ABSTRACT
Oral candidiasis (OC) is the most common opportunistic fungal infection among immunocompromised individuals. This systematic review and meta-analysis reports on the contribution of non-albicans Candida species in causing OC among human immunodeficiency virus (HIV)-infected individuals in sub-Saharan Africa between 2005 and 2015. Thirteen original research articles on oral Candida infection/colonization among HIV-infected African populations were reviewed. The prevalence of OC ranged from 7.6% to 75.3%. Pseudomembranous candidiasis was found to range from 12.1% to 66.7%. The prevalence of non-albicans Candida species causing OC was 33.5% [95% confidence interval (CI) 30.9–36.39%]. Of 458 non-albicans Candida species detected, C. glabrata (23.8%; 109/458) was the most common, followed by C. tropicalis (22%; 101/458) and C. krusei (10.7%; 49/458). The overall fluconazole resistance was 39.3% (95% CI 34.4–44.1%). Candida albicans was significantly more resistant than non-albicans Candida species to fluconazole (44.7% vs 21.9%; p < 0.001). One-quarter of the cases of OC among HIV-infected individuals in sub-Saharan Africa were due to non-albicans Candida species. Candida albicans isolates were more resistant than the non-albicans Candida species to fluconazole and voriconazole. Strengthening the capacity for fungal diagnosis and antifungal susceptibility testing in sub-Saharan Africa is mandatory in order to track the azole resistance trend.
Introduction
Oral candidiasis (OC) is one of the most common fungal opportunistic infections in immunocompromised individuals [Citation1]. OC occurs in up to 95% of human immunodeficiency virus (HIV)-infected individuals during the course of their illness [Citation2,Citation3], and is a prognostic indicator for acquired immune deficiency syndrome (AIDS) [Citation4,Citation5]. In sub-Saharan Africa, there is an increased prevalence of severe immunocompromised conditions, which is associated with a higher incidence of opportunistic infections [Citation6]. Worldwide, it is estimated that 70% of the HIV-infected individuals living in sub-Saharan Africa [Citation6] are at risk of infection with OC.
OC is mainly caused by Candida albicans [Citation7], which accounts for up to 81% of cases among HIV-infected individuals [Citation8]. It is documented that between 17% and 75% of healthy individuals can be colonized by Candida species [Citation9,Citation10]. However, non-albicans Candida species have been implicated in colonization of the oral cavity, eventually causing infection in 20–40% of immunocompromised individuals [Citation10–Citation12].
The increased prevalence of OC among African HIV-infected individuals ranges from 18% [Citation13,Citation14] to >60% [Citation15–Citation17], and this has resulted in increased use of antifungal agents for both prophylactic and treatment purposes [Citation18]. Furthermore, there is an increasing number of reports of Candida species that are resistant to azole antifungal agents [Citation19,Citation20]. This list of resistant species includes C. krusei, C. inconspicua, and C. norvegensis, which are all intrinsically resistant to fluconazole and have been isolated from patients with systemic candidiasis [Citation20,Citation21]. There have also been increased reports of fluconazole resistance in C. glabrata isolates, which manifests following the use of azole antifungal agents [Citation19,Citation21]. However, data on the spectrum of Candida species and the respective antifungal susceptibility profiles among HIV-infected individuals from sub-Saharan Africa are still limited. This systematic review and meta-analysis aimed to report the incidence of the non-albicans species in OC among the HIV-infected African population of sub-Saharan Africa between 2005 and 2015.
