?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
In the present study, water electrolysis was employed for Hydroxy gas (HHO) production as a gaseous additive. The engine test was performed using the Diesel, B5, and B20 as pilot fuels. HHO was imported into the engine's combustion chamber at three volumetric flow rates of 3, 4, and 5 cc/s through the inlet manifold as the low-level HHO rate.The engine test setup was a single-cylinder dual-fueled diesel engine at a constant speed (1500 rpm) and full load condition. According to the results, HHO by 3 and 4 cc/s did not have a significant effect on BP, BTE, and BSFC. Using HHO gas by 5 cc/s significantly increased BP by about 2.5, 3.1, and 0.5% compared with Diesel, B5 and B20, respectively, and decreased BSFC by about 11, 7.8, and 13.5% compared with Diesel, B5, and B20, respectively.HHO gas by 5 cc/s significantly decreased CO2 by about 7, 6.3, and 10.6% compared with Diesel, B5, and B20, respectively, and decreased CO emissions by about 6, 14.3, and 21.2% compared with Diesel, B5 and B20, respectively. However, the use of HHO gas and biodiesel increased NOx emission by about 16, 13.7, and 10.5% compared with Diesel, B5, and B20, respectively.
Introduction
Recently, fossil fuels and their impact on pollutions have led researchers to find clean fuel resources. Hydrogen is the only renewable fuel that does not have carbon content (Knop et al., Citation2008). Obviously, by reducing carbon in the fuel reduces engine emissions (except NOx) (Saravanan et al., Citation2007). Hydrogen, as a clean and environmentally friendly fuel (Dincer, Citation2007; Yilmaz et al., Citation2010), is an accessible and endless fuel source (Arat, Citation2019). According to the International Energy Agency (IEA), ‘Global Trends and Prospects for Hydrogen', the commercial value of hydrogen gas for 2022 is estimated to be more than 154 billion$ (Arat, Citation2019).
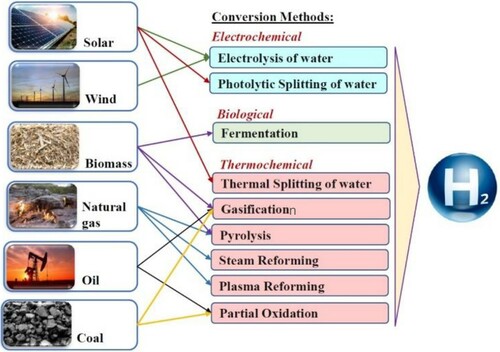
Hydrogen can be generated by electrochemical, biological, and thermochemical methods. Figure presents different hydrogen production methods. The most desirable method for producing hydrogen is water electrolysis (Baltacioglu et al., Citation2019) with renewable energy sources such as solar and wind resources (Trujillo-Olivares et al., Citation2019). Solar energy and water are available almost worldwide (Cammack et al., Citation2001; Ramadhas, Citation2016). The cost of producing hydrogen must be reduced to the use of water electrolysis. Also, the durability, reliability, and safety of the system must be increased (Vickers, Citation2017; Yadav Milind et al., Citation2011).
In the water electrolysis process, hydrogen is produced along with oxygen. The generated gas is called hydroxy gas (HHO) (Arjun et al., Citation2019). The HHO can be produced from all surfaces of the electrodes using wet cell type generators. But the main problem with these generators is heat generation and reduction of the production yield. In dry cell type generators, water flows between the electrodes and produces less heat. These types of generators are more efficient type (Sudrajat et al., Citation2018). They usually use NaOH or KOH to ionize water as an electrolyte (Ismail et al., Citation2018).
Hydroxy gas contains 1 mol of hydrogen and 2 moles of oxygen (Selvi Rajaram et al., Citation2014). A diesel engine that uses diesel fuel with gaseous fuel, such as natural gas, biogas, or hydrogen, is called a ‘dual-fuel diesel engine' (Akbarian et al., Citation2018). Studies indicate that HHO gas is more flammable than diesel fuel (Selvi Rajaram et al., Citation2014). Therefore, can improve the combustion process in diesel engines. The use of hydrogen increases the H/C ratio of the fuel and reduces carbon-based emissions (Szwaja & Grab-Rogalinski, Citation2009). Also, the diffusion rate of hydrogen gas in the air is extremely high, thus reducing the inhomogeneity of diesel fuel injection and creating a more uniform fuel–air mixture (Ismail et al., Citation2019).
Also, biodiesel fuel can improve diesel engines’ performance and emissions (Faizollahzadeh Ardabili et al., Citation2018). Like hydrogen, Biodiesel is a renewable and clean fuel (Reece & Peterson, Citation1995). The use of biodiesel significantly reduces greenhouse gas (GHG) emissions (Rimkus et al., Citation2018). Studies in the field of biodiesel combustion have shown that biodiesel fuel can reduce the emission of particulate matter (PM), unburned hydrocarbons (UHC), and carbon monoxide (CO) (Uludamar et al., Citation2016). Biodiesel produces lower brake power (BP) than diesel fuel duel to the lower low heat value (LHV) compared with that of diesel fuel. Researchers are now looking to solve this problem. One way is to use additives with high-energy content such as hydrogen (Arat, Citation2019; Rimkus et al., Citation2018).
Various studies have recently been developed on employing HHO gas with biodiesel fuel in a diesel engine (Table ). As can be seen, almost all researchers agree that the use of HHO gas in diesel engines increases braking power (BP), braking torque (BT), and braking thermal efficiency (BTE), and reduces brake specific fuel consumption (BSFC). Most researchers also found that HHO gas in diesel engines reduces CO, UHC, and PM emissions and increases NOx and CO2 emissions.
Table 1. studies developed for employing HHO as a gaseous additive for biodiesel fuel in diesel engine.
Reviewing the articles showed that the use of HHO gas in diesel engines improves engine performance and reduces emissions. HHO gas is a new and sustainable energy source that can provide energy security in the present and future. According to the authors’ best knowledge, the effect of low-level HHO as an additive and the interactions of HHO and biodiesel has not been reported yet. In the present study, a dry cell HHO gas generator was designed and manufactured. Then, the effects of using HHO gas and diesel–biodiesel blends (B5 and B20) on a dual fuel diesel engine were investigated. The diesel engine was connected to an electric power generator and the electricity needed to produce HHO gas came from the diesel engine. Therefore, net braking power (BP) was studied as a criterion for system evaluation.
