?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Objective: Refractory infection is an important factor affecting the progression of medication-related osteonecrosis of the jaw (MRONJ) from clinical stage I to stage II/III. The aim of this study was to explore the distribution of bacteria and their association with the inflammatory pathway of stage II/III MRONJ.
Materials and Methods: Nine specimens of fresh inflammation tissue, located next to the necrotic bone or sequestrum, were collected from MRONJ patients. Nine specimens from normal oral mucosa were collected from healthy patients. The 16S rRNA gene sequencing method was used to determine the distribution characteristics of the bacterial colony. The protein microarray analysis was used to detect the expression of inflammatory cytokines.
Results: The average relative abundance of Bacteroidetes, Spirochaetes, Synergistetes, and Tenericutes was higher, while Proteobacteria and Actinobacteria were lower in the MRONJ group. Most pro-inflammatory cytokines were up-regulated in the MRONJ group; yet, only IFNγ, TNFα, and IL8 showed statistical differences (P < 0.05). Porphyromonas and Treponema were positively correlated with IL8, and Mogibacterium was positively correlated with IFNγ and TNFα.
Conclusions: IL8/IFNγ/TNFα pro-inflammatory effect caused by Porphyromonas, Treponema, and Mogibacterium may be the leading cause of advancing MRONJ and thus may be used as a new target for infection control.
Introduction
Medication-related osteonecrosis of the jaw (MRONJ) is the progressive death of the jaw caused by long-term application of antiresorptive and/or antiangiogenic drugs. Diagnosis of MRONJ is performed based on patient’s pharmacological history as well as clinical and radiographic features (judgment of the jawbone or mucosal or cutaneous fistula that perforated the jawbone) [Citation1]. Low bone turnover, bone and soft tissue toxicity, microfractures, inflammation, and infection are all possible causes of MRONJ [Citation2–4]. Microorganisms in the oral cavity can directly affect the jawbone through an odontogenic infection, like pulpitis or periodontitis [Citation5]. Previous studies have suggested that surgical and dental treatment may promote bacteria colonization and further stimulate the immune response [Citation6].
So far, many preclinical and clinical studies have examined the association between bacterial infection and systemic antiresorptive drugs and ONJ [Citation7,Citation8]. Patients with MRONJ have significantly higher oral bacteria count compared to healthy people, even after receiving systemic antibiotic therapy [Citation9]. Previous studies have found that genus Streptococcus is highly prevalent in all patients with periodontal disease with or without a history of bisphosphonate (BP) therapy and patients with MRONJ, while genera of Parvimonas, Fusobacterium, Eubacterium, Gemella, Leptotrichia, and Selenomonas have been reported to have a higher frequency in MRONJ cohort [Citation10]. Animal studies have suggested that the abolishment of pathogenic bacteria, such as Fusobacterium nucleatum with broad-spectrum antibiotics, may speed up the epithelial healing in an animal model of MRONJ [Citation11].
The infection symptoms evidenced by pain and erythema in the exposed bone region, with or without purulent drainage in MRONJ patients, are important indicators of clinical stage I and II/III stages. Yet, the standard therapeutic regimen for controlling infection is mainly based on empirical medication with long-term administration, which may be harmful to the patients and further aggravate their condition [Citation12]. Bacterial infections of jawbone lesions in MRONJ patients often recur shortly after discontinuation of antibiotics [Citation13]. Most MRONJ patients with infection symptoms require surgical treatment, causing bone defects, occlusal dysfunction, and other problems.
In this study, we focused on the deep biopsies of MRONJ lesions next to the sequestrum in an advanced stage (II/III). Bacteria from the samples were then analyzed using 16S rRNA sequencing data. The expression levels of ten kinds of inflammatory cytokines were detected by inflammatory factors protein chip. The correlation between these bacteria and related molecular pathways of inflammation was also analyzed and verified using correlation analysis.
