ABSTRACT
Background
Antifungal resistance is a major problem, commonly caused by drug-efflux pump overexpression. To evaluate if chitosan could be effective in drug-resistant Candida infections, we investigated the effects of efflux pumps on antifungal activity of chitosan.
Materials and Methods
The minimal fungicidal concentration (MFC) of oligomer (7–9 kD) and polymer (900–1,000 kD) chitosan against Saccharomyces cerevisiae and Candida albicans were evaluated by broth and agar dilution methods. The MFCs of S. cerevisiae with single deletion of efflux pump genes, with deletion of seven efflux pumps (AD∆), and AD∆ overexpressing