Material and method
Design, manufacture, and evaluation of HHO generator
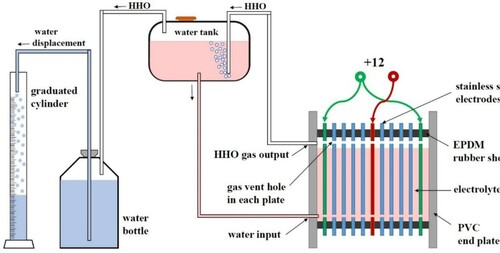
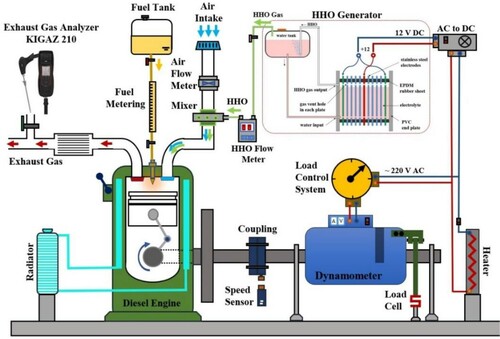
In this study, a dry cell type HHO generator was designed and manufactured. 38 electrode plates with dimensions of 20×20×1 mm were made of stainless steel 316L. Among the electrodes were rubber gaskets (2 mm thick). The gaskets were made of corrosion-resistant rubber (Ethylene Propylene Diene Monomer), which prevented adjacent plates’ contact. One plate was attached to the negative charge (cathode), and two plates to the positive charge (anode), and the rest were neutral. The electrodes from the top and bottom had holes 5 mm in diameter. The electrolyte flowed freely between all cells. The generated HHO gas was collected at the top of the generator through holes in the upper electrodes. The generated gas was passed through a trap of water to ensure system safety. Figure presents a schematic of the HHO generator.
The alkaline electrolyte was prepared by adding NaOH to distilled water. The HHO generator had a capacity of 1.5 liters of electrolyte. The electrolysis process was performed using (∼ 220 v) alternating current generated from the diesel engine. Using an AC to DC converter (A30 and V12 Model 12-360-5 China) AC power was converted to direct power. A KYORITSU KEW MATE 2000 multimeter measured the voltage and current intensity. The amount of HHO gas produced was calculated by employing discharged water (using a graduated cylinder and chronometer) (Najafi & Ardabili, Citation2018).
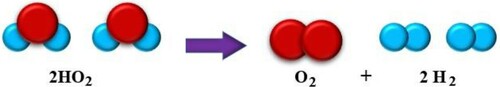
The generator's efficiency is the ratio of the HHO gas energy produced to the electricity consumed (to produce it) (EL-Kassaby et al., Citation2016). The HHO gas contains 2 moles of hydrogen and 1 moles of oxygen (Figure ), so the mole fraction of hydrogen and oxygen in it is 0.66 and 0.33, respectively. Thus, hydrogen accounts for 66% of the volume of HHO gas (). Since only H2 gas is combustible, the efficiency of the HHO generator is calculated according to Equation (1):
(1)
(1) That VH2 is the volume of hydrogen (m3) in the hydroxy gas, LHVH2 is low heat value of hydrogen equal to 119.93×106 J/kg, and
is the density of hydrogen gas in conventional conditions equal to 0.0838 kg/m3 (Table ). V is the voltage (volts), I the current of the current (amps) and t the time (seconds) which were measured experimentally.
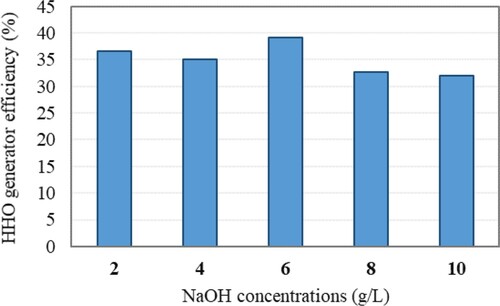
Since the electrolyte concentration affects HHO production, directly, the amount of NaOH was added to distilled water at five levels of 2, 4, 6, 8, and 10 g/L. Figure presents the efficiency of the HHO gas generator at different catalyst concentrations. It was observed that increasing the catalyst concentration increases the HHO gas generator's efficiency initially and then decreases. The highest efficiency of the HHO generator at 6 g/L concentration was 39.12%. Therefore, in this study 6 g/L concentration of NaOH catalyst was used.
Fuel preparation
In this study, biodiesel fuel was produced by transesterification using waste cooking oil (WCO), methanol alcohol (with alcohol to oil ratio 6:1), and NaOH catalyst (by 1% of the oil weight) at a temperature of 65°C for 30 min by the stirring intensity of 600 rpm (Faizollahzadeh Ardabili et al., Citation2018; Hajlari et al., Citation2019). Finally, after neutralization and washing processes, biodiesel was generated with a purity of 98.5%. Figure shows the biodiesel production steps. The thermochemical properties of biodiesel and diesel fuel were measured according to the ASTM standard. Table presents the prepared fuels’ properties.
Table 2. Properties of biodiesel, diesel, and hydrogen.
Biodiesel and diesel fuels were blended at two ratios of 5 and 20% vol which were called B5 and B20, respectively. In each fuel sample, HHO gas was added at rates of 3, 4, and 5 cc/s (0.18, 0.24, and 0.3 L/min). B5, B20, and pure diesel (D) ware used as control fuels. Table shows the amount of diesel, biodiesel, and HHO fuels.
Table 3. The amount of diesel, biodiesel and HHO fuels.
Engine test equipment
The engine tests were performed by a Kirloskar DA10 single-cylinder diesel engine (Figure ). The specifications of the engine are described in detail in Table . The engine shaft was connected to a jb/t-200 electric generator. The generator's legs were completely suspended, and its body was attached to a 200 kN load cell (model: LNron FG-5100) through an arm 30 cm long. The generated electricity was transmitted to a heater through a variable electrical resistance (model: TDGC2-5KVA). By changing the state of the variable electrical resistance, the engine was placed under load.
Table 4. Kirloskar DA10 diesel engine specifications.
By measuring the force by the load cell (F), the braking torque of the relation was calculated. By measuring the rotational speed of the engine (by a magnetic sensor and Autonics MP5W-4N pulse meter), the braking power of Equation (2) was calculated:
(2)
(2) where
is the force (N),
is the rotational speed of the engine (rpm) and
refers to the brake power (kW).
The flow of liquid fuel consumption () was measured by a calibrated cylinder method. By measuring the fuel consumption time of 4 cc, the fuel consumption flow was calculated. A GS-84-04C G4 flowmeter measured the volumetric flow rate of hydroxy gas (HHO). KIGAZ 210 (Kimo Instruments“ Chevry-Cossigny“ France) portable gas analyzer was used to measure exhaust gases. The gas analyzer measured the CO2, CO, and NOX emissions values with an accuracy of 0.1 vol.%, 1, and 1 ppm, respectively.
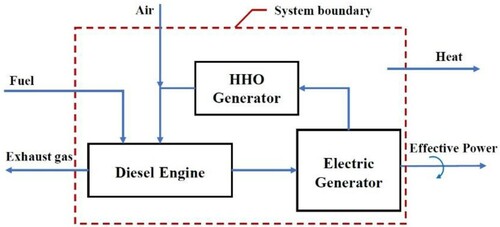
In engine tests, diesel, B5, and B20 were the sources of energy. The electricity needed to produce HHO gas was provided by an electric generator connected to the engine. Therefore, if we consider the diesel-generator motor set as a thermodynamic system, the electricity required to produce HHO gas will be internal energy. Figure shows the diesel-generator set as a thermodynamic system.
Special brake fuel consumption (g/kWh) was calculated from Equation (3):
(3)
(3) where
is volumetric of fuel consumption (cc/s) that is measured experimentally.
The density of diesel, B5 and B20 fuels is 0.840, 0.8423 and 0.8491 kg/m3, respectively (Table ).
Brake thermal efficiency (%) was calculated from Equation (4):
(4)
(4) where the LHVf of the low heat value of diesel, B5, and B20 fuels is 42.49, 42.43, and 40.01 MJ/kg, respectively (Table ).