Materials and methods
Tissue collection
A total of 9 consecutive patients (age range: 49–80) with MRONJ (experimental group) admitted to the Department of Oral and Maxillofacial Surgery, Peking University School and Hospital of Stomatology (PKUSHS) between December 2018 and April 2019 were recruited in the study. The inclusion criteria were the following: 1) the MRONJ was diagnosed according to the American Association of Oral & Maxillofacial Surgeons definition [1]; 2) patients who have not used antibiotics systematically and have not used mouthwash containing antibiotics within one month before specimen collection; 3) those who signed the informed consent that was reviewed and approved by the Ethical Committee of PKUSHS (PKUSSIRB-201,949,114). Nine patients with jawbone lesions without infection were used as a control group (ages 29–80).
Biopsies were collected from fresh inflammation tissue located next to the necrotic bone or sequestrum (Supplementary Figure 1). Fresh tissue was then placed in a sterile vial on ice and stored in liquid nitrogen (−80°C) until further use.
DNA extraction
DNA was extracted from each mucosa sample using an improved protocol based on the manual of QIAamp Fast DNA Stool Mini Kit (Qiagen, Germany). In detail, 1 ml of InhibitEX Buffer and a proper amount of glass beads (0.5 mm diameter, Qiagen) was added to each 200 mg of the mucosa. The mixture was homogenized and shaken for 1 min twice with a Homogeneous instrument (FASTPREP-24, Aosheng Biotech, China). Afterward, the DNA purification was performed according to the manufacturer’s instructions.
16S rRNA gene sequencing
The V3-V4 region of the bacteria 16S ribosomal RNA genes was amplified using barcoded primers 341 F 5ʹ-CCTACGGGRSGCAGCAG-3ʹ and 806 R 5ʹ-GGACTACVSGGGTATCTAAT-3ʹ [Citation14], using the following PCR conditions: 95°C 3 min, followed by 30 cycles at 98°C for 20s, 58°C for 15s, and 72°C for 20s and a final extension at 72°C for 5 min. PCR reactions were performed in 30 μL mixture containing 15 μL of 2× KAPA Library Amplification ReadyMix, 1 μL of each primer (10 μM), 50ng of template DNA, and ddH2O. Negative controls consisting of empty sterile storage tubes were processed for DNA extraction, amplification was performed using the same procedures, and reagents were used for the mucosa samples. There was no detectable amplification in the negative controls.
Amplicons were extracted from 2% agarose gels and purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, USA) according to the manufacturer’s instructions and quantified using Qubit®2.0 (Invitrogen, USA). All quantified amplicons were pooled to equalize sequencing concentrations using Illumina MiSeq/PE250 (Illumina, Inc., CA, USA). The paired-end reads of 425bp were overlapped on their 3ʹ ends for concatenation into original longer tags by using PANDAseq (https://github.com/neufeld/pandaseq, version 2.9). DNA extraction, sequencing and library construction and sequencing were conducted at Realbio Genomics Institute (Shanghai, China).
Process of sequencing data
Assembled tags, trimmed of barcodes and primers, were further checked on their rest lengths and average base quality. A 16S tags were restricted between 220 bp and 500 bp, such that the average Phred score of bases was > 20 (Q20) and there were not more than three ambiguous N. The copy number of tags were enumerated, and redundant tags were removed. Only the tags with a frequency >1, which tend to be more reliable and had a representative tag, were clustered into Operational Taxonomic Units (OTUs). OTUs were clustered with 97% similarity using UPARSE (http://drive5.com/uparse/). Chimeric sequences were identified and removed using Usearch (version 7.0.1090). Each representative tag was assigned to taxa by RDP Classifer (http://rdp.cme.msu.edu/) against the RDP database (http://rdp.cme.msu.edu/) using a confidence threshold of 0.8. OTU profiling table and alpha diversity analyses were achieved by python scripts of QIIME (version 1.9.1).