Material and methods
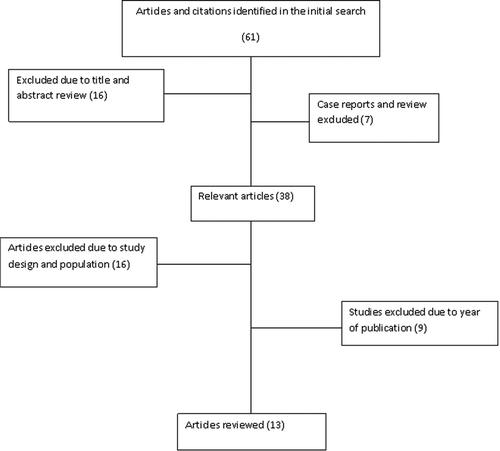
A literature search of English-language articles undertaking research on oral Candida colonization and/or infection was performed using PubMed/MEDLINE, Google Scholar, Web of Knowledge, Google Health, Embase, and POPLINE. The search terms included were ‘oral thrush’, ‘oral candidiasis’, ‘oral Candida’, ‘oral Candida colonization’, and ‘candidiasis of buccal cavity’, plus African country names in different combinations. New links shown in the abstract were followed to retrieve more abstracts. Thus, a total of 61 abstracts was obtained. All abstracts were carefully reviewed independently by two authors. Sixteen abstracts were excluded since nine were general reports on HIV/AIDS oral manifestations; three were restricted to pediatric populations; and four only described general opportunistic infections, Candida infections, or genetic variations of innate immunity and OC. None of the excluded abstracts contained details of oral Candida species, pattern of clinical presentation, or antifungal susceptibility. Further analysis excluded one case report and six review articles. The analysis led to 38 articles being obtained on studies on OC that had been conducted in Africa. All 38 articles were carefully reviewed, and a further 25 articles were excluded as they assessed OC among HIV-infected African children or neonates (n = 12), were clinical trials (n = 2), involved immunocompetent individuals (n = 1), comprised a retrospective cohort study (n = 1), or had been conducted before 2005 (n = 9) (Figure 1). The remaining 13 relevant articles were reviewed independently by two authors. A wide selection of data was extracted from each article and transferred on to a spreadsheet. The data extracted included year of publication, region (country), study population, sampling technique, patient gender, method for Candida species identification, use of highly active antiretroviral therapy (HAART), CD4 cell count, prevalence of oral fungal colonization and infection, and the antifungal susceptibility testing scheme.
Data were examined manually and analyzed to obtain the proportion of oral Candida colonization and infection. A meta-analysis model was used to calculate the pooled (weighted) proportion of OC, non-albicans Candida species, and fluconazole resistance among C. albicans and non-albicans Candida species. A proportion test was conducted using STATA v.11 to establish the statistical differences between the prevalences of oral Candida infection among the HIV-infected African population. A p value of <0.05 at a 95% confidence interval (CI) was used to define statistical significance.
Ethical approval
Ethical clearance for conducting this study was granted by the joint CUHAS/BMC research ethics and review committee, with certificate number CREC/048/2014.
Results
In total, 13 articles from Nigeria, South Africa, Ethiopia, Uganda, Cameroon, Tanzania, and Ghana were included in this review.
The majority of the articles (n = 12; 85.7%) reported on OC, four (28.6%) on both OC and Candida colonization, and two (14.3%) only on oral Candida species colonization ().
Table 1. Summary of the published articles on oral candidiasis among human immunodeficiency virus (HIV)-infected African populations.
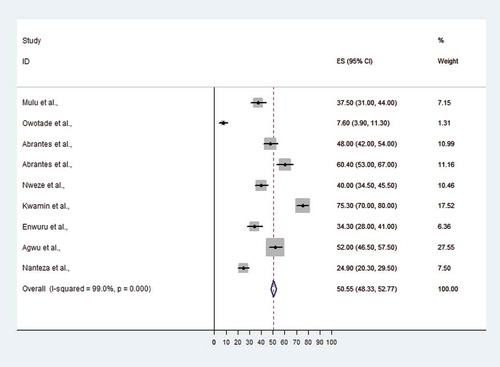
In six articles that reported on oral Candida species colonization among HIV-infected individuals, the prevalence ranged from 0.25% in Nigeria [Citation26] to 82.3% in Ethiopia [Citation22]. With the exception of one article from Tanzania that did not report on OC prevalence [Citation25], the prevalence of oral Candida infection was reported to range from 7.6% in Nigeria to 75.3% in Ghana among HIV-infected individuals. The pooled prevalence of OC among HIV-infected Africans was 50.6% (95% CI 48.3–52.8%) (Figure 2). The lowest OC prevalence was detected in South Africa (7.6%, 95% CI 3.9–11.3%) and the highest OC prevalence was observed in Ghana (75.3%, 95% CI 70–80%) ().
Figure 2. Proportional estimate (ES) with 95% confidence interval (CI) of oral candidiasis (OC) among human immunodeficiency virus (HIV)-infected patients from Africa. The midpoint of each horizontal line segment shows the proportional estimate of OC of each study, while the rhombic mark shows the pooled proportions for all studies.
No clear data were given regarding OC and HIV treatment status. Nine articles involving 2,239 individuals provided data on HIV treatment status. Among the 2,239 individuals, 1,407 (62%) did not receive HAART. Five articles [Citation15,Citation16,Citation26,Citation27,Citation30] had detailed data on OC distribution among individuals receiving and not receiving HAART. Only one article showed that HAART was associated with a significantly lower isolation rate of Candida species [Citation26].