Lambda (λ) for the HHO/Biodiesel/Diesel advantages of Equation (5) was calculated (Le Anh Tuan & Van Tai, Citation2011):
(5)
(5) where,
,
and
are the airflow, the liquid fuel rate, and the HHO mass flow rate (g/s), respectively.
is the stoichiometric ratio of air to HHO gas
.
is the stoichiometric ratio of air to fuels (D, B5, and B20), according to the chemical formula, they were calculated, which are 14.3, 14.19 and 13.91, respectively (Table ).
Table 5. Uncertainty analysis of the measurement parameters used in the study.
Table 6. Design summary.
Given that and
, the equivalence ratio (
) is the opposite of lambda (
). Therefore, the equivalence ratio of Equation (6) was calculated:
(6)
(6) where
and
are the inlet airflow and the inlet fuel flow rate (cc/s), respectively, which is measured experimentally.
and
are air and fuel densities (kg/m3).
In all experimental tests, the accuracy of the measured data was validated. Due to device measurement error, data uncertainty was calculated according to Equation (7) (Barford, Citation1985; Kline, Citation1953):
(7)
(7) where δR is the amount of uncertainty, R is the response function, x1, x2, … and x2 are independent variables, and δ1, δ2, … and δn are symbols of the uncertainty of each independent variable.
The accuracy of measuring motor load (), motor speed (
) and volumetric fuel consumption (
) is ±1 N, ±5 rpm, and ±0.1 cc/s, respectively. As a result, the uncertainty of BP, BSFC, and BTE was calculated to be 1.014%, 1.033%, and 1.275%, respectively (Table ).
Engine tests were performed at full load and constant speed 1500 rpm. HHO gas was used in three levels 3, 4, and 5 cc/s as a supplement to diesel, B5, and B20 fuels. HHO gas entered the engine's combustion chamber through the inlet manifold. By changing the voltage of the electrolysis system and using the calibration table, the HHO gas flow rate was measured and controlled.
Results and discussion
Engine performance
Exhaust gas temperature
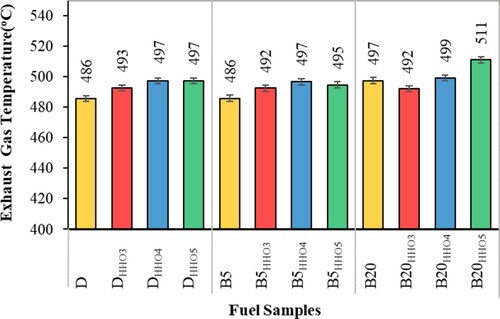
Figure shows the temperature variation of the exhaust gases using HHO and various biodiesel–diesel fuel blends. According to Figure , biodiesel increases the EGT due to the complete combustion and higher heat release rate (MohamedMusthafa et al., Citation2011). EGT depends on the amount of oxygen in the fuel (Dhamodaran et al., Citation2017). Biodiesel contains 10-12% oxygen, while diesel fuel without oxygen (Faizollahzadeh Ardabili et al., Citation2018). The oxygen in biodiesel fuel causes complete combustion, increasing exhaust gas temperature (Ghazali et al., Citation2015). The biodiesel cetane number is higher than diesel (Table ) (Faizollahzadeh Ardabili et al., Citation2019). As a result, biodiesel's use shortens the ignition delay time (Abu-Hamdeh & Alnefaie, Citation2015). Thus, some of the fuel that is not burned in the main combustion stage burns at the end of the combustion stage and releases the combustion chamber's temperature (Yu et al., Citation2002). Other researchers have reported expansion of exhaust gas temperature using biodiesel–diesel fuel blends (Abu-Hamdeh & Alnefaie, Citation2015; Banapurmath et al., Citation2008; Ghazali et al., Citation2015; Tamilselvan et al., Citation2017). Interestingly, the trend of changes in exhaust gas temperature (Figure ) is consistent with the amounts of oxygen in fuels (Table ). By adding HHO gas, the exhaust gas temperature increases (Kumar et al., Citation2003). Also, enriching the biodiesel–diesel fuel blends with high-calorie HHO gas (Table ) causes more intense combustion and increases the combustion temperature in the cylinder (Kumar et al., Citation2003; Rahman et al., Citation2017). Also, the oxygen in HHO gas increases the combustion efficiency of biodiesel–diesel fuels, in which case the energy released rate from the fuels increases and the combustion temperature in-cylinder increases. In general, the simultaneous use of two fuels, HHO gas and biodiesel, in a dual-fuel diesel engine significantly increased the EGT. The highest amount of EGT was related to pilot fuel B20 by HHO rate 5 cc/s (B20HHO5).
Engine brake power
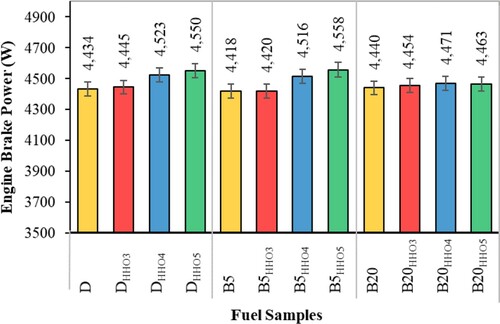
Engine BP is the useful power of the engine output shaft. As shown in Figure , the engine's BP is generally increased by using HHO gas. Due to the uncertainty of the engine's BP (Table ), the addition of HHO gas in the amount of 3 cc/s, did not have a significant effect on the BP compared to diesel, B5 and B20 fuels. But with the addition of 4 and 5 cc/s of HHO gas, the engine's BP increased compared to diesel and B5 fuels. The highest BP increase is related to DHHO5 and B5HHO5 fuels, which have increased by 2.61 and 3.16% compared to D and B5 fuels, respectively. This can be related to the higher calorific value of HHO gas (Table ) (Sandalcı et al., Citation2019). Also, excess oxygen imported with HHO gas can complete combustion and thus increase BP (Baltacioglu et al., Citation2016). Masjuki et al. (Citation2016) developed a study for comparing the effect of HHO with that of the Diesel and B20. According to the results, HHO increased BP about 2% in comparison with control fuel. The main reason was claimed to be the differences in the calorific value of the HHO and Diesel fuel (Masjuki et al., Citation2016). The similar findings also reported by Baltacioglu et al. (Citation2019) in investigation of the effects of HHO at 1 L/min level on performance and emission characteristics of a diesel engine fueled by biodiesel and diesel as pilot fuels. According to the results, the presence of HHO significantly increased the BP compared with control fuel (Baltacioglu et al., Citation2019).
The calorific value of HHO gas is higher than that of Diesel, B5, and B20, and its use in a dual-fuel diesel engine can compensate for the low heat value of pilot liquid fuels (Deheri et al., Citation2020; Sandalcı et al., Citation2019). The results of Figure show that using HHO gas increases the energy entering the engine, which compensates for the low energy of Diesel and B5 fuels and improves it. But, the use of the low value of HHO gas (3 cc/s) does not compensate for the low thermal value of B20 fuel. Therefore, it doesn't affect the production of BP. Also, the results showed that adding biodiesel to diesel fuel did not have a significant effect on BP. Data on the brake power of Diesel, B5, and B20 fuels are in the uncertainty range. The thermal value of biodiesel fuel is low, which reduces the BP. While biodiesel contains oxygen, it improves combustion and increases BP. As a result, with biodiesel's addition to diesel fuel, the BP generated does not change significantly.