Protein microarray analysis
Human protein antibody arrays (Quantibody® Human Inflammation Array 1, Cat. # QAH-INF-1, RayBiotech, Inc., Guangzhou, China) were provided by RayBiotech. There were both positive control spots and negative control spots on the array. The positive control spots were standardized amounts of biotinylated IgGs printed directly onto the array. The signal intensity data was calculated as the fluorescence intensity minus local background and normalized to the positive control signals. The significance of the differences in the expression of ten cytokines between MRONJ samples and control samples were evaluated via protein microarray analysis. The expression ratio represents the tendency of a difference in protein expression.
Statistical analysis
The statistical analysis was performed by GraphPad Prism 8 with a two-tailed Student’s t- test. All data were presented as mean ± SD.Pearson’s correlation analysis was used to examine associations between cytokines andbacteria at the genus level using the corrplot package in R. Values of P < 0.05 were considered to be statistically significant.
Results
A total of 18 patients, including 9 patients with MRONJ and 9 patients in the control group, were enrolled in this study. The mean age of the two groups was 62.8 years and 53.9 years, respectively. There were more females than males in both groups. Underlying diseases were osteoporosis (1) and cancer (8) in the MRONJ group, and cystic lesion of the jawbone (6) and ameloblastoma (3) in the control group. In the MRONJ group, seven patients were in Stage II and two patients in Stage III. As for the location of MRONJ, a mandible (7) presented more lesions than a maxilla (2). The patients’ basic information are shown in and Supplementary Table 1.
Table 1. Demographics of the MRONJ study population
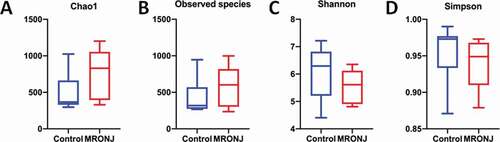
16S rRNA gene amplicons were sequenced from the 18 qualified samples. After processing, 447,587 sequences were generated (mean, 24,866 1799; range, 21,396 to 27,212). Using the minimum (21,396) and a 97% similarity level, OTUs were subsampled and detected. The alpha diversity analysis included community richness (Chao1 and observed species) and community evenness (Shannon index and Simpson index). In general, the community richness in the MRONJ group was higher than in the control group, while the community evenness was lower; however, the four indexes showed no statistical differences ().
Figure 1. The α-diversity indices of the bacterial composition and distribution within the oral mucosal microenvironment were not different in patients with MRONJ and healthy controls. The community richness (A, B) and community evenness (C, D)
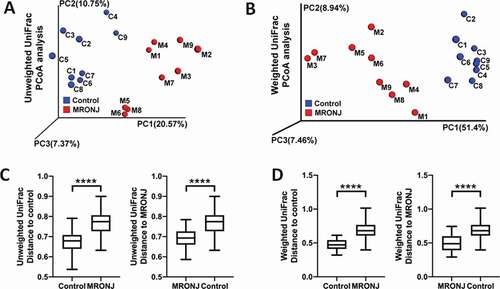
Beta diversity was analyzed to measure the differences in diversity of genera between the two groups. PCoA results showed that the MRONJ group could be easily discriminated from the control group based on both weighted unifrac and unweighted unifrac (). Further, the results of permutational multivariate analysis of variance (PERMANOVA) indicated significant differences between the two groups (P < 0.001, ).
