ABSTRACT
Objectives
This study aims to clarify the effect of ferroptosis by P. gingivalis on periodontal epithelium impairment and potential mechanisms.
Materials and methods
The expression of epithelial junction proteins (CDH1, OCLN, ZO-1), FTL and GPX4 in healthy and periodontitis tissues was analyzed using bioinformatics analysis and validated in vivo. An in vitro model was constructed to evaluate ferroptosis by mitochondria morphology, content of iron and GSH, and level of lipid peroxidation, FTL, GPX4 and SLC7A11. The iron concentration was changed with iron chelator DFO and iron supplementation FAC. The epithelial impairment was assessed by protein expression. To investigate the mechanism, si-MYB (a negative transcription factor of SLC7A11) and GPX4 inhibitor RSL3 were employed.
Results
CDH1, OCLN, ZO-1 and GPX4 expression was decreased, while FTL expression was elevated in periodontitis tissues. Infected cells showed ferroptosis change of the mitochondria with higher level of lipid peroxidation, iron, FTL and lower level of GPX4, GSH, SLC7A11. FAC augmented ferroptosis and weakened epithelial junction, while DFO exhibited a counteractive effect. Silencing MYB rescued SLC7A11, GPX4 and epithelial junction proteins, which was hindered by RSL3.
Conclusions
Our study demonstrated that P. gingivalis weakened the oral epithelial barrier by causing ferroptosis via inhibiting SLC7A11/GSH/GPX4 axis.
Introduction
Periodontitis is a prominent global oral health issue and the leading cause of adult tooth loss [Citation1–3]. Initiated by the dysbiosis of periodontal pathogens like Porphyromonas gingivalis (P. gingivalis) [Citation4], this inflammatory disease severely affects the supporting tissues of the teeth, including the weakening of the superficial epithelial barrier [Citation5,Citation6]. Oral epithelium, recognized as the primary defense mechanism of periodontal tissue, relies on epithelial junction proteins such as cadherin 1 (CDH1), occludin (OCLN) and zonula occludens 1 (ZO-1) to maintain its integrity [Citation7]. Understanding the molecular mechanisms involved in this destructive process is pivotal for the development of novel strategies aimed at protecting periodontal tissues. However, current knowledge falls short of providing a comprehensive elucidation.
Ferroptosis has recently been linked with inflammation as a new clue to unveiling the cellular and molecular basis for tissue damage [Citation8–10]. Ferroptosis is an iron-dependent form of cell death [Citation11], characterized by an excessive accumulation of iron in most affected cells. In response to the elevated intracellular iron levels, excess iron ions are sequestered within ferritin, a major iron storage protein, to maintain iron homeostasis [Citation12]. Therefore, the expression of ferritin can serve as an indicator of iron ion content to some extent. Interestingly, both animal and clinical experiments have shown higher iron content in periodontitis tissue compared to healthy periodontal tissue [Citation13,Citation14]. Moreover, changes in iron content have also been suggested to correlate with the severity of periodontitis [Citation15–17]. These findings provide compelling evidence supporting the hypothesis that ferroptosis may be the underlying mechanism behind periodontal tissue damage caused by periodontitis.
In addition to iron overload, the breakdown of the antioxidant system is a crucial aspect of ferroptosis. The Fenton reaction, triggered by the excessive iron and the coexistence of peroxidating polyunsaturated fatty acids, leads to the destructive peroxidation of cell membrane lipids [Citation11]. Glutathione peroxidase 4 (GPX4), a key antioxidant molecule, plays a vital role in detoxifying lipid hydroperoxides with the assistance of its cofactor glutathione (GSH) [Citation11,Citation18,Citation19]. The synthesis of GSH is limited by the availability of cysteine, which enters cells in its oxidized form, cystine, via the action of the system xc− transporter. The system xc− comprises solute carrier family 7 member 11 (SLC7A11) and solute carrier family 3 member 2 (SLC3A2) [Citation20,Citation21]. The absence of SLC7A11 was found to promote liver damage and cancer cell death associated with ferroptosis [Citation22,Citation23]. Consequently, the inactivation of the SLC7A11-GSH-GPX4 defense pathway can lead to the accumulation of lipid peroxidation and subsequent cell ferroptosis.
To date, the role of ferroptosis has been found in fibroblasts within periodontitis tissue [Citation24] and in cell models induced by P. gingivalis [Citation25–27]. However, there is a lack of evidence regarding whether and how ferroptosis contributes to epithelial junction impairment in periodontitis. In this study, we aimed to investigate the role and potential mechanism of ferroptosis in oral epithelial damage caused by P. gingivalis infection. By uncovering the mechanisms of tissue damage associated with periodontitis, we can gain a better understanding of its pathogenesis and potentially identify novel therapeutic approaches.
Materials and methods
Bioinformatic analysis
To discover the difference of gene expression profile between gingival tissues from patients with periodontitis and healthy control, data were obtained from the NCBI GEO database (https://www.ncbi.nlm.nih.gov/geo/). Two datasets (GSE10334 and GSE16134) were obtained. The data were analyzed with GEO2R to identify differentially expressed genes (DEGs). For the DEGs, we set cut-off criteria as P-value <0.05, and fold change (>1.5 or <−1.5). The correlation between FTL and epithelial junction proteins was performed using Spearman correlation analysis.
Bacterial culture
P. gingivalis ATCC 33277 was routinely cultured [Citation28]. Briefly, P. gingivalis was cultured on tryptone soy broth agar plates and broth medium with yeast, vitamin K and hemin at 37°C in an anaerobic system (80% N2, 10% H2 and 10% CO2). P. gingivalis at logarithmic growth phase was collected by centrifugation at 3500 rpm for 10 min and resuspended to an optical density of 1.0 (OD 600 nm) with cell culture medium.