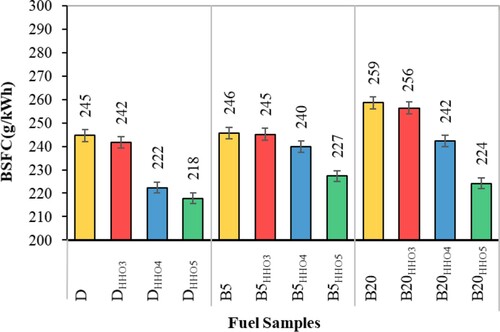
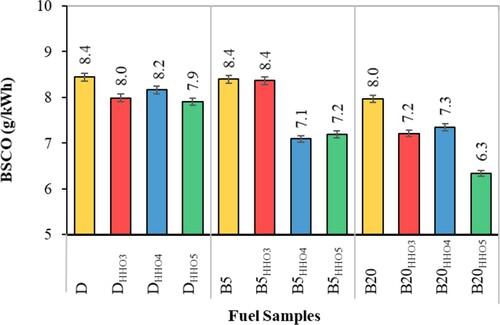
Brake specific fuel consumption
The brake special fuel consumption (BSFC) indicates the amount of fuel consumption on the mechanical energy produced by internal combustion engines. Variation of BSFC for different HHO gas and the biodiesel–diesel fuel blends is shown in Figure . As can be seen, the amount of BSFC increases as the amount of biodiesel in blend fuels increases. The BSFC for diesel, B5, and B20 was 245, 246, and 259 g/kWh, respectively. According to Table , BSFC data on diesel and B5 fuel do not differ significantly. But the amount of BSFC associated with B20 is significantly higher than diesel and B5 fuels. The main reason is biodiesel's low calorific value compared to diesel fuel (Table ) (Faizollahzadeh Ardabili et al., Citation2018). As can be seen, the amount of BSFC for HHO-enriched fuels is lower than for Diesel, B5, and B20 fuels. This is due to the high heat value content of HHO gas compared to biodiesel and diesel fuels (Table ), air-hydrogen mixing, and high hydrogen flame speed (Saravanan et al., Citation2008b), which has improved the combustion of the biodiesel–diesel fuel blends (Rahman et al., Citation2017). The results showed that adding 3 and 4 cc/s of HHO gas to D, B5, and B20 fuels did not make a significant difference in BSFC. But by adding 5 cc/s of HHO gas, the amount of BSFC is significantly reduced. The lowest BSFC values were for DHHO5, B5HHO5, and B20HHO5 fuels, which were 218, 227, and 224 g/kWh, respectively. DHHO5, B5HHO5, and B20HHO5 fuels reduced BSFC by 13.5%, 7.7%, and 11%, respectively, compared to D, B5, and B20 fuels. Therefore, in this study, the minimum amount of HHO gas that can significantly reduce the amount of BSFC was obtained, 5cc/s. Interestingly, the addition of HHO to B5 and B20 fuels corrects and improves biodiesel's low heat value, thereby reducing the amount of BSFC. The similar results were conducted by Masjuki et al. (Citation2016) to compare the effect of HHO on the performance of a dual fuel diesel engine with that of the diesel and B20. According to the results, HHO successfully reduced the BSFC about 5% compared with the control fuel (Masjuki et al., Citation2016). Baltacioglu et al. (Citation2019) reported a smooth reduction of BSFC by employing HHO at 1 L/min compared to the control fuel (Baltacioglu et al., Citation2019).
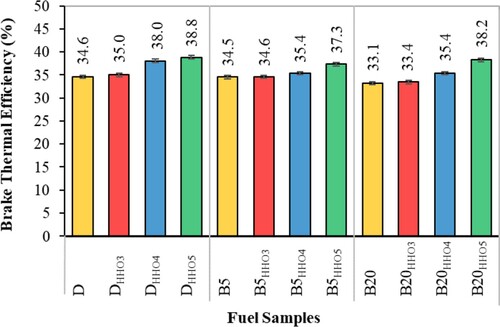
Brake thermal efficiency
The brake thermal efficiency (BTE) indicates the conversion of the thermal energy in the fuel into mechanical energy. Figure shows the effect of HHO gas and biodiesel/diesel blends on the variation of BTE. As can be seen, the BTE is reduced by using biodiesel and increased by using HHO gas. Interestingly, these changes in BTE are consistent with the caloric content of biodiesel and HHO gas (Table ). The BTE increases with HHO gas use, which is due to the better mixing of hydrogen-air and faster combustion and further diffusion of the flame (Saravanan et al., Citation2008a). Also, the oxygen in HHO gas causes complete combustion of biodiesel and Diesel (Kalam et al., Citation2003). Combustion of hydrogen produces higher temperatures than biodiesel and diesel fuels. Therefore, enriching biodiesel/diesel fuels with hydrogen increases the BTE (Baltacioglu et al., Citation2016; Yilmaz et al., Citation2010). The results showed that using HHO gas in the amount of 3 cc/s, increasing the BTE is not significant, but by increasing HHO by 4 and 5 cc/s, the BTE increases significantly. Baltacioglu et al. (Citation2019) reported a considerable increase of BTE (by about 2.9%) in the presence of HHO at 1 L/min in comparison with the control fuel (Baltacioglu et al., Citation2019). The main reason was the higher calorific value of the Hydrogen which led to an increase in BTE value.
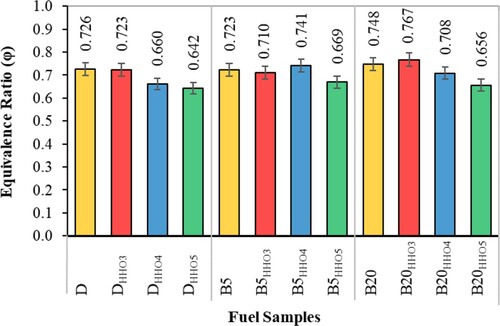
Equivalence ratio (Ø)
Figure shows the changes in the equivalence ratio using HHO gas and biodiesel/diesel fuel blends. Accordingly, with increasing HHO gas, the values of the equivalence ratio () decrease. HHO gas causes better air–fuel mixing (Saravanan et al., Citation2008b). As a result, biodiesel/diesel fuel blends’ combustion is improved (Rahman et al., Citation2017), reducing fuel consumption per kWh of braking power (Figure ). Reducing the actual fuel consumption compared to its stoichiometric consumption reduces the equilibrium ratio (
). As can be seen, adding 5 cc/s of HHO gas to D, B5, and B20 fuels significantly reduces the equivalence ratio's value. Adding 3 and 4 cc/s of HHO gas does not make a significant difference in
. The lowest
values were for DHHO5, B5HHI5, and B20HHO5 fuels, which were 0.642, 0.669, and 0.656, respectively.