Figure 2. The β diversity of microbiota and the key bacterial alteration within the oral mucosal microenvironment were significantly different in patients with MRONJ and healthy controls. Unweighted and Weighted PCoA results (A, B) and Unifrac ANOSIM analysis (C, D)
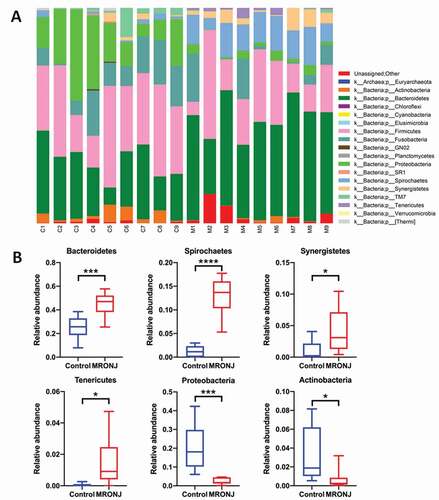
The relative abundance of microbial taxa characterized the microbial community composition. Eighteen phyla, 29 classes, 50 orders, 88 families, and 127 genera were detected. At the phylum level, the main genera were Bacteroidetes, Firmicutes, Proteobacteria, Fusobacteria, and Spirochaetes ()), which occupied about 90% of the microbial community composition. Compared with the control group, the average relative abundance of Bacteroidetes, Spirochaetes, Synergistetes, and Tenericutes was higher in the MRONJ group, while Proteobacteria and Actinobacteria were lower ()).
Figure 3. The microbial community composition was characterized by the relative abundance of microbial taxa. (A) The relative abundance of bacterial taxa at the phylum level in each patient. (B) The relative abundance of Bacteroidetes, Spirochaetes, Synergistetes, Tenericutes, Proteobacteria, and Actinobacteria between the MRONJ and healthy groups were detected using real-time PCR. *, P < 0.05;**, P < 0.01;***, P < 0.001
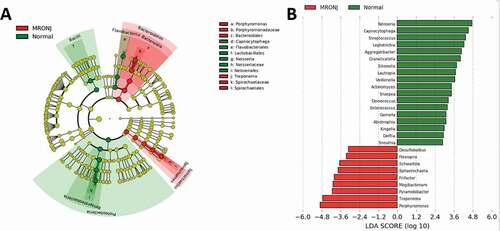
LEfSe (Linear discriminant analysis Effect Size) analysis, which detected differences in the predominance of bacteria communities between the two groups, was used to further investigate the microbiota changes. Generally, the classification tree exhibited the hierarchical relationship among all taxonomic units from phylum to genus (from inner to outer). The genera of the top 20 relative abundances were marked in ). At the genus level, 27 kinds of bacteria showed significant differences between the two groups (LDA score > 3.0, P< 0.05; ).
Figure 4. Taxonomic differences of fecal microbiota in PD and healthy groups. (A) Cladogram generated by LEfSe analysis. B, Linear discriminant analysis (LDA) effect size (LEfSe) analysis revealed significant bacterial differences in microbiota between the MRONJ and healthy controls. The LDA scores (log10)>3 and P < 0.05 are listed
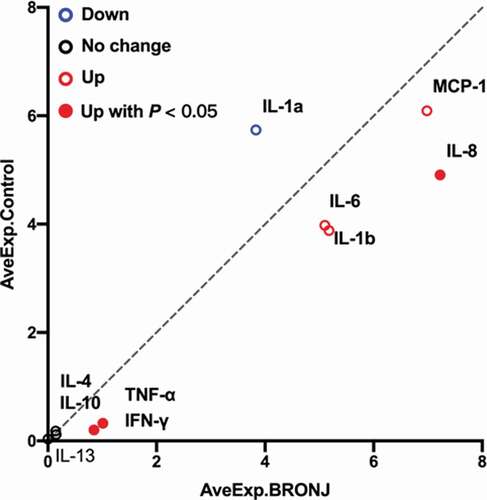
The expression levels of ten cytokines in the 18 samples were detected by using the Quantibody® Human Inflammation Array 1. As shown in , IFNγ, TNFα, IL1β, IL6, IL8, and MCP1 were up-regulated, and IL1α was down-regulated in the MRONJ group; there were no differences in IL4, IL10, and IL13 between groups. However, only IFNγ, TNFα, and IL8 showed statistical differences (P < 0.05) ().