P. gingivalis-induced experimental periodontitis model
Experimental periodontitis was induced by a ligature/P. gingivalis method. Briefly, 3-week-old SPF class SD male rats (76–100 g) were randomly divided into experimental and control groups. P. gingivalis-soaked 5–0 silk ligatures were ligated sub-gingivally around the left maxillary first molar on day 0. Before ligation, the silk was soaked in bacterial solution at a concentration of 1 × 109 CFU/mL. One week after ligation, the 109 CFU/mL P. gingivalis solution was coated to ligatures every other day for 4 weeks. The mice were then euthanized and sample materials were taken for follow-up experiments.
Micro-computed tomography (μCT)
All maxillary biopsies were fixed in 4% paraformaldehyde were subjected to be scanned by μCT. Three-dimensional digitized images of the palatal view were collected for each sample using reconstruction software, according to the manufacturer’s procedures. Alveolar bone loss was analyzed. Linear measurements were taken from the cemento-enamel junction (CEJ) to the alveolar bone crest (ABC) according to tomographic method.
Histological analysis of periodontal tissue
The maxillae were decalcified in 10% EDTA solution for 4 weeks. Specimens were dehydrated in progressively increased concentration ethanol and embedded in paraffin blocks. Sections were then prepared for hematoxylin and eosin (HE) staining and images were obtained.
Immunohistochemistry analysis
After being deparaffinized, rehydrated and antigen retrieved, the sample slices were quenched with endogenous peroxidase and blocked with 3% bovine serum albumin for 30 min. Then, sections were, respectively, incubated with the following antibodies at 4°C for overnight: primary antibodies of ZO-1, CDH1, OCLN, FTL and GPX4 (Abcam). Incubation with the secondary antibody at room temperature was followed by hematoxylin counterstaining.
Cell culture
Human immortalized oral epithelial cells (HIOECs), an immortalized cell line obtained from the normal oral mucosa, were generously provided by Professor Wantao Chen from Ninth People’s Hospital, Shanghai Jiao Tong University. HIOECs were cultured as previously described [Citation28]. Briefly, the cells were cultured in Defined Keratinocyte-SFM (Gibco) with growth supplement at 37°C with 5% CO2 in a humidified atmosphere.
Immunofluorescent assay
Confluent HIOECs monolayers were washed with PBS and fixed for 30 min at 4°C with 4% paraformaldehyde. After washing three times with PBS, slides were incubated with 1% BSA in PBS for 1 h at room temperature for blocking [Citation29]. The washing was followed by incubation overnight at 4°C with the antibodies ZO-1, CDH1 and OCLN (Abcam). The slides were then incubated with Alexa Fluor 488-conjugated secondary antibody (Proteintech) at 37°C for 2 h. Nucleus of HIOECs were stained with DAPI (Beyotime) for 5 min at room temperature.
Transmission electron microscope (TEM)
HIOECs (2 × 106 cells) were plated in 60-mm culture dishes. Cells were then treated with P. gingivalis (MOI 50) for 24 h. Cells were digested and fixed with 2% glutaraldehyde and 1% osmium tetroxide. The cell samples were then cut into ultrathin sections, stained with 2% uranyl acetate, dehydrated, embedded and stained with lead citrate.
Flow cytometry
To visualize the lipid peroxidation, cells were seeded in 24-well plates and treated with the designed conditions. After different treatments, the cells were stained with 5 μM C11-BODIPY581/591 probe (Dojindo) in accordance with the manufacturer’s instructions. After 30 min at 37°C in the dark, the cells were harvested by trypsin and washed with PBS and the analysis of fluorescence was performed by FACS analysis (BD Biosciences). Data were collected from at least 10,000 cells [Citation30].
Fluorescent probes
To detect intracellular and mitochondrial Fe2+, FerroOrange and Mito-FerroGreen (Dojindo) were used according to the manufacturer’s protocol. HIOECs were in 12-well plates in three replicates and allowed to attach overnight and then washed with PBS for 3 times. Before P. gingivalis infection for 24 h, cells were pretreated with DFO for 2 h. After HBSS washing, the cells were stained with a final concentration of 1 μM FerroOrange or 5 μM Mito-FerroGreen for 30 min at 37°C.
GSH assay
The GSH levels in HIOECs were detected with a Reduced GSH Assay Kit (Nanjing Jiancheng) according to the manufacturer’s instructions. Briefly, after P. gingivalis infection, living HIOECs were harvested with PBS and sonicated, followed by centrifuging the homogenate at 4000 rpm for 10 min at 4°C. Then, the supernatant was collected and mixed with prescribed reagents of the kit at room temperature for 5 min. Finally, a microplate reader was carried out to obtain the absorbance at 405 nm.
Quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from HIOECs using RNAiso Plus reagent (TAKARA) according to the manufacturer’s protocol. Next, the cDNA was reversely transcribed with the kit (Beyotime). Then, qRT-PCR amplification experiments (TAKARA) were carried out in an Applied Biosystems 7500 Real-Time PCR System. The primers (Sangon Biotech) were listed as below: SLC7A11 forward, 5'-GGTCCATTACCAGCCTTTGTACG-3'; SLC7A11 reverse, 5'-AATGTAGCGTCCAACTGCCAG-3'; GPX4 forward, 5'-GAGGCACGACCGAAGTAAACTAC-3'; GPX4 reverse, 5'-CCGAACTGGT-TTCACGGGAA-3'; GAPDH forward, 5'-GAAGGTGAAGGTCCGAGTC-3'; GAPDH reverse, 5'-GAAGATGGTGATGGCATTTC-3'. GAPDH was selected as the endogenous control.