Emission characteristics
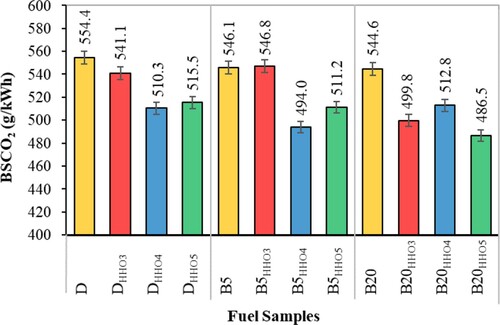
Break specific CO2 emissions
CO2 is the most critical pollutant in the environment. Figure shows break specific CO2 emissions using HHO gas and biodiesel/diesel fuel blends. As can be seen, break specific CO2 emissions increase significantly with HHO gas. The main reason for the increase break specific CO2 emissions is the improvement in combustion (Aklouche et al., Citation2017). By injecting HHO gas, some oxygen gas enters the combustion chamber, and carbon oxidation is improved, resulting in more complete combustion (Deheri et al., Citation2020). The higher temperature in-cylinder is a sign of complete combustion (Figure ) (Rahman et al., Citation2017; Verma et al., Citation2018). The results of this study showed that using HHO gas at values 4 and 5 cc/s significantly reduced break specific CO2 emissions. The lowest emissions of break specific CO2, related to DHHO4, B5HHO4, and B20HHO5 fuels, are 510.3, 494, and 486.5 g/kWh, respectively. B20HHO5 fuel significantly reduces break specific CO2 emissions by about 12.25% compared with diesel fuel as control.
Break specific CO emissions
CO is a toxic and dangerous gas which is generated by the incomplete combustion of hydrocarbon fuels (Akbarian et al., Citation2018). Figure shows CO emissions using HHO gas and biodiesel/diesel fuel blends. Accordingly, break specific CO emissions are significantly reduced by using HHO gas, because HHO gas does not contain carbon (Baltacioglu et al., Citation2016). Also, break specific CO emissions depend on the fuel equivalent ratio (Saravanan et al., Citation2007). As shown in Figure , the values of the equivalence ratio () decrease with increasing HHO gas. Decreasing the equivalence ratio means more inlet air into the cylinder. Obviously, despite the high oxygen content, the combustion in-cylinder is more complete and the break specific CO is reduced.
Also, because biodiesel fuel contains 10–12% oxygen, so by adding it to diesel fuel, the amount of oxygen in the combustion chamber increases, and as a result, the combustion is more complete, and CO emissions are reduced. The findings show that the simultaneous use of HHO gas and biodiesel intensifies the effect of increasing oxygen in the combustion chamber and significantly reduces break specific CO emissions. The highest break specific CO reduction was related to the B20HHO5 fuel, in which the ratio of biodiesel to diesel was 20:80, and the HHO gas flow rate was 5 cc/s. B20HHO5 fuel significantly reduces break specific CO emissions by about 25% compared to pure diesel fuel. Rimkus et al. (Citation2018) also reported a significant reduction of CO emissions by employing 0.14–0.18 Vol.% of HHO in the engine's input air. According to the results, the presence of the HHO reduced the CO emission especially at higher engine loads, due to the catalyzing effects of hydrogen in the fuel combustion (Rimkus et al., Citation2018).
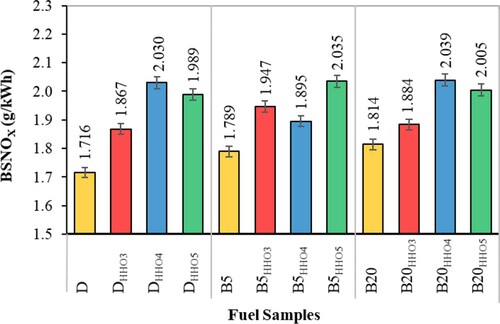
Nitrogen oxides (NOx)
NOX play an essential role in creating and spreading environmental pollution due to their toxicity. The formation of NOX during combustion depends on three factors: oxygen concentration, the temperature of the combustion chamber, and residence time (Premkartikkumar et al., Citation2014). Figure shows the NOX emission using HHO gas in a biodiesel/diesel fuel blend. Accordingly, the amount of NOX emissions increases significantly with HHO gas. The presence of HHO in the combustion chamber increases the intensity of combustion as well as increasing the temperature (Figure ), resulting in NOX emissions (Kumar et al., Citation2003). Also, the presence of oxygen in B5 and B20 fuels (Table ) increases the concentration of oxygen in the combustion chamber [37], resulting in more intense combustion and increases NOX emissions. On the other hand, the differences in cetane number of biodiesel and diesel (Table ) affects the ignition delay (Heywood; Lavoie et al., Citation1970; Lyn & Valdmanis, Citation1968; Owen & Coley, Citation1995; Wong & Steere, Citation1982) and accordingly, increases the retention time of fuel in the combustion chamber and led to form more NOX. The findings show that using of HHO gas and biodiesel simultaneously increases the NOX emissions. The highest NOX is for B20HHO4 fuel with 2.039 g/kWh, which is significantly higher than for B20 and D fuels. The increase of NOx emission was also reported by Masjuki et al. (Citation2016) for comparing the effect of HHO on the emission characteristics of a dual fuel diesel engine with that of the Diesel and B20. According to the results, HHO caused an increase in NOx emission comparison with control fuel (Masjuki et al., Citation2016). Baltacioglu et al. (Citation2019) reported an increase of NOx emission by employing HHO at 1 L/min compared to the control fuel (Baltacioglu et al., Citation2019). In another study, Rimkus et al. (Citation2018) investigated the effect of 0.14–0.18% of the volume of HHO in the input air to the engine. According to the results, the presence of the HHO increased the NOx emission especially in the lower engine loads. The main reason was the higher concentration of HHO in the combustion chamber that increases the combustion chamber's temperature and led to the formation of the NOx (Rimkus et al., Citation2018).
Optimization
The optimization process was performed by response surface methodology (RSM) as a mathematical-based analysis method. The RSM method's theoretical description is presented in our previous studies in (Faizollahzadeh Ardabili et al., Citation2018). The optimization was performed in terms of increasing the engine's performance and reducing engine exhaust emissions. In the optimization process, the biodiesel concentration (cc) in the pilot fuel samples and HHO rate (cc/s) were considered as the independent variables which affect the BP (W), BSFC (gr/kWh), and engine emissions (gr/kWh) as the dependent variables. Table and Table present the optimization parameters. Dataset was analyzed using Central Composite Design (CCD).
Table 7. Optimization parameters.
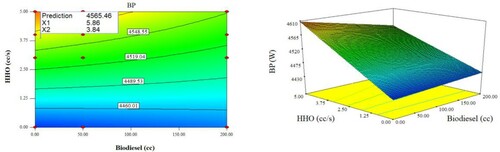
Figure presents the optimal levels for the independent variables in the presence of the dependent variables.
According to Figure , the best condition for the highest performance as well as the lowest engine emission has happened in the biodiesel concentration of 5.86 cc and HHO rate 3.84 cc/s. this condition could successfully generate BP of 4565.46 W and efficiency of 36.75%, consume BSFC of 227.855 gr/kWh and produce engine emissions including O2, CO, CO2, and NOx of 787.4, 7.96, 523.2, and 1.9 gr/kWh, respectively.
As is clear from Figure , increasing the HHO rate increases the BP but increasing the biodiesel concentration reduces the BP, especially at higher HHO rates. This can be due to biodiesel's lower heating value (Faizollahzadeh Ardabili et al., Citation2019). Increasing the HHO rate also reduces the BSFC value due to its higher heating value, but increasing the biodiesel concentration increases the BSFC, especially at higher HHO rates. Nb is also increased by increasing the HHO rate and reduced by increasing the biodiesel concentration.