Figure 5. Scatter plot generated from protein microarray data reflecting differential expression proteins (DEPs) between the MRONJ and healthy groups. The basic statistics used for significance analysis are moderated t-statistic. Red presents up-regulation, blue presents down-regulation, and black shows no difference
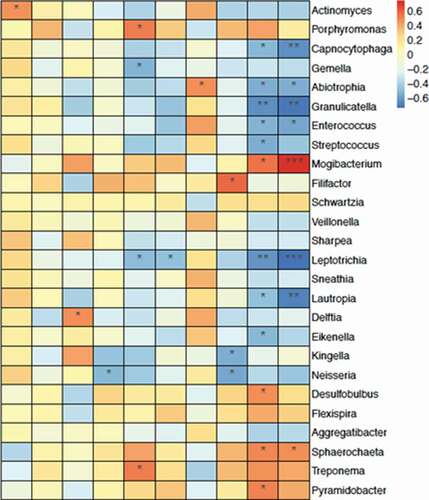
To investigate the relationship between cytokines andbacteria at the genus level, correlation analysis was performed by setting the expression levels of ten cytokines as environmental factors. Among the three differently expressed cytokines, IL8 was positively correlated with Porphyromonas and Treponema, and negatively correlated with Leptotrichia. Both IFNγ and TNFα were positively correlated with Mogibacterium, and negatively correlated with Granulicatella. These five genera possessed a significantly different relative abundance between the MRONJ group and the control group ().
Discussion
Premedication dental evaluation to complete periodontitis and periapical periodontitis treatment or the removal of unretained teeth before chemotherapy are effective preventive strategies for reducing the incidence of MRONJ [Citation15]. Alveolar bone inflammation caused by substantial microorganisms in the oral cavity contributes to the occurrence of MRONJ. Tooth extraction is the most common local risk factor associated with MRONJ, and teeth sockets are usually filled with bacteria and inflammatory cells after tooth extraction [Citation6]. Impaired angiogenesis that results from the indirect effect of antiresorptive drugs or a direct effect of antiangiogenic drugs, combined with local bacteria-based inflammation status, can easily interfere with the growth and migration of gingival epithelial cells by down-regulating the production of keratinocyte growth factor, therefore delaying the healing process [Citation11]. The application of antibiotics often temporarily relieves acute inflammation, which indicates that the inflammatory state caused by bacteria has an essential impact on MRONJ disease. In patients with MRONJ, when these specimens were located deeply, next to the necrotic bone or sequestrum, they were less affected by the oral flora, reducing the noise signal of sequencing and obtaining more realistic results of MRONJ disease-related bacteria. Thus, obtaining bacterial information and the corresponding inflammatory state in the deeper part of the MRONJ lesion may help develop a more accurate and effective anti-infective treatment against MRONJ.
According to De Ceulaer et al., MRONJ could be considered as a bisphosphonate-induced Actinomyces infection [Citation16]. Most specimens (83/101) from oncological patients who had the histological confirmation of MRONJ revealed the presence of Actinomyces infection [Citation17]. In this study, we found different bacteria distribution in MRONJ lesions and normal oral mucosa. We discovered that Porphyromonas, Treponema, and Mogibacterium detected in necrotic bone were associated with the progression of MRONJ [Citation18,Citation19]. A previous study used Porphyromonas in combination with alendronate to establish bisphosphonate-related osteonecrosis of the jaw (BRONJ) model in rats, which further suggested these specific bacteria might be a possible risk factor for MRONJ [Citation20]. Treponema is a constituent of healthy oral flora, which has a vital role in periodontal disease etiology and pathogenesis; its reduction prompts the disorder of microbiota [Citation21]. Mogibacterium is a pathogenic anaerobe that has been associated with acute dental abscess [Citation22]. Granulicatella is one of the common health-associated commensals [Citation23]. The changing trends of the relative abundance of these genera observed in the present study were consistent with the occurrence of MRONJ.