Western blot
HIOECs were lysed using RIPA buffer containing a protease inhibitor, followed by total protein quantitation using a BCA Protein Assay Kit (Beyotime). Equal amounts of protein were loaded and fractionated by SDS/PAGE gels and transferred to PVDF membranes. The membranes were then blocked with 5% skimmed milk and incubated with primary antibodies at 4°C overnight. After incubation of secondary antibody, Odyssey CLX was used to detect the expression of proteins. The primary antibodies used in this study included anti-CDH1, anti-OCLN, anti-SLC7A11, anti-FTL, anti-GPX4 (Abcam) and anti-GAPDH (Proteintech).
RNA interference
MYB, a transcription factor, negatively regulates SLC7A11. The siRNAs that targeted MYB and non-targeting control siRNA (si-NC) and its negative control were designed and synthesized by GenePharma. The sequences of siRNA targeting MYB are listed as follows: sense, 5'-GGACGAACUG-AUUAUGCU ATT-3'; anti-sense, 5'-UAGCAUUAUCAG UGUCGUCCTT-3'. Briefly, HIOECs were transfected with target siRNA or si-NC via Golden Tran-R (Golden Transfer Science and Technology) according to the manufacturer’s instructions. The transfected HIOECs were subsequently infected with P. gingivalis. Cells were harvested at 24 or 48 h after transfection for subsequent qRT-PCR or western blot analysis, respectively. The gene expression was assessed by qRT-PCR, and the protein expression level was assessed by western blot.
Statistical analysis
All experiments were repeated independently for at least 3 times under each condition unless otherwise indicated. The data statistical analysis was performed using SPSS 20.0 software. Experimental data were expressed as mean ± standard deviation (mean ± SD). One-way analysis of variance (one-way ANOVA) was used for comparison between multiple groups, followed by Tukey's multiple comparisons. The Student’s t-test was used to compare the differences between two independent groups. The Spearman statistical analysis was used for non-parametric basis correlations. Differences were considered statistically significant at P < 0.05.
Results
A negative relationship between FTL and epithelial junction proteins in periodontitis epithelial tissues
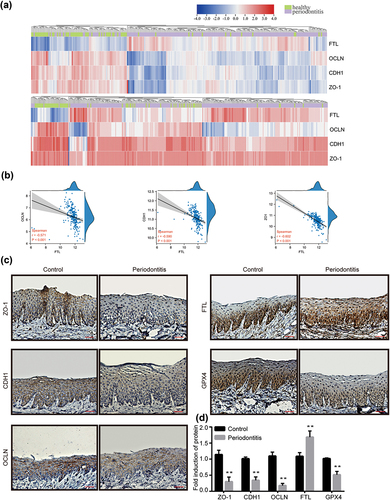
To explore the expression and the possible relationship of genes involved in epithelial junctions and iron metabolism in clinical samples of periodontitis, we obtained GSE10334 and GSE16134 datasets from GEO database. DEGs related to epithelial junction in gingival tissues from patients with periodontitis were listed. The expression of epithelial junction proteins OCLN, CDH1 and ZO-1 were found to be significantly changed. Meanwhile, FTL was found to be significantly increased in the periodontitis gingival tissues. The differential expressions of FTL, OCLN, CDH1 and ZO-1 in healthy and periodontitis gingival tissues are shown (). The correlation analysis showed a negative correlation between FTL and epithelial junction proteins ().
Figure 1. The relationship between epithelial junction proteins and FTL as well as protein expression in periodontitis gingiva tissues. (a) Heatmaps of FTL, OCLN, CDH1 and ZO-1 expression in healthy and periodontitis gingiva tissues in the GSE10334 (upper panel) and GSE16134 (lower panel). Red for up-regulated and blue for down-regulated gene expression. (b) The correlation of FTL with differentially expressed epithelial junction proteins in periodontitis tissues of OCLN, CDH1 and ZO-1. (c) Representative immunohistochemistry images of epithelial junction proteins (ZO-1, CDH1, OCLN), FTL and GPX4 in rat gingiva tissues. (d) Analysis of immunohistochemistry staining of epithelial junction proteins (ZO-1, CDH1, OCLN), FTL and GPX4. Data are presented as mean ± SD, n = 6. Scale bars, 100 μm. **P < 0.01 vs control.
P.gingivalis altered the expression of epithelial junction proteins, FTL and GPX4 in rat epithelial tissues
To evaluate the level of alveolar bone loss, we applied the μCT to measure the distance between CEJ and ABC of the first molars (M1) at three sites and averaged the measurement as a datapoint for each sample. These data displayed a clear increase in alveolar bone loss in the periodontitis group (Fig. S1 A). HE staining analysis exhibited the rat gingival structure around M1. In the subepithelial connective tissues, inflammatory corpuscles such as neutrophil and lymphocyte were observed in the gingival tissues. A remarkable difference was observed between the two groups (Fig. S1 B). The expression levels of differently expressed proteins were then tested in the periodontal tissues of control and periodontitis rats using immunohistochemistry. The expression of epithelial junction proteins (ZO-1, CDH1 and OCLN) and GPX4, a key regulator in anti-oxidation pathway, were significantly lower in periodontitis group compared to control group, while the expression of FTL was higher in the periodontitis group ().
P.gingivalis infection decreased the expression level of ZO-1, CDH1 and OCLN in vitro
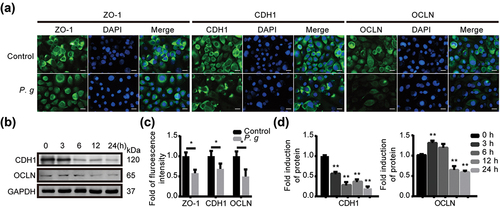
To further explore the influence on the epithelial junction by P. gingivalis infection, we infected HIOECs with P. gingivalis in different conditions and detected the expression of epithelial junction proteins by immunofluorescence () and western blot (). As images show, the protein expression levels of ZO-1, CDH1 and OCLN were significantly reduced within 24 h. Thus, we confirmed that P. gingivalis infection weakened the epithelial junction of oral epithelial cells.