HHO also affected the O2 emission. By the way, increasing the HHO rate significantly increased O2 emission. This phenomenon is also observed by Ismail et al., which is related to the extra oxygen content of HHO (Ismail et al., Citation2018). But, biodiesel did not indicate a significant effect on O2 emission. Increasing the HHO rate and biodiesel concentration successfully reduced the CO emission. Uludamar also reported a reduction for CO and CO2 emissions in the presence of HHO (Uludamar, Citation2018). The main reason was claimed to be the fast its fast flame expansion in the presence of HHO gas, and also lack of carbon in HHO reduced the availability of carbon in the combustion outcome (Jhang et al., Citation2016). Increasing the HHO rate also increased the NOx emission. One of the main reasons for that is the higher temperature of the combustion chamber in the HHO gas presence due to the faster burning rate. This was also reported by Zhou et al. (Citation2014).
Conclusion
The present study's main aim is to investigate the performance and emission of a dual-fuel diesel engine using HHO as induced fuel and biodiesel/diesel blends as pilot fuel. Using HHO gas with 3 cc/s flow rate does not significantly affect BP, but using it in the amount of 4 and 5 cc/s increases the BP compared to diesel and B5 fuels. The highest BP increase is related to DHHO5 and B5HHO5 fuels, which increased by 2.61% and 3.16% compared to Diesel and B5 fuels, respectively. Adding 5 cc/s of HHO gas, reduced BSFC significantly. The lowest BSFC values for DHHO5, B5HHO5, and B20HHO5 fuels are 218, 227, and 224 g/kWh, respectively. Using HHO gas by 3 cc/s has no significant effect on the BTE, but HHO by 4 and 5 cc/s significantly increased the BTE of the engine. HHO gas by 4 and 5cc/s also reduced the CO2 emissions significantly. Simultaneous use of HHO gas and biodiesel intensified the effect of increasing oxygen in the combustion chamber and significantly reduced CO emissions. The highest CO reduction was related to B20HHO5 fuel. The use of HHO and biodiesel gas in a dual fuel diesel engine increased the NOx emission. The highest NOX was related to B20HHO4 fuel by 2.039 g/kWh, which was significantly higher than for B20 and diesel fuel. According to the obtained results, the simultaneous use of HHO gas and biodiesel fuel in a dual fuel diesel engine enhances fuel combustion and improves performance and emissions of the engine. The minimum amount of HHO gas that has a significant effect on BSFC reduction and emissions is 5 cc/s. Therefore, the use of HHO gas can be considered as a promising technique to increase performance and reduce emissions of dual-fuel diesel engines, without any change in the structure of diesel engines.
Nomenclatures
| ASTM | = | American Society for Testing and Materials |
| BP | = | Brake Power |
| BSFC | = | Brake Specific Fuel Consumption |
| BTE | = | Brake Thermal Efficiency |
| B5 | = | Diesel fuel including 5% biodiesel |
| B20 | = | Diesel fuel including 20% biodiesel |
| B20HHO5 | = | 20%Biodiesel+80%Diesel+5cc/s HHO |
| CO | = | Carbon Monoxide |
| CO2 | = | Carbon Dioxide |
| D | = | Diesel Fuel |
| DFDE | = | Dual Fuel Diesel Engine |
| GHG | = | Greenhouse gas |
| H2 | = | Hydrogen |
| HHO | = | Hydroxy Gas |
| ICEs | = | Internal Combustion Engines |
| IEA | = | International Energy Agency |
| L/min | = | Liter per Minute |
| NOx | = | Nitrogen Oxides |
| PM | = | Particulate Matter |
| Ppm | = | Parts per million |
| rpm | = | Revolutions per Minute |
| WCOME | = | Waste Cooking Oil Methyl Ester |
Acknowledgement
Open Access Funding by the Publication Fund of the TU Dresden.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Abu-Hamdeh, N. H., & Alnefaie, K. A. (2015). A comparative study of almond biodiesel-diesel blends for diesel engine in terms of performance and emissions. BioMed research international
- Akbarian, E., Najafi, B., Jafari, M., Faizollahzadeh Ardabili, S., Shamshirband, S., & Chau, K.-W. (2018). Experimental and computational fluid dynamics-based numerical simulation of using natural gas in a dual-fueled diesel engine. Engineering Applications of Computational Fluid Mechanics, .12(1), 517–534. https://doi.org/https://doi.org/10.1080/19942060.2018.1472670
- Aklouche, F., Loubar, K., Bentebbiche, A., Awad, S., & Tazerout, M. (2017). Experimental investigation of the equivalence ratio influence on combustion, performance and exhaust emissions of a dual fuel diesel engine operating on synthetic biogas fuel. Energy Conversion and Management, 152, 291–299. https://doi.org/https://doi.org/10.1016/j.enconman.2017.09.050
- Arat, H. T. (2019). Simulation of diesel hybrid electric vehicle containing hydrogen enriched CI engine. International Journal of Hydrogen Energy, 44(20), 10139–10146. https://doi.org/https://doi.org/10.1016/j.ijhydene.2018.10.004
- Arjun, T., Atul, K., Muraleedharan, A. P., Walton, P. A., Bijinraj, P., & Raj, A. A. (2019). A review on analysis of HHO gas in IC engines. Materials Today: Proceedings, 11, 1117–1129. https://doi.org/https://doi.org/10.1016/j.matpr.2018.12.046
- Baltacioglu, M. K., Arat, H. T., Özcanli, M., & Aydin, K. (2016). Experimental comparison of pure hydrogen and HHO (hydroxy) enriched biodiesel (B10) fuel in a commercial diesel engine. International Journal of Hydrogen Energy, 41(19), 8347–8353. https://doi.org/https://doi.org/10.1016/j.ijhydene.2015.11.185
- Baltacioglu, M. K., Kenanoglu, R., & Aydın, K. (2019). HHO enrichment of bio-diesohol fuel blends in a single cylinder diesel engine. International Journal of Hydrogen Energy. 44(34), 18993–19004. https://doi.org/10.1016/j.ijhydene.2019.02.060.
- Banapurmath, N., Tewari, P., & Hosmath, R. S. (2008). Combustion and emission characteristics of a direct injection, compression-ignition engine when operated on Honge oil, HOME and blends of HOME and diesel. International Journal of Sustainable Engineering, 1(2), 80–93. https://doi.org/https://doi.org/10.1080/19397030802221265
- Barford, N. C. (1985). Experimental measurements: Precision, error and truth. Wiley.
- Cammack, R., Frey, M., & Robson, R. (2001). Hydrogen as a fuel: Learning from nature. CRC Press.
- Deheri, C., Acharya, S. K., Thatoi, D. N., & Mohanty, A. P (2020). A review on performance of biogas and hydrogen on diesel engine in dual fuel mode. Fuel, 260, 116337. https://doi.org/https://doi.org/10.1016/j.fuel.2019.116337
- Dhamodaran, G., Krishnan, R., Pochareddy, Y. K., Pyarelal, H. M., Sivasubramanian, H., & Ganeshram, A. K. (2017). A comparative study of combustion, emission, and performance characteristics of rice-bran-, neem-, and cottonseed-oil biodiesels with varying degree of unsaturation. Fuel, 187, 296–305. https://doi.org/https://doi.org/10.1016/j.fuel.2016.09.062.