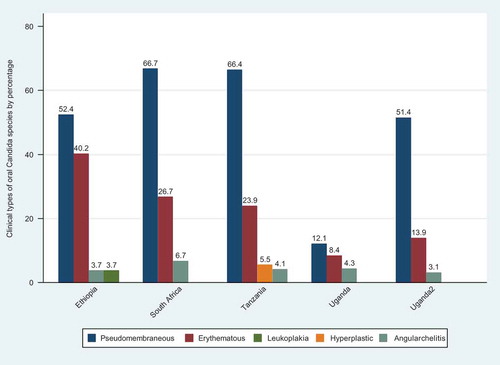
Pseudomembranous candidiasis was the most prevalent form of OC reported in these studies. The prevalence of pseudomembranous candidiasis ranged from 12.1% in Uganda to 66.7% in South Africa. The prevalence of erythematous candidiasis (chronic atrophic candidiasis) was highest in Ethiopia, at 40.2%. Candida leukoplakia and hyperplastic candidiasis were reported by a single article each, one from Ethiopia and one from Tanzania ().
Of 1,795 Candida isolates analyzed, C. albicans was the most common species (n = 1,337; 74.5%, 95% CI 72.2–76.8%), and non-albicans Candida species accounted for 458 (25.5%, 95% CI 21.5–29.5%) of isolates. The prevalence of non-albicans Candida species colonizing the oral cavity of the immunocompromised African population was found to range from 6.7% to 58.9% in Nigeria.
Of 458 non-albicans Candida species detected, C. glabrata was the most frequent isolate (23.8%; 109/458), followed by C. tropicalis (22%; 101/458) and C. krusei (10.7%; 49/458) ().
Table 2. Candida species distributions according to different studies.
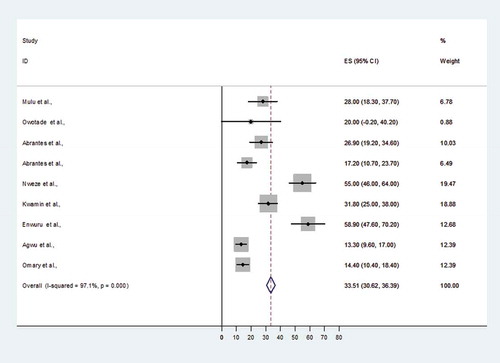
The prevalence of non-albicans Candida species causing OC ranged from 13.3% (95% CI 9.6–17%) to 58.9% (95% CI 47.6–70.2%) and both reports involved Nigerian subjects (). When the data for non-albicans Candida species causing OC among HIV-infected Africans were pooled, the overall prevalence was 33.5% (95% CI 30.9–36.39%) ().
Figure 4. Proportional estimate (ES) of non- albicans Candida species causing oral candidiasis (OC) with 95% confidence interval (CI). The midpoint of each horizontal line segment shows the proportional estimate of non-albicans Candida species in each study, while the rhombic mark shows the pooled proportions for all studies.
Seven articles reported on the occurrence of mixed Candida species. Altogether, 1,914 HIV-infected patients were studied, with 236 (12.3%) having mixed Candida species. In total, 201 individuals (85.2%) had a mixture of C. albicans and a non-albicans Candida species.
There were many variations on the breakpoints used in the determination of the antifungal susceptibility. Of the 13 articles analyzed, only five reported on antifungal susceptibility pattern. All five reported the minimum inhibitory concentrations (MICs) by broth microdilution techniques. The Clinical and Laboratory Standards Institute (CLSI) breakpoints were used for interpretation of the drug susceptibility of echinocandins, intraconazole, fluconazole, and amphotericin B (). One multicenter study undertaken in South Africa and Cameroon [Citation17] used the previously suggested breakpoints for flucytosine [Citation31], voriconazole [Citation32], and posaconale [Citation33] (). A study conducted in Ethiopia by Mulu et al. [Citation22] used 2 µg/ml as the breakpoint for amphotericin B, as previously reported by Brito et al. [Citation34]. In the study by Mulu et al. [Citation22], the MIC for micafungin was defined as the lowest concentration in which at least 50% of growth of the sample was inhibited.
Table 3. Breakpoints for minimum inhibitory concentration determination.