Figure 2. P. gingivalis infection depressed the expression of epithelial junction proteins in vitro. (a) Representative images of immunofluorescence with ZO-1, CDH1 and OCLN in HIOECs treated with P. gingivalis at MOI of 50 for 24 h. (b) Western blot analysis of CDH1 and OCLN in HIOECs with P. gingivalis infection at MOI of 50 for different times. (c) Analysis of immunofluorescence staining of ZO-1, CDH1 and OCLN. (d) Analysis of the protein density of CDH1 and OCLN. Data are presented as mean ± SD, n = 3, repeated three times. Scale bars, 50 μm. P. g, P. gingivalis; *P < 0.05, **P < 0.01 vs control.
P.gingivalis infection induced ferroptosis in vitro
Considering mitochondrion is one of the important features of ferroptosis, we observed the ultrastructure of oral epithelial cells under P. gingivalis infection with TEM. It was shown that the mitochondrial volume was decreased. Meanwhile, the membrane density was increased and the crest was decreased or even disappeared (). As is known, ferroptosis is driven by the peroxidation of specific membrane lipids [Citation31]. The mitochondria membrane functions to maintain cellular homeostasis with other organelle [Citation32]. To explore the cause of mitochondrial morphological changes, we detected the lipid peroxidation level by flow cytometry. The lipid peroxidation level was significantly higher in infected cells compared to the control ().
Figure 3. P. gingivalis induced ferroptosis in vitro. (a) Morphological changes of the mitochondria in HIOECs after P. gingivalis infection for 24 h. (b) Representative images of lipid peroxidation level in HIOECs with BODIPY C11 by flow cytometry (upper panel), and analysis of the lipid peroxidation level (lower panel). (c) Representative immunofluorescence images of iron level in cytoplasm and mitochondria of HIOECs treated with P. gingivalis at MOI of 50 for 24 h using FerroOrange and FerroGreen probe, respectively. (d) Analysis of the immunofluorescence intensity. (e) Western blot of SLC7A11, FTL and GPX4 in HIOECs treated with P. gingivalis at MOI of 50 for different times. (f) Analysis of protein expression level. Data are presented as mean ± SD, n = 3, repeated three times. Scale bars in (a), 1 μm. Scale bars in (c), 50 μm. P. g, P. gingivalis; *P< 0.05, **P < 0.01 vs control.
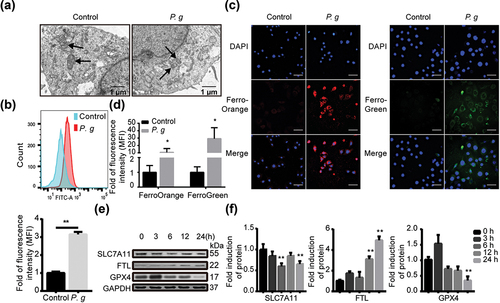
Ferrous ions have a greater capacity to oxidize and can be converted faster than ferric ions in Fenton reactions [Citation33]. They could trigger Fenton-dependent ferroptosis [Citation34]. So fluorescent probes were used for ferrous ion content detection. The ferrous ion content in infected cells was significantly higher than the control, regardless of cytoplasm or mitochondria (). Further, the expression level of FTL was significantly increased as the infection time was prolonged. The expression level of GPX4 and SLC7A11 were significantly decreased as the infection time was prolonged ().
The effect of iron overload on epithelial cells
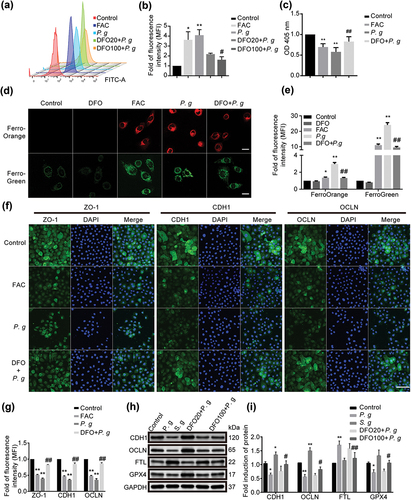
We further focused on investigating the effect of iron overload on cell ferroptosis. Iron chelator deferoxamine (DFO) and the iron supplementation ammonium ferric citrate (FAC) were utilized as negative and positive control, respectively. In this study, DFO significantly reduced the lipid peroxidation level () and the content of ferrous ions in P. gingivalis-infected HIOECs (). The GSH content clearly increased in cells that intervened with DFO compared to cells without DFO (). DFO also attenuated the decrease in the protein expression of ZO-1, CDH1 and OCLN by immunofluorescence (). Protein expression of CDH1, OCLN, FTL and GPX4 treated with P. gingivalis or Streptococcus gordonii (S. gordonii) was detected by western blot (). Conversely, FAC significantly augmented intracellular iron levels and elevated lipid oxidation, leading to a notable reduction in the expression of key epithelial junction proteins. The results showed that iron overload by P. gingivalis could induce epithelial cells ferroptosis and epithelial junctions impairment.