- Dincer, I. (2007). Environmental and sustainability aspects of hydrogen and fuel cell systems. International Journal of Energy Research, 31(1), 29–55. https://doi.org/https://doi.org/10.1002/er.1226
- EL-Kassaby, M. M., Eldrainy, Y. A., Khidr, M. E., & Khidr, K. I. (2016). Effect of hydroxy (HHO) gas addition on gasoline engine performance and emissions. Alexandria Engineering Journal. 55(1), 243–251. https://doi.org/https://doi.org/10.1016/j.aej.2015.10.016
- Faizollahzadeh Ardabili, S., Najafi, B., Alizamir, M., Mosavi, A., Shamshirband, S., & Rabczuk, T. (2018). Using SVM-RSM and ELM-RSM approaches for optimizing the production process of methyl and ethyl esters. Energies11(11), 2889. https://doi.org/https://doi.org/10.3390/en11112889.
- Faizollahzadeh Ardabili, S., Najafi, B., & Shamshirband, S. (2019). Fuzzy logic method for the prediction of cetane number using carbon number, double bounds, iodic, and saponification values of biodiesel fuels. Environmental Progress & Sustainable Energy.38(2), 584–599. https://doi.org/https://doi.org/10.1002/ep.12960
- Ghazali, W. N. M. W., Mamat, R., Masjuki, H., Najafi, G. J. R., & Reviews, S. E. (2015). Effects of biodiesel from different feedstocks on engine performance and emissions: A review. Renewable and Sustainable Energy Reviews, 51, 585–602. https://doi.org/https://doi.org/10.1016/j.rser.2015.06.031.
- Hajlari, S. A., Najafi, B., & Ardabili, S. (2019). Castor oil, a source for biodiesel production and its impact on the diesel engine performance. 28, 1–10.
- Ismail, T. M., Ramzy, K., Abelwhab, M., Elnaghi, B. E., Abd El-Salam, M., & Ismail, M. (2018). Performance of hybrid compression ignition engine using hydroxy (HHO) from dry cell. Energy Conversion and Management, 155, 287–300. https://doi.org/https://doi.org/10.1016/j.enconman.2017.10.076
- Ismail, T. M., Ramzy, K., Elnaghi, B. E., Mansour, T., Abelwhab, M., El-Salam, M. A., & Ismail, M. (2019). Modelling and simulation of electrochemical analysis of hybrid spark-ignition engine using hydroxy (HHO) dry cell. Energy Conversion and Management, 181, 1–14. https://doi.org/https://doi.org/10.1016/j.enconman.2018.11.067
- Jannatkhah, J., Najafi, B., & Ghaebi, H. (2020). Energy and exergy analysis of combined ORC–ERC system for biodiesel-fed diesel engine waste heat recovery. Energy Conversion and Management , 209, 112658. https://doi.org/https://doi.org/10.1016/j.enconman.2020.112658
- Jhang, S.-R., Chen, K.-S., Lin, S.-L., Lin, Y.-C., & Cheng, W. (2016). Reducing pollutant emissions from a heavy-duty diesel engine by using hydrogen additions. Fuel, 172, 89–95. https://doi.org/https://doi.org/10.1016/j.fuel.2016.01.032
- Kalam, M., Husnawan, M., & Masjuki, H. M. (2003). Exhaust emission and combustion evaluation of coconut oil-powered indirect injection diesel engine. Renewable Energy, 28(15), 2405–2415. https://doi.org/https://doi.org/10.1016/S0960-1481(03)00136-8
- Kline, S. J. (1953). Describing uncertainty in single sample experiments. Mech. Engineering, 75, 3–8.
- Knop, V., Benkenida, A., Jay, S., & Colin, O. (2008). Modelling of combustion and nitrogen oxide formation in hydrogen-fuelled internal combustion engines within a 3D CFD code. International Journal of Hydrogen Energy, 33(19), 5083–5097. https://doi.org/https://doi.org/10.1016/j.ijhydene.2008.06.027
- Kumar, M. S., Ramesh, A., & Nagalingam, B. (2003). Use of hydrogen to enhance the performance of a vegetable oil fuelled compression ignition engine. International Journal of Hydrogen Energy, 28(10), 1143–1154. https://doi.org/https://doi.org/10.1016/S0360-3199(02)00234-3
- Lavoie, G. A., Heywood, J. B., & Keck, J. C. (1970). Experimental and theoretical study of nitric oxide formation in internal combustion engines. Combustion science and technology, 1(4), 313–326. https://doi.org/https://doi.org/10.1080/00102206908952211
- Le Anh, T., Nguyen, D. K., & Tai, C. V. (2011). Simulation study on potential addition of HHO gas in a motorcycle engine using AVL Boost.School of Transportation Engineering, Hanoi University of Science and Technology
- Lyn, W., & Valdmanis, E. (1968). The effects of physical factors on ignition delay (No. 0148-7191).SAE Technical Paper. https://doi.org/10.4271/680102.
- Masjuki, H., Ruhul, A., Mustafi, N. N., Kalam, M., Arbab, M., & Fattah, I. R. (2016). Study of production optimization and effect of hydroxyl gas on a CI engine performance and emission fueled with biodiesel blends. International Journal of Hydrogen Energy, 41(33), 14519–14528. https://doi.org/https://doi.org/10.1016/j.ijhydene.2016.05.273
- MohamedMusthafa, M., Sivapirakasam, S., & Udayakumar, M. (2011). Comparative studies on fly ash coated low heat rejection diesel engine on performance and emission characteristics fueled by rice bran and pongamia methyl ester and their blend with diesel. 36(5), 2343–2351. https://doi.org/https://doi.org/10.1016/j.energy.2010.12.047
- Najafi, B., & Ardabili, S. F. (2018). Application of ANFIS, ANN, and logistic methods in estimating biogas production from spent mushroom compost (SMC). Resources, Conservation and Recycling, 133, 169–178. https://doi.org/https://doi.org/10.1016/j.resconrec.2018.02.025
- Owen, K., & Coley, T. (1995). Automotive fuels reference bookSociety of Automotive Engineers. Inc., Warrendale, PA, 648 .
- Parthasarathy, M., Lalvani, J. I. J., Dhinesh, B., & Annamalai, K. (2016). Effect of hydrogen on ethanol–biodiesel blend on performance and emission characteristics of a direct injection diesel engine. Ecotoxicology and environmental safety, 134, 433–439. https://doi.org/https://doi.org/10.1016/j.ecoenv.2015.11.005
- Premkartikkumar, S., Annamalai, K., & Pradeepkumar, A. (2014). Effectiveness of oxygen enriched hydrogen-HHO gas addition on DI diesel engine performance, emission and combustion characteristics. Thermal Science, 18(1), 259–268. https://doi.org/https://doi.org/10.2298/TSCI121014078P
- Rahman, M. A., Ruhul, A., Aziz, M., & Ahmed, R. (2017). Experimental exploration of hydrogen enrichment in a dual fuel CI engine with exhaust gas recirculation. International Journal of Hydrogen Energy, 42(8), 5400–5409. https://doi.org/https://doi.org/10.1016/j.ijhydene.2016.11.109
- Ramadhas, A. S. (2016). Alternative fuels for transportation. CRC Press.