The incidence of fluconazole resistance among Candida species was found to range from 5% in Tanzania to 40% in South Africa. The highest rate (13%) of Candida species that were resistant to echinocandins (micafungin) was detected in Cameroon ().
Table 4. Antifungal resistance patterns for Candida albicans and non-albicans Candida species from different countries.
Among C. albicans, micafungin resistance ranged from 0% to 4%, while for non-albicans Candida species it ranged from 0% to 51.6% (). In total, 252 C. albicans samples were tested for susceptibility to voriconazole. The resistance rate was found to range from 1.8% to 54.7%, while for non-albicans Candida species it ranged from 1.7% to 6% (). Overall, C. albicans was significantly more resistant than non-albicans Candida species to voriconazole (104/252 vs 4/115; p < 0.001).
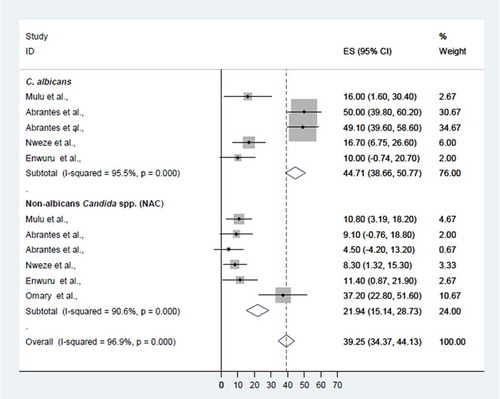
When the data for fluconazole resistance were pooled, the overall fluconazole resistance rate was 39.3% (95% CI 34.4–44.1%), while the rate of fluconazole resistance among C. albicans was significantly higher than that among non-albicans Candida species (44.7%, 95% CI 38.7–50.8% vs 21.9%, 95% CI 15.1–28.7%; p < 0.001) ().
Figure 5. Proportional estimate (ES) of fluconazole resistance by Candida species. The midpoint of each horizontal line segment shows the proportional estimate of fluconazole-resistant Candida species of each study, while the rhombic mark shows the pooled proportions for all studies by Candida species with 95% confidence interval (CI).
Discussion
OC is the leading opportunistic infection among immunocompromised individuals. Sub-Saharan Africa has the world’s highest prevalence of HIV/AIDS patients, with an estimated 24.7 million cases [Citation35]. In the current review, up to 82% of HIV-infected patients were orally colonized by Candida species. A similar prevalence has been reported previously in southern India [Citation36,Citation37] and in North America [Citation38]. The overall prevalence in the current review is much higher than that in previous reports from Italy, Brazil, and China [Citation39–Citation41]. The variations in prevalence across the world are considered to be due to differences in diagnostic techniques, geographic and/or ethnic differences, and oral hygiene [Citation38,Citation39].
Oral Candida colonization among HIV-infected individuals predicted the subsequent development of OC [Citation7,Citation15,Citation42], mainly owing to the impaired immune system in these patients [Citation43]. In the current review, the highest prevalence of OC among HIV-infected populations was 75%, in Ghana. The incidence of OC was considered relatively stable as it was comparable to a review undertaken between 1984 and 2000 [Citation6].
OC has different clinical presentations with diverse histopathological features [Citation44]. In the current review, pseudomembranous candidiasis (or thrush) was the most common clinical presentation of OC among HIV-infected populations in sub-Saharan Africa. Pseudomembranous candidiasis has also been noted as the most common clinical manifestation of acute OC among immunocompromised individuals in the UK [Citation1,Citation45].
Chronic erythematous candidiasis, which is commonly detected in patients wearing dentures [Citation1], was also commonly found in AIDS patients in a study conducted in Ethiopia by Mulu et al. [Citation22]. This clinical form of OC is characterized by localized chronic erythematous tissues on the dorsum of the tongue, palate, or buccal mucosa [Citation1,Citation46]. Among HIV-infected individuals, erythematous candidiasis is associated with the chronic use of corticosteroids and topical and systemic antibiotics [Citation47]. Its increased prevalence has also been associated with the shedding of the pseudomembranes in persistent or acute pseudomembranous candidiasis [Citation46].