Previous studies revealed that the removal of pre-existing periodontal inflammatory conditions reduces local inflammation levels, including IL1β, IL6, and IL17. Moreover, improving the local condition of periodontitis before teeth extraction could ameliorate MRONJ in animal model research [Citation24]. Elevation of pro-inflammatory cytokines, including TNF-α IFN-γ and IL-1β, and soluble Sema4D production accompanied by Sema4D-expressing γδT cells were detected in mouse MRONJ-like lesions [Citation25]. It has also been reported that IL17 increased in the serum in the anti-resorptive treatment of minipigs. After allogeneic bone marrow, mesenchymal stem cell (BMMSC) transplantation via intravenous infusion, mucosal healing, and bone reconstruction was observed [Citation26]. The immunomodulation potential of BMMSCs may cause the systematic suppression of the proinflammation status. This study found that Porphyromonas and Treponema were positively correlated with IL8, and Mogibacterium was positively correlated with both IFNγ and TNFα. It was previously reported that pretreatment of macrophage-like cells with alendronate, which altered the production of TLR ligand-induced monocyte chemoattractant protein-1 (MCP-1), may help explain why MCP-1, one of the markers expressed by Porphyromonas gingivalis, was not significantly increased in MRONJ [Citation27].
This study comprehensively described the bacterial flora of drug-related jaw bone necrosis disease, especially in advanced-stage patients, and the utilization of protein microarray to detect inflammatory factors that have an essential role during the MRONJ pathogenesis. The IL8/IFNγ/TNFα pro-inflammatory effect caused by Porphyromonas, Treponema, and Mogibacterium may be the leading cause of advancing MRONJ. Our data further the understanding of the intrinsic bacterial-inflammatory mechanism and reveal a new target for infection control of MRONJ disease.
Supplemental Material
Download Zip (107.6 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Ruggiero SL, Dodson TB, Fantasia J, et al. Association of, S. Maxillofacial, American association of oral and maxillofacial surgeons position paper on medication-related osteonecrosis of the jaw–2014 update. J Oral Maxillofac Surg. 2014;72(10):1938–7.
- Saia G, Blandamura S, Bettini G, et al. Occurrence of bisphosphonate-related osteonecrosis of the jaw after surgical tooth extraction. J Oral Maxillofac Surg. 2010;68(4):797–804.
- Rao NJ, Wang JY, Yu RQ, et al. Role of periapical diseases in medication-related osteonecrosis of the jaws. Biomed Res Int. 2017;2017:1560175.
- Li CL, Lu WW, Seneviratne CJ, et al. Role of periodontal disease in bisphosphonate-related osteonecrosis of the jaws in ovariectomized rats. Clin Oral Implants Res. 2016;27(1):1–6.
- Song M, Alshaikh A, Kim T, et al. Preexisting periapical inflammatory condition exacerbates tooth extraction-induced bisphosphonate-related osteonecrosis of the jaw lesions in mice. J Endod. 2016;42(11):1641–1646.
- Schipmann S, Metzler P, Rossle M, et al. Osteopathology associated with bone resorption inhibitors - which role does Actinomyces play? A presentation of 51 cases with systematic review of the literature. J Oral Pathol Med. 2013;42(8):587–593.
- Aghaloo TL, Kang B, Sung EC, et al. Periodontal disease and bisphosphonates induce osteonecrosis of the jaws in the rat. J Bone Miner Res. 2011;26(8):1871–1882.
- Bolette A, Lecloux G, Rompen E, et al. Influence of induced infection in medication-related osteonecrosis of the jaw development after tooth extraction: A study in rats. J Craniomaxillofac Surg. 2019;47(2):349–356.
- De Bruyn L, Coropciuc R, Coucke W, et al. Microbial population changes in patients with medication-related osteonecrosis of the jaw treated with systemic antibiotics. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018;125(3):268–275.
- Pushalkar S, Li X, Kurago Z, et al. Oral microbiota and host innate immune response in bisphosphonate-related osteonecrosis of the jaw. Int J Oral Sci. 2014;6(4):219–226.
