, Yalta K, Yontar C, et al. Levosimendan improves renal function in patients with acute decompensated heart failure: comparison with dobutamine. Cardiovasc Drugs Ther. 2007 Dec 5;21(6):431–435.
- Yilmaz MB, Yontar C, Erdem A, et al. Comparative effects of levosimendan and dobutamine on right ventricular function in patients with biventricular heart failure. Heart Vessels. 2009 Jan;24(1):16–21.
- Pan H-Y, Zhu J-H, Gu Y, et al. Comparative effects of recombinant human brain natriuretic peptide and dobutamine on acute decompensated heart failure patients with different blood BNP levels. BMC Cardiovasc Disord. 2014 Mar;4(14):31.
- Pan H-Y, Zhu J-H, Gu Y, et al. Comparative effects of recombinant human brain natriuretic peptide and dobutamine on acute decompensated heart failure patients with differsent blood BNP levels. BMC Cardiovasc Disord. 2014 Dec;14(1):31.
- Bocchi EA, Moura LZ, Issa VS, et al. Effects of the recombinant form of the natural human B-type natriuretic peptide and levosimendan on pulmonary hyperventilation and chemosensivity in heart failure: ventilatory response in heart failure. Cardiovasc Ther. 2013 Apr;31(2):100–107.
- Qiu J, Jia L, Hao Y, et al. Efficacy and safety of levosimendan in patients with acute right heart failure: a meta-analysis. Life Sci. 2017 Sep;1(184):30–36.
- Pang PS, Cleland JGF, Teerlink JR, et al. A proposal to standardize dyspnoea measurement in clinical trials of acute heart failure syndromes: the need for a uniform approach. Eur Heart J. 2008 Mar;29(6):816–824.