In general, among African HIV-infected individuals, non-albicans Candida species contributed about 33.5% of OC. The prevalence of non-albicans Candida species was within the range that was observed in Brazil and New Delhi, India [Citation40,Citation48]. As previously documented in Greece, Spain, and New Zealand [Citation18,Citation49,Citation50], the predominant non-albicans Candida species detected were C. glabrata (24%), C. tropicalis (22%), and C. krusei (11%). The high prevalence of C. glabrata and C. krusei among HIV-infected populations from sub-Saharan Africa is of public health importance because of the fluconazole resistance pattern that is normally associated with these species [Citation4,Citation5,Citation51]. Contrary to previous reports from the USA and Finland, where non-albicans Candida species were commonly detected in co-infection with C. albicans and associated with treatment failure [Citation52,Citation53], in most of the studies in sub-Saharan Africa non-albicans Candida species were sensitive to azole and dual presentation was not reported.
The prevalence of non-albicans Candida species associated with OC has been linked to a history of fluconazole use [Citation25,Citation28]. However, in the current review, the majority of non-albicans Candida species were significantly more sensitive to fluconazole than they were to C. albicans. This could be because the non-albicans Candida species that are intrinsically resistant to fluconazole contributed only 35% of non-albicans Candida species in this review. Therefore, the use of fluconazole may not be the only reason for non-albicans Candida species infection. HIV infection with significant depression of the immune system may contribute to the ability of non-pathogenic non-albicans Candida species to cause OC in this population.
In Africa, fluconazole is considered to be the drug of choice in both the treatment and prophylactic prevention of fungal infections in HIV-infected individuals and people with AIDS [Citation25,Citation28,Citation57]. The use of fluconazole has been associated with the development of resistance [Citation22,Citation25]. This could explain the observed high rate of fluconazole resistance among C. albicans.
It is documented that the overexpression of drug efflux pumps by C. albicans due to inappropriate use of azole antifungals leads to the development of resistance to several azole antifungal agents [Citation58,Citation59]. This could explain the high rate of voriconazole resistance among C. albicans. However, this mechanism does spare amphotericin B [Citation58], which is expensive and not available in most centers in developing countries. This was confirmed in this review, where the rate of amphotericin B resistance was found to range from 0% to 8.5% among C. albicans. With increased inappropriate use of azole antifungal agents [Citation60], resistant strains of C. albicans and non-albicans Candida species could be selected, underscoring the importance of monitoring antifungal resistance and limiting over-the-counter availability of antimycotic drugs.
Despite the good-quality data summarized in this review, differences in diagnostic techniques and incomplete data reported by most of the studies may have compromised the findings. Most of the studies did not report the HIV disease stage, the use of antiretrovirals, or trimethoprim/sulphamethoxazole prophylaxis. All these factors are known to have an effect on the manifestation of OC.
In conclusion, about one-quarter of the cases of OC among HIV-infected individuals in sub-Saharan Africa are due to non-albicans Candida species. In HIV-infected individuals, C. albicans was more resistant than non-albicans Candida species to fluconazole and voriconazole. There is a need to strengthen the capacity for fungal diagnosis and antifungal susceptibility testing in sub-Saharan African in order to be able to track the resistance trend of Candida species in developing countries. Data from these centers will be used to guide the appropriate use of azoles so that they can be preserved for future generations.
Acknowledgments
The authors acknowledge the contribution of Well Cornell Medical Library through Mr Yanga Machumi and the Institute of Medical Microbiology, University Medical Center Göttingen, Germany, for support in accessing the full articles.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
Martha F. Mushi
Martha F. Mushi, B.Sc, M.Sc is a Lecturer of Microbiology and Immunology and a consultant medical Microbiologist at Catholic University of Health and Allied Sciences and Bugando Medical Centre. She is the chairperson of MYCAFRICA; a network to promote medical mycology in Africa. She has published over 30 publications in the field of infectious diseases. Currently she is a PhD student at CUHAS focus in the epidemiology of azole resistant Candida and Aspergillus species in Tanzania.
Oliver Bader
Dr. Oliver Bader heads the mycology group at the Institute for Medical Microbiology at the University Medical Center Göttingen. His research interests include the epidemiology, mechanisms and diagnostics of fungal infections and their drug susceptibility patterns.
Liliane Taverne-Ghadwal
Dr. med. Liliane Taverne-Ghadwal: She has been trained at the Institute for Medical Microbiology, University Medical Centre Göttingen. She has interest on the epidemiology and diagnostics of fungal infections and has written her doctoral thesis on oral mycosis in HIV patients from Chad. Besides that from 2009 she is has been working and being trained as an anesthesiologist at the Medical University Göttingen and since 2015 at the Evangelical Klinikum Bethel in Bielefeld.