- Mawardi H, Giro G, Kajiya M, et al. A role of oral bacteria in bisphosphonate-induced osteonecrosis of the jaw. J Dent Res. 2011;90(11):1339–1345.
- Akashi M, Kusumoto J, Takeda D, et al. A literature review of perioperative antibiotic administration in surgery for medication-related osteonecrosis of the jaw. Oral Maxillofac Surg. 2018;22(4):369–378.
- Ji X, Pushalkar S, Li Y, et al. Antibiotic effects on bacterial profile in osteonecrosis of the jaw. Oral Dis. 2012;18(1):85–95.
- Wang Y, Qian PY. Conservative fragments in bacterial 16S rRNA genes and primer design for 16S ribosomal DNA amplicons in metagenomic studies. PLoS One. 2009;4(10):e7401.
- Owosho AA, Liang STY, Sax AZ, et al. Medication-related osteonecrosis of the jaw: an update on the memorial sloan kettering cancer center experience and the role of premedication dental evaluation in prevention. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018;125(5):440–445.
- De Ceulaer J, Tacconelli E, Vandecasteele SJ. Actinomyces osteomyelitis in bisphosphonate-related osteonecrosis of the jaw (BRONJ): the missing link? Eur J Clin Microbiol. 2014;33(11):1873–1880.
- Cerrato A, Zanette G, Boccuto M, et al. Actinomyces and MRONJ: A retrospective study and a literature review. J Stomatol Oral Maxillofac Surg. 2020. DOI:https://doi.org/10.1016/j.jormas.2020.07.012
- Ermer MA, Kottmann SC, Otten JE, et al. In vitro investigation of the antimicrobial effect of three bisphosphonates against different bacterial strains. J Oral Maxillofac Surg. 2018;76(3):553–560.
- Wei X, Pushalkar S, Estilo C, et al. Molecular profiling of oral microbiota in jawbone samples of bisphosphonate-related osteonecrosis of the jaw. Oral Dis. 2012;18(6):602–612.
- Hidaka K, Mikuni-Takagaki Y, Wada-Takahashi S, et al. Low-intensity pulsed ultrasound prevents development of bisphosphonate-related osteonecrosis of the jaw-like pathophysiology in a rat model. Ultrasound Med Biol. 2019;45(7):1721–1732.
- Velusamy SK, Sampathkumar V, Ramasubbu N, et al. Aggregatibacter actinomycetemcomitans colonization and persistence in a primate model. Proc Natl Acad Sci U S A. 2019;116(44):22307–22313.
- Robertson D, Smith AJ. The microbiology of the acute dental abscess. J Med Microbiol. 2009;58(Pt 2):155–162.
- Hong B-Y, Sobue T, Choquette L, et al. Chemotherapy-induced oral mucositis is associated with detrimental bacterial dysbiosis. Microbiome. 2019;7(1):66.
- Kim T, Kim S, Song M, et al. Removal of pre-existing periodontal inflammatory condition before tooth extraction ameliorates medication-related osteonecrosis of the jaw–like lesion in mice. Am J Pathol. 2018;188(10):2318–2327.
- Movila A, Mawardi H, Nishimura K, et al. Possible pathogenic engagement of soluble Semaphorin 4D produced by gamma delta T cells in medication-related osteonecrosis of the jaw (MRONJ). Biochem Biophys Res Commun. 2016;480(1):42–47.
- Li Y, Xu J, Mao L, et al. Allogeneic mesenchymal stem cell therapy for bisphosphonate-related jaw osteonecrosis in Swine. Stem Cells Dev. 2013;22(14):2047–2056.
- Masuda T, Deng X, Tamai R. Mouse macrophages primed with alendronate down-regulate monocyte chemoattractant protein-1 (MCP-1) and macrophage inflammatory protein-1 alpha (MIP-1 alpha) production in response to Toll-like receptor (TLR) 2 and TLR4 agonist via Smad3 activation. Int Immunopharmacol. 2009;9(9):1115–1121.