- Reece, D., & Peterson, C. (1995). Acute toxicity of biodiesel to freshwater and marine organisms. National Renewable Energy Lab., Golden, CO (United States)
- Riazi, M., & Albahri, T. (2005). Prediction of Reid vapor pressure of petroleum fuels. Petroleum science and technology, 23(1), 75–86. https://doi.org/https://doi.org/10.1081/LFT-20009686225
- Rimkus, A., Matijošius, J., Bogdevičius, M., Bereczky, Á, & Török, Á. (2018). An investigation of the efficiency of using O2 and H2 (hydrooxile gas-HHO) gas additives in a ci engine operating on diesel fuel and biodiesel. 152, 640–651. https://doi.org/https://doi.org/10.1016/j.energy.2018.03.087
- Sandalcı, T., Işın, Ö, Galata, S.Karagöz, Y., & Güler, İ. (2019). Effect of hythane enrichment on performance, emission and combustion characteristics of an CI engine. International journal of hydrogen energy, 44(5), 3208–3220. https://doi.org/https://doi.org/10.1016/j.ijhydene.2018.12.069
- Saravanan, N., & Nagarajan, G. (2010). Performance and emission studies on port injection of hydrogen with varied flow rates with diesel as an ignition source. Applied Energy , 87(7), 2218–2229. https://doi.org/https://doi.org/10.1016/j.apenergy.2010.01.014
- Saravanan, N., Nagarajan, G., Dhanasekaran, C., & Kalaiselvan, K. (2007). Experimental investigation of hydrogen port fuel injection in DI diesel engine. International Journal of Hydrogen Energy, 32(16), 4071–4080. https://doi.org/https://doi.org/10.1016/j.ijhydene.2007.03.036
- Saravanan, N., Nagarajan, G., Kalaiselvan, K., & Dhanasekaran, C. (2008a). An experimental investigation on hydrogen as a dual fuel for diesel engine system with exhaust gas recirculation technique. Renewable Energy , 33(3), 422–427. https://doi.org/https://doi.org/10.1016/j.renene.2007.03.015
- Saravanan, N., Nagarajan, G., Sanjay, G., Dhanasekaran, C., & Kalaiselvan, K. (2008b). Combustion analysis on a DI diesel engine with hydrogen in dual fuel mode. Fuel, 87(17-18), 3591–3599. https://doi.org/https://doi.org/10.1016/j.fuel.2008.07.011
- Selvi Rajaram, P., Kandasamy, A., & Arokiasamy Remigious, P. (2014). Effectiveness Of oxygen enriched hydrogen-Hho Gas addition On direct injection diesel engine performance, emission And combustion characteristics. Thermal Science, 18(1). https://doi.org/https://doi.org/10.2298/TSCI121014078P
- Sudrajat, A., Handayani, E. M., Tamaldin, N., & Yamin, A. K. M. (2018). Principle of generator HHO hybrid multistack type production technologies to increase HHO gas volume. (Ed.),(Eds.). SHS Web of Conferences. Vol. 49, p. 02016
- Szwaja, S., & Grab-Rogalinski, K. (2009). Hydrogen combustion in a compression ignition diesel engine. International journal of hydrogen energy , 34(10), 4413–4421. https://doi.org/https://doi.org/10.1016/j.ijhydene.2009.03.020
- Tamilselvan, P., Vignesh, K., & Nallusamy, N. (2017). Experimental investigation of performance, combustion and emission characteristics of CI engine fuelled with chicha oil biodiesel. International Journal of Ambient Energy, 38(7), 752–758.
- Thangaraj, S., & Govindan, N. (2018). Evaluating combustion, performance and emission characteristics of diesel engine using karanja oil methyl ester biodiesel blends enriched with HHO gas. International Journal of Hydrogen Energy, 43(12), 6443–6455. https://doi.org/https://doi.org/10.1016/j.ijhydene.2018.02.036
- Trujillo-Olivares, I., Soriano-Moranchel, F., Álvarez-Zapata, L. A., de Guadalupe González-Huerta, R., & Sandoval-Pineda, J. M. (2019). Design of alkaline electrolyser for integration in diesel engines to reduce pollutants emission. International Journal of Hydrogen Energy, 44(47), 25277–25286. https://doi.org/https://doi.org/10.1016/j.ijhydene.2019.07.256
- Uludamar, E. (2018). Effect of hydroxy and hydrogen gas addition on diesel engine fuelled with microalgae biodiesel. International journal of hydrogen energy, 43(38), 18028–18036. https://doi.org/https://doi.org/10.1016/j.ijhydene.2018.01.075
- Uludamar, E., Yıldızhan, Ş, Aydın, K., & Özcanlı, M. (2016). Vibration, noise and exhaust emissions analyses of an unmodified compression ignition engine fuelled with low sulphur diesel and biodiesel blends with hydrogen addition. International journal of hydrogen energy, 41(26), 11481–11490. https://doi.org/https://doi.org/10.1016/j.ijhydene.2016.03.179
- Verma, S., Das, L., Kaushik, S., & Tyagi, S. (2018). An experimental investigation of exergetic performance and emission characteristics of hydrogen supplemented biogas-diesel dual fuel engine. International Journal of Hydrogen Energy, 43(4), 2452–2468. https://doi.org/https://doi.org/10.1016/j.ijhydene.2017.12.032
- Vickers, N. (2017). Animal Communication: When I’m Calling You, will You Answer Too?. Current biology , 27(14), R713–R715. https://doi.org/https://doi.org/10.1016/j.cub.2017.05.064
- Wong, C., & Steere, D. (1982). The effects of diesel fuel properties and engine operating conditions on ignition delay. SAE transactions, 3873–3892. doi:https://doi.org/10.2307/44634401
- Yadav Milind, S., Sawant, S., Anavkar Jayesh, A., & Chavan Hemant, V. (2011). Investigations on generation methods for oxy-hydrogen gas, its blending with conventional fuels and effect on the performance of internal combustion engine. Journal of Mechanical Engineering Research, 3(9), 325–332. https://doi.org/https://doi.org/10.5897/JMER.9000031
- Yilmaz, A. C., Uludamar, E., & Aydin, K. (2010). Effect of hydroxy (HHO) gas addition on performance and exhaust emissions in compression ignition engines. international journal of hydrogen energy, 35(20), 11366–11372. https://doi.org/https://doi.org/10.1016/j.ijhydene.2010.07.040
- Yu, C., Bari, S., & Ameen, A. (2002). A comparison of combustion characteristics of waste cooking oil with diesel as fuel in a direct injection diesel engine. Proceedings of the Institution of Mechanical Engineers, Part D: Journal of Automobile Engineering, 216(3), 237–243. https://doi.org/https://doi.org/10.1243/0954407021529066
- Zhou, J., Cheung, C., & Leung, C., & Part D: Journal of Automobile Engineering. (2014). Combustion, performance and emissions of a diesel engine with H2, CH4 and H2–CH4 addition. nternational journal of hydrogen energy, 39(9), 4611–4621. https://doi.org/https://doi.org/10.1016/j.ijhydene.2013.12.194