Christine Bii
Dr Christine C. Bii, BSc, MSc, PhD Medical Mycology is a Principle Research Officer at the Center for Microbiology Research, Kenya Medical Research Institute, Nairobi Kenya. She heads the Medical Mycology Research in the Institute and her focus is in opportunistic fungal infections and emerging antifungal resistance. She has published widely in the area of Cryptococcus, Candida, PCP and mycotoxins and mentored over 50 post graduate students.
Uwe Groß
Prof. Dr. med. Uwe Groß is Head of the Institute for Medical Microbiology of the University Medical Center Goettingen, Germany. He is coordinating the Goettingen International Health Network that is cooperating with partners from sub-Saharan Africa, South-East Asia and South America in the field of infectious diseases. He has published several research papers on the epidemiology, diagnosis and pathogenesis of infections caused by bacteria, fungi, and parasites.
Stephen E. Mshana
Prof. Stephen E. Mshana, MD, M.Med, PhD, Fell Med. Ed: Professor of Clinical Microbiology and Consultant Clinical Microbiologist at the Catholic University of Health and Allied Sciences (CUHAS)/Bugando Medical Centre (BMC), Mwanza Tanzania. Throughout his career, Prof. Mshana has contributed to and co-authored more than 100 scientific articles on the field of clinical microbiology focus mainly on antimicrobial resistance.
References
- Akpan A, Morgan R. Oral candidiasis. Postgrad Med J. 2002;78:1–10.
- Feigal DW, Katz MH, Greenspan D, et al. The prevalence of oral lesions in HIV-infected homosexual and bisexual men: three San Francisco epidemiological cohorts. Aids. 1991;5:519–526.
- Dupont B, Graybill J, Armstrong D, et al. Fungal infections in AIDS patients. J Med Vet Mycol. 1992;30:19–28.
- Thanyasrisung P, Kesakomol P, Pipattanagovit P, et al. Oral Candida carriage and immune status in Thai human immunodeficiency virus-infected individuals. J Med Microbiol. 2014;63:753–759.
- Meurman J, Siikala E, Richardson M, et al. Non-Candida albicans Candida yeasts of the oral cavity. In: Mendez-Vilas A., editor. Communicating current research and educational topics and trends in applied microbiology. Microbiology book series. Badajoz: 2007. p. 719–731.
- Hodgson T, Rachanis C. Oral fungal and bacterial infections in HIV‐infected individuals: an overview in Africa. Oral Dis. 2002;8:80–87.
- Guida R. Candidiasis of the oropharynx and esophagus. Ear Nose Throat J. 1988;67:834-836, 838-840.
- Sangeorzan JA, Bradley SF, He X, et al. Epidemiology of oral candidiasis in HIV-infected patients: colonization, infection, treatment, and emergence of fluconazole resistance. Am J Med. 1994;97:339–346.
- Bastiaan RJ, Reade PC. The prevalence of Candida albicans in the mouths of tobacco smokers with and without oral mucous membrane keratoses. Oral Surg Oral Med Oral Pathol. 1982;53:148–151.
- Mushi MF, Mtemisika CI, Bader O, et al. High oral carriage of non-albicans Candida spp. among HIV-infected individuals. Int J Infect Dis. 2016;49:185–188.
- Kuhn DM, Mukherjee PK, Clark TA, et al. Candida parapsilosis characterization in an outbreak setting. Emerg Infect Dis. 2004;10:1074–1081.
- Li L, Redding S, Dongari-Bagtzoglou A. Candida glabrata, an emerging oral opportunistic pathogen. J Dent Res. 2007;86:204–215.
- Mayanja B, Morgan D, Ross A, et al. The burden of mucocutaneous conditions and the association with HIV‐1 infection in a rural community in Uganda. Trop Med Int Health. 1999;4:349–354.
- Matee M, Scheutz F, Moshy J. Occurrence of oral lesions in relation to clinical and immunological status among HIV‐infected adult Tanzanians. Oral Dis. 2000;6:106–111.
- Nweze EI, Ogbonnaya UL. Oral Candida isolates among HIV-infected subjects in Nigeria. J Microbiol Immunol Infect. 2011;44:172–177.
- Kwamin F, Nartey NO, Codjoe FS, et al. Distribution of Candida species among HIV-positive patients with oropharyngeal candidiasis in Accra, Ghana. J Infect Dev Ctries. 2013;7:041–045.