Figure 4. Effect of iron overload on epithelial cells. (a) Representative images of lipid peroxidation in HIOECs treated with FAC (200 μM) or P. gingivalis at MOI of 50 for 24 h with or without a pretreatment of iron chelator DFO (20 μM or 100 μM) for 2 h before infection using BODIPY C11 by flow cytometry. (b) Analysis of lipid peroxidation level in HIOECs. (c) GSH level in HIOECs treated with FAC (200 μM) or P. gingivalis with or without DFO (100 μM) using GSH kit with a microplate reader. (d) Representative fluorescence images of iron level in cytoplasm and mitochondria of HIOECs treated with P. gingivalis at MOI of 50 for 24 h with or without DFO (100 μM) using FerroOrange and FerroGreen probe, respectively. Scale bars, 20 μm. (e) Analysis of fluorescence intensity of HIOECs treated with P. gingivalis with or without DFO (100 μM). (f) Representative images of immunofluorescence of ZO-1, CDH1 and OCLN in HIOECs treated with FAC (200 μM) or P. gingivalis (MOI = 50) only or a pretreatment of DFO (100 μM). The nucleus was stained with DAPI. Scale bars, 100 μm. (g) Analysis of immunofluorescence intensity of ZO-1, CDH1 and OCLN in HIOECs. (h) Western blot of CDH1, OCLN, FTL and GPX4 treated with P. gingivalis or S. gordonii for 24 h. HIOECs were infected with P. gingivalis (MOI = 50) only or pretreated with DFO (20 μM or 100 μM) for 2 h before a 24 hours-infection. (i) Analysis of protein expression level of CDH1, OCLN, FTL and GPX4 treated with P. gingivalis or S. gordonii. Data are presented as mean ± SD, n = 3, repeated three times. P. g, P. gingivalis; S. g, S. gordonii; *P < 0.05, **P < 0.01 vs control, ##P < 0.01, #P < 0.05 vs P. gingivalis.
P.gingivalis weakened the epithelial junction mediated by ferroptosis via inhibiting GPX4
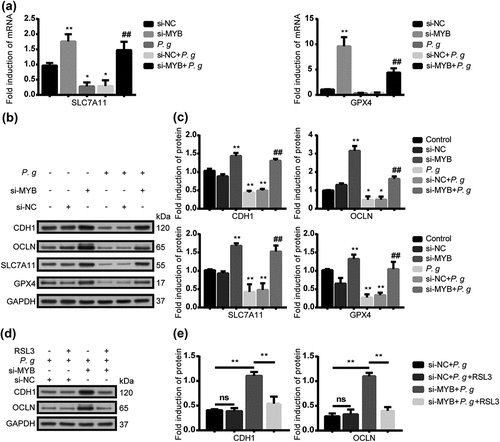
By manipulating iron concentrations in experimental groups, we could examine the impact of iron levels on cellular oxidative stress. Higher iron levels increase intracellular free iron and intensify oxidative stress, resulting in weakened epithelial cell connections. Conversely, reducing iron levels lowers oxidative stress and maintains strong cell connections. Our study aims to uncover the intricate relationship between oxidative stress regulation and epithelial cell connectivity. The infection decreased the expression of GPX4, reduced the content of GSH as well as decreased the expression of SLC7A11. We supposed that the inhibition of GPX4 might have resulted from the suppression of SLC7A11. NCBI (https://www.ncbi.nlm.nih.gov/) and UCSC (https://genome.ucsc.edu/) were applied to find the potential promoter of SLC7A11. In addition, PROMO (https://alggen.lsi.upc.es/cgi-bin/promo_v3/promo/promoinit.cgi?dirDB=TF_8.3) was subsequently used to predict the transcription factors of SLC7A11. Other studies have suggested that MYB played an oncogene role. In recent years, some scholars have found that MYB was also involved in the regulation of inflammation caused by bacteria [Citation35]. However, the role of MYB in the regulation of SLC7A11/GPX4 was not found yet. After silencing MYB, the mRNA () and protein expressions () of CDH1 and OCLN as well as SLC7A11 and GPX4 were significantly increased. To further confirm the role of GPX4 in the regulation of epithelial junction molecules induced by P. gingivalis, we used RAS-selective-lethal-3 (RSL3), a small-molecule inhibitor of GPX4. The expression of epithelial junction proteins in cells inhibited by RSL3 after transfection was lower than that without RSL3, suggesting that GPX4 participated in the degeneration of epithelial junction by P. gingivalis ().
Figure 5. P. gingivalis impaired the epithelial junction proteins mediated by ferroptosis via MYB/SLC7A11/GPX4. (a) The mRNA level of SLC7A11 and GPX4 in HIOECs treated with P. gingivalis at MOI of 50 with the interference of si-NC or si-MYB by qRT-PCR analysis. (b) Western blot of CDH1, OCLN, SLC7A11 and GPX4 in HIOECs treated with P. gingivalis at MOI of 50 with the interference of si-NC or si-MYB. (c) Analysis of protein expression level of CDH1, OCLN, SLC7A11 and GPX4. (d) Western blot of CDH1 and OCLN in HIOECs treated with P. gingivalis at MOI of 50 with the interference of si-NC or si-MYB. After transfection with siRNA, the cells were pretreated with or without GPX4 inhibitor RSL3 (1 μM) for 2 h before P. gingivalis infection. (e) Analysis of protein expression level of CDH1 and OCLN. Data are presented as mean ± SD, n = 3, repeated three times. P. g, P. gingivalis; *P < 0.05, **P < 0.01 vs control, ## P < 0.05 vs P. gingivalis.
Discussion
Periodontitis is a common infectious disease that occurs in the periodontal support tissues. The oral epithelium is the first line of defense against periodontal infection. This study found that P. gingivalis could induce impairment of oral epithelial junction as well as oral epithelial cells ferroptosis, including shrunken mitochondria, enhanced intracellular iron content and lipid peroxidation, high expression level of FTL, and weakened GSH and GPX4. DFO, an iron chelator, was found to attenuate such damage and might be applied as a candidate for periodontitis therapy in the perspective of iron metabolism. To the best of our knowledge, this is the first time to demonstrate a possible link between destruction of oral epithelium and ferroptosis under P. gingivalis infection.