- Dos Santos Abrantes PM, McArthur CP, Africa CWJ. Multi-drug resistant oral Candida species isolated from HIV-positive patients in South Africa and Cameroon. Diagn Microbiol Infect Dis. 2014;79:222–227.
- Belazi M, Velegraki A, Koussidou‐Eremondi T, et al. Oral Candida isolates in patients undergoing radiotherapy for head and neck cancer: prevalence, azole susceptibility profiles and response to antifungal treatment. Oral Microbiol Immunol. 2004;19:347–351.
- White TC, Marr KA, Bowden RA. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin Microbiol Rev. 1998;11:382–402.
- Fournier P, Schwebel C, Maubon D, et al. Antifungal use influences Candida species distribution and susceptibility in the intensive care unit. J Antimicrob Chemother. 2011;66:2880–2886.
- Moran GP, Sullivan DJ, Coleman DC. Emergence of non-Candida albicans Candida species as pathogens. Washington (DC): Candida and candidiasis ASM Press; 2002. p. 37–53.
- Mulu A, Kassu A, Anagaw B, et al. Frequent detection of ‘azole’ resistant Candida species among late presenting AIDS patients in northwest Ethiopia. BMC Infect Dis. 2013;13:82.
- Owotade FJ, Patel M, Ralephenya TR, et al. Oral Candida colonization in HIV-positive women: associated factors and changes following antiretroviral therapy. J Med Microbiol. 2013;62:126–132.
- Nanteza M, Tusiime JB, Kalyango J, et al. Association between oral candidiasis and low CD4+ count among HIV positive patients in Hoima Regional Referral Hospital. BMC Oral Health. 2014;14:143.
- Hamza OJ, Matee MI, Moshi MJ, et al. Species distribution and in vitro antifungal susceptibility of oral yeast isolates from Tanzanian HIV-infected patients with primary and recurrent oropharyngeal candidiasis. BMC Microbiol. 2008;8:1.
- Esebelahie NO, Enweani IB, Omoregie R. Candida colonisation in asymptomatic HIV patients attending a tertiary hospital in Benin City, Nigeria. Libyan J Med. 2013;18(8):20322.
- Owotade FJ, Patel M. Virulence of oral Candida isolated from HIV-positive women with oral candidiasis and asymptomatic carriers. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;118:455–460.
- Enwuru C, Ogunledun A, Idika N, et al. Fluconazole resistant opportunistic oro-pharyngeal candida and non-candida yeast-like isolates from HIV infected patients attending ARV clinics in Lagos, Nigeria. Afr Health Sci. 2008;8:142–148.
- Agwu E, Ihongbe JC, McManus BA, et al. Distribution of yeast species associated with oral lesions in HIV-infected patients in Southwest Uganda. Med Mycol. 2012;50:276–280.
- Yitayew B, Woldeamanuel Y, Asrat D, et al. Oral Candida carriage among HIV infected and non-‐infected individuals in Tikur Anbesa specialized hospital. Addis Ababa, Ethiopia: GJMEDPH; 2015;4:2.
- Pfaller M, Espinel-Ingroff A, Canton E, et al. Wild-type MIC distributions and epidemiological cutoff values for amphotericin B, flucytosine, and itraconazole and Candida spp. as determined by CLSI broth microdilution. J Clin Microbiol. 2012;50:2040–2046.
- Pfaller M, Diekema D, Rex J, et al. Correlation of MIC with outcome for Candida species tested against voriconazole: analysis and proposal for interpretive breakpoints. J Clin Microbiol. 2006;44:819–826.
- Messer SA, Diekema DJ, Hollis RJ, et al. Evaluation of disk diffusion and Etest compared to broth microdilution for antifungal susceptibility testing of posaconazole against clinical isolates of filamentous fungi. J Clin Microbiol. 2007;45:1322–1324.
- Brito GNB, Inocêncio AC, Querido SMR, et al. In vitro antifungal susceptibility of Candida spp. oral isolates from HIV-positive patients and control individuals. Braz Oral Res. 2011;25:28–33.
- Barchiesi F, Maracci M, Radi B, et al. Point prevalence, microbiology and fluconazole susceptibility patterns of yeast isolates colonizing the oral cavities of HIV-infected patients in the era of highly active antiretroviral therapy. J Antimicrob Chemother. 2002;50:999–1002.
- Girish Kumar C, Menon T, Rajasekaran S, et al. Carriage of Candida species in oral cavities of HIV infected patients in South India. Mycoses. 2009;52:44–48.