In this study, P. gingivalis was used as a typical periodontal pathogen to study its effect on epithelial junction. Meanwhile, S. gordonii, one of the early colonizers of dental plaque biofilm, was first used as a control to verify the speciality of P. gingivalis in the preliminary study. Compared with the decreased expression of epithelial junction proteins in oral epithelial cells infected with P. gingivalis, the expression of these proteins was increased in cells infected with S. gordonii (data not shown). This result indicated that P. gingivalis could disrupt epithelial junction while some other oral pathogens could not. Similarly, S. gordonii was found to increase or maintain the expression of tight junction components, which was consistent with our results [Citation36,Citation37]. Besides, S. gordonii was also applied in the evaluation of ferroptosis-associated proteins (). It was shown that ferroptosis biomarkers expression such as FTL and GPX4 was not significantly changed under S. gordonii infection. Together, these results demonstrated the special characteristics of P. gingivalis on epithelial junction and ferroptosis compared with other oral pathogens.
Ferroptosis depends on iron-mediated oxidative damage and is critically regulated by a series of reactions such as iron accumulation, free radicals generation and lipid peroxidation. So far, multiple experimental methods were used to demonstrate the occurrence of ferroptosis [Citation31,Citation38]. The alteration of mitochondria morphology was considered as one of the features of ferroptosis. By application of TEM, mitochondria in cells of ferroptosis was observed to show dense and shrunken form with vestigial cristae [Citation39,Citation40], which are consistent with the mitochondria morphology in our study. Cells undergoing ferroptosis were also determined by increased iron content, lipid peroxidation and reduced GSH content [Citation41–43]. Our results demonstrated that P. gingivalis could induce abnormal content of intracellular iron, lipid peroxidation and GSH in oral epithelial cells, which is consistent with other studies.
Based on the above findings, we further explored the mechanism of ferroptosis induced by P. gingivalis in epithelial cells and the intrinsic link with the impairment of epithelial junctions. Lipid peroxidation can be limited by antioxidant pathways. The system xc−-GSH-GPX4-dependent antioxidant defense is a major pathway to reduce the accumulation of lipid hydroperoxides, which was one of the major reason to trigger ferroptosis. The system xc− embeds on the surface of the cell membrane and imports cysteine (the oxidized form of cysteine) in mammalian cells. System xc− is a heterodimer composed of two subunits, SLC3A2 and SLC7A11. As shown, a two-ways regulation of SLC7A11 constituted a fine-tuning mechanism to control GSH levels during ferroptosis [Citation20]. Genetic deletion of SLC7A11 led to lipid peroxidation and ferroptosis in some cells or tissues [Citation44]. For example, lung adenocarcinoma cells exhibited sensitivity to SLC7A11 inhibitor-induced iron death [Citation45]. Similarly, we found that up-regulation of SLC7A11 could reduce lipid peroxidation and attenuate ferroptosis in oral epithelial cells infected by P. gingivalis. These suggested that SLC7A11 might play a crucial role in the process of cell oxidation and ferroptosis. SLC7A11 is a vital regulator of GPX4 [Citation42]. The expression and activity of GPX4 are dependent on the presence of GSH and selenium. GSH is synthesized by cysteine, glycine and glutamate. The cysteine is the major limiting factor in this process [Citation42,Citation46]. In the present study, the function of SLC7A11/GSH/GPX4, a classical antioxidant pathway, was found to be notably diminished with P. gingivalis infection.
As we have learned, P. gingivalis is the best characterized bacterium of studied pathogens which can accumulate iron on the surface of bacterial cells [Citation47]. This can explain why P. gingivalis increased the intracellular iron content after invading oral epithelial cells in our study. In addition, ferritin is the main iron storage protein. We detected the expression of ferritin and the content of iron ion to jointly illustrate the overall iron levels of cells. Excess iron ions in the cytoplasm transport electrons in the progress of constant valence state conversion and lead to free radical-driven reactions to damage cells, including lipid peroxidation, a hallmark of ferroptosis. The pretreatment of DFO at a concentration of 100 μM significantly reduced the lipid peroxidation level and partly restored the expression level of epithelial junction proteins decreased by ferroptosis (). In addition, the increase in lipid peroxidation level declined the expression of epithelial junction proteins. These results jointly provided a novel idea for clinical therapy that DFO might be applied to treat diseases such as periodontitis caused by iron overload-mediated lipid peroxidation. In vivo experiments should be performed to clarify the role of DFO in the treatment of periodontitis in the future.
As shown in , the expression of epithelial junction proteins was weakened after infection of P. gingivalis with increased intracellular iron content, increased lipid peroxidation and diminished function of antioxidant pathway SLC7A11/GSH/GPX4. Enhancement of SLC7A11 expression could reverse the expression of epithelial junction proteins. The application of RSL3, an inhibitor of GPX4, could weaken the reversal. Thus, P. gingivalis impaired oral epithelial junction by inducing cellular ferroptosis.
Figure 6. Proposed model that P. gingivalis induces ferroptosis and thereby impairs oral epithelial junction. P. gingivalis suppresses SLC7A11 by intervening its transcription factor MYB. Thus, it decreases GSH level and induces GPX4 loss of function. Meanwhile, excess iron followed with P. gingivalis infection causes accumulation of lipid peroxidation which should originally be cleaned by GPX4. The imbalance causes intracellular ferroptosis as well as impairment of the epithelial junction.
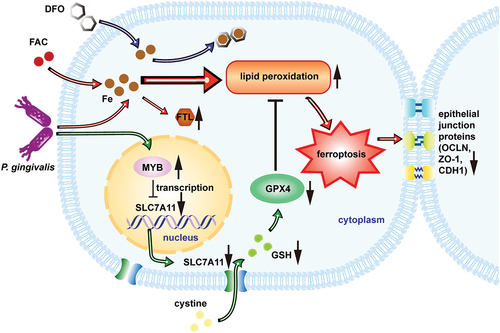
In conclusion, we discovered that P. gingivalis infection caused iron overload environment with the disability of antioxidant system and herein resulted in ferroptosis, which could attenuate the expression of epithelial junction proteins. Furthermore, we also applied a kind of iron chelator to provide new ideas and new targets for the clinical prevention and treatment of periodontitis and other diseases with iron overload-mediated lipid peroxidation.
Author contributions
The authors XS, ZL and YP designed the experiments. XS and Jiabo Li performed the experiments. XS and ZL analyzed the data. SZ, QL and FG helped perform the analysis with constructive discussions. XS wrote the initial draft of the manuscript. Jinwen Liu and FG revised the manuscript. YP administered funding acquisition. All authors contributed to the article and approved the submitted version.
Ethics statement
The animal studies were reviewed and approved by the animal project review committee of China Medical University (CMU2020387).
Supplemental Material
Download MS Word (5.9 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request. Datasets presented in this study can be found in online repositories. The repository and accession numbers can be found below: NCBI (https://www.ncbi.nlm.nih.gov/), GSE10334 and GSE16134.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/20002297.2024.2334578.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Lu HX, Tao DY, Lo ECM, et al. The 4th national oral health survey in the mainland of China: background and methodology. Chin J Dent Res. 2018;21(3):161–12. doi: 10.3290/j.cjdr.a41079
- Holde GE, Oscarson N, Trovik TA, et al. Periodontitis prevalence and severity in adults: a cross-sectional study in Norwegian circumpolar communities. J Periodontol. 2017;88(10):1012–1022. doi: 10.1902/jop.2017.170164
- Eke P, Dye B, Wei L, et al. Update on prevalence of periodontitis in adults in the United States: NHANES 2009 to 2012. J Periodontol. 2015;86(5):611–622. doi: 10.1902/jop.2015.140520
- Socransky S, Haffajee A, Cugini M, et al. Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25(2):134–144. doi: 10.1111/j.1600-051X.1998.tb02419.x
- Chen W, Alshaikh A, Kim S, et al. Porphyromonas gingivalis impairs oral epithelial barrier through targeting GRHL2. J Dent Res. 2019;98(10):1150–1158. doi: 10.1177/0022034519865184
- Hao T, Zhang R, Zhao T, et al. Porphyromonas gingivalis infection promotes inflammation via inhibition of the AhR signalling pathway in periodontitis. Cell Proliferation. 2023;56(2):e13364. doi: 10.1111/cpr.13364
- Imafuku K, Iwata H, Natsuga K, et al. Zonula occludens-1 distribution and barrier functions are affected by epithelial proliferation and turnover rates. Cell Proliferation. 2023;56(9):e13441. doi: 10.1111/cpr.13441
- Amaral EP, Costa DL, Namasivayam S. A major role for ferroptosis in mycobacterium tuberculosis –induced cell death and tissue necrosis. J Exp Med. 2019;216(3):556–570. doi: 10.1084/jem.20181776
- Dar HH, Tyurina YY, Mikulska-Ruminska K, et al. Pseudomonas aeruginosa utilizes host polyunsaturated phosphatidylethanolamines to trigger theft-ferroptosis in bronchial epithelium. J Clin Invest. 2018;128(10):4639–4653. doi: 10.1172/JCI99490
- Tuo QZ, Lei P, Jackman KA, et al. Tau-mediated iron export prevents ferroptotic damage after ischemic stroke. Mol Psychiatry. 2017;22(11):1520–1530. doi: 10.1038/mp.2017.171
- Dixon SJ, Lemberg KM, Lamprecht MR, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060–1072. doi: 10.1016/j.cell.2012.03.042
- Jacobs D, Watt GD, Frankel RB, et al. Fe2+ binding to apo and holo mammalian ferritin. Biochemistry. 1989;28(23):9216–9221. doi: 10.1021/bi00449a038
- Schroeder HE, Lindhe J. Conditions and pathological features of rapidly destructive, experimental periodontitis in dogs. J Periodontol. 1980;51(1):6–19. doi: 10.1902/jop.1980.51.1.6
- Mu Q, Chen L, Gao X, et al. The role of iron homeostasis in remodeling immune function and regulating inflammatory disease. Sci Bull. 2021;66(17):1806–1816. doi: 10.1016/j.scib.2021.02.010
- Costa S, Ribeiro C, Moreira A, et al. High serum iron markers are associated with periodontitis in post-menopausal women: a population-based study (NHANES III). J Clin Periodontol. 2022;49(3):221–229. doi: 10.1111/jcpe.13580
- Costa S, Moreira A, Costa C, et al. Iron overload and periodontal status in patients with sickle cell anaemia: a case series. J Clin Periodontol. 2020;47(6):668–675. doi: 10.1111/jcpe.13284
- Meuric V, Lainé F, Boyer E, et al. Periodontal status and serum biomarker levels in HFE haemochromatosis patients. A case-series study. J Clin Periodontol. 2017;44(9):892–897. doi: 10.1111/jcpe.12760
- De Domenico I, Ward D, Kaplan J. Specific iron chelators determine the route of ferritin degradation. Blood. 2009;114(20):4546–4551. doi: 10.1182/blood-2009-05-224188
- Kontoghiorghes G, Marcus R, Huehns E. Desferrioxamine suppositories. Lancet. 1983;2(8347):454. doi: 10.1016/S0140-6736(83)90413-0
- Chen X, Kang R, Kroemer G, et al. Broadening horizons: the role of ferroptosis in cancer. Nat Rev Clin Oncol. 2021;18(5):280–296. doi: 10.1038/s41571-020-00462-0
- Tu H, Tang L, Luo X, et al. Insights into the novel function of system xc- in regulated cell death. Eur Rev Med Pharmacol Sci. 2021;25(3):1650–1662. doi: 10.26355/eurrev_202102_24876