- Jeddy N, Ranganathan K, Devi U, et al. A study of antifungal drug sensitivity of Candida isolated from human immunodeficiency virus infected patients in Chennai, South India. J Oral Maxillofac Pathol. 2011;15:182.
- Vargas KG, Joly S. Carriage frequency, intensity of carriage, and strains of oral yeast species vary in the progression to oral candidiasis in human immunodeficiency virus-positive individuals. J Clin Microbiol. 2002;40:341–350.
- Campisi G, Pizzo G, Milici ME, et al. Candidal carriage in the oral cavity of human immunodeficiency virus–infected subjects. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;93:281–286.
- Junqueira JC, Vilela SF, Rossoni RD, et al. Oral colonization by yeasts in HIV-positive patients in Brazil. Rev Inst Med Trop Sao Paulo. 2012;54:17–24.
- Liu X, Liu H, Guo Z, et al. Association of asymptomatic oral candidal carriage, oral candidiasis and CD4+ lymphocyte count in HIV‐positive patients in China. Oral Dis. 2006;12:41–44.
- Lott TJ, Holloway BP, Logan DA, et al. Towards understanding the evolution of the human commensal yeast Candida albicans. Microbiology. 1999;145:1137–1143.
- Samaranayake L, MacFarlane T. Host factors and oral candidosis. Oral Candidosis. 1990;66–103.
- Feller L, Khammissa R, Chandran R, et al. Oral candidosis in relation to oral immunity. J Oral Pathol Med. 2014;43:563–569.
- Samaranayake L. Nutritional factors and oral candidosis. J Oral Pathol Med. 1986;15:61–65.
- Lehner T. Oral candidosis. Dent Pract Dent Rec. 1967;17:209–216.
- Scully C, Ei-Kabir M, Samaranayake LP. Candida and oral candidosis: a review. Crit Rev Oral Biol Med. 1994;5:125–1257.
- Maheshwari M, Kaur R, Chadha S. Candida species prevalence profile in HIV seropositive patients from a major tertiary care hospital in New Delhi, India. J of Pathogens. 2016;2016.
- Dronda F, Alonso-Sanz M, Laguna F, et al. Mixed oropharyngeal candidiasis due to Candida albicans and non-albicans Candida strains in HIV-infected patients. Europ J Clin Microbiol Infect Dis. 1996;15:446–4452.
- Cannon R, Chaffin W. Oral colonization by Candida albicans. Crit Rev Oral Biol Med. 1999;10:359–383.
- Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the infectious diseases society of America. Clinical Infect Dis. 2015;civ933.
- Cartledge J, Midgley J, Gazzard B. Non-albicans oral candidosis in HIV-positive patients. J Antimicrob Chemother. 1999;43:419–422.
- Meurman J, Siikala E, Richardson M, et al. Non-Candida albicans Candida yeasts of the oral cavity. Commun Curr Res Educ Top Trends Appl Microbiol. 2007;1:719–731.
- Wayne P. Clinical and Laboratory Standards Institute: reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard. In: CLSI document M27-A3 and Supplement S.3.2008
- Barrio EE, Ruesga M, Vidal MV, et al. Comparative evaluation of ATB Fungus 2 and Sensititre YeastOne panels for testing in vitro Candida antifungal susceptibility. Revista Iberoamericana De Micología. 2008;25:3–6.
- Arendrup MC, Garcia-Effron G, Lass-Flörl C, et al. Echinocandin susceptibility testing of Candida species: comparison of EUCAST EDef 7.1, CLSI M27-A3, Etest, disk diffusion, and agar dilution methods with RPMI and isosensitest media. Antimicrob Agents Chemother. 2010;54:426–439.
- Maenza JR, Keruly JC, Moore RD, et al. Risk factors for fluconazole-resistant candidiasis in human immunodeficiency virus-infected patients. J Infect Dis. 1996;173:219–225.
- Albertson GD, Niimi M, Cannon RD, et al. Multiple efflux mechanisms are involved in Candida albicans fluconazole resistance. Antimicrob Agents Chemother. 1996;40:2835–2841.
- Niimi M, Firth NA, Cannon RD. Antifungal drug resistance of oral fungi. Odontology. 2010;98:15–25.
- Mushi MF, Masewa B, Jande M, et al. Prevalence and factor associated with over-the-counter use of antifungal agents’, in Mwanza City, Tanzania. Tanzania J of Health Res. 2017;19.1