- Wang H, An P, Xie E, et al. Characterization of ferroptosis in murine models of hemochromatosis. Hepatology. 2017;66(2):449–465. doi: 10.1002/hep.29117
- Koppula P, Zhuang L, Gan B. Cystine transporter SLC7A11/xCT in cancer: ferroptosis, nutrient dependency, and cancer therapy. Protein Cell. 2021;12(8):599–620. doi: 10.1007/s13238-020-00789-5
- Xing L, Dong W, Chen YL, et al. Fibroblast ferroptosis is involved in periodontitis-induced tissue damage and bone loss. Int Immunopharmacol. 2023;114:114. doi: 10.1016/j.intimp.2022.109607
- Yang WY, Meng X, Wang YR, et al. PRDX6 alleviates lipopolysaccharide-induced inflammation and ferroptosis in periodontitis. Acta Odontol Scand. 2022;80(7):535–546. doi: 10.1080/00016357.2022.2047780
- Wang HW, Qiao XT, Zhang C, et al. Long non-coding RNA LINC00616 promotes ferroptosis of periodontal ligament stem cells via the microRNA-370/transferrin receptor axis. Bioengineered. 2022;13(5):13070–13081. doi: 10.1080/21655979.2022.2076508
- Zhao YH, Li J, Guo W, et al. Periodontitis-level butyrate-induced ferroptosis in periodontal ligament fibroblasts by activation of ferritinophagy. Cell Death Discovery. 2020;6(1). doi: 10.1038/s41420-020-00356-1
- Geng F, Liu J, Guo Y, et al. Persistent exposure to porphyromonas gingivalis promotes proliferative and invasion capabilities, and tumorigenic properties of human immortalized oral epithelial cells. Front Cell Infect Microbiol. 2017;7:57. doi: 10.3389/fcimb.2017.00057
- Zhang S, Li C, Liu J, et al. Fusobacterium nucleatum promotes epithelial-mesenchymal transition through regulation of the lncRNA MIR4435-2HG/miR-296-5p/Akt2/SNAI1 signaling pathway. FEBS J. 2020;287(18):4032–4047. doi: 10.1111/febs.15233
- Mei H, Zhao L, Li W, et al. Inhibition of ferroptosis protects house ear institute-organ of corti 1 cells and cochlear hair cells from cisplatin-induced ototoxicity. J Cell Mol Med. 2020;24(20):12065–12081. doi: 10.1111/jcmm.15839
- Stockwell B. Ferroptosis turns 10: emerging mechanisms, physiological functions, and therapeutic applications. Cell. 2022;185(14):2401–2421. doi: 10.1016/j.cell.2022.06.003
- Wong Y, Kim S, Peng W, et al. Regulation and function of mitochondria-lysosome membrane contact sites in cellular homeostasis. Trends Cell Biol. 2019;29(6):500–513. doi: 10.1016/j.tcb.2019.02.004
- Brillas E, Sirés I, Oturan MA. Electro-Fenton process and related electrochemical technologies based on Fenton’s reaction chemistry. Chem Rev. 2009;109(12):6570–6631. doi: 10.1021/cr900136g
- Xing G, Meng L, Cao S, et al. PPARα alleviates iron overload-induced ferroptosis in mouse liver. EMBO Rep. 2022;23(8):e52280. doi: 10.15252/embr.202052280
- Li X, He S, Li R, et al. Pseudomonas aeruginosa infection augments inflammation through miR-301b repression of c-myb-mediated immune activation and infiltration. Nat Microbiol. 2016;1(10):16132. doi: 10.1038/nmicrobiol.2016.132
- Ye P, Harty D, Commandeur Z, et al. Binding of streptococcus gordonii to oral epithelial monolayers increases paracellular barrier function. Microb Pathog. 2013;56:53–59. doi: 10.1016/j.micpath.2012.11.004
- Dickinson B, Moffatt C, Hagerty D, et al. Interaction of oral bacteria with gingival epithelial cell multilayers. Mol Oral Microbiol. 2011;26(3):210–220. doi: 10.1111/j.2041-1014.2011.00609.x
- Van der Meeren L, Verduijn J, Krysko D, et al. High-throughput mechano-cytometry as a method to detect apoptosis, necroptosis, and ferroptosis. Cell Proliferation. 2023;56(6):e13445. doi: 10.1111/cpr.13445
- Xu M, Tao J, Yang Y, et al. Ferroptosis involves in intestinal epithelial cell death in ulcerative colitis. Cell Death Dis. 2020;11(2):86. doi: 10.1038/s41419-020-2299-1
- Yoshida M, Minagawa S, Araya J, et al. Involvement of cigarette smoke-induced epithelial cell ferroptosis in COPD pathogenesis. Nat Commun. 2019;10(1):3145. doi: 10.1038/s41467-019-10991-7
- Li J, Cao F, Yin HL, et al. Ferroptosis: past, present and future. Cell Death Dis. 2020;11(2):88. doi: 10.1038/s41419-020-2298-2
- Chen X, Li J, Kang R, et al. Ferroptosis: machinery and regulation. Autophagy. 2021;17(9):2054–2081. doi: 10.1080/15548627.2020.1810918
- Jiang X, Stockwell BR, Conrad M. Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol. 2021;22(4):266–282. doi: 10.1038/s41580-020-00324-8
- Sato H, Shiiya A, Kimata M, et al. Redox imbalance in cystine/glutamate transporter-deficient mice. J Biol Chem. 2005;280(45):37423–37429. doi: 10.1074/jbc.M506439200
- Hu K, Li K, Lv J, et al. Suppression of the SLC7A11/glutathione axis causes synthetic lethality in KRAS-mutant lung adenocarcinoma. J Clin Invest. 2020;130(4):1752–1766. doi: 10.1172/JCI124049
- Tang D, Chen X, Kang R, et al. Ferroptosis: molecular mechanisms and health implications. Cell Res. 2021;31(2):107–125. doi: 10.1038/s41422-020-00441-1
- Olczak T, Simpson W, Liu X, et al. Iron and heme utilization in Porphyromonas gingivalis. FEMS Microbiol Rev. 2005;29(1):119–144. doi: 10.1016/j.femsre.2004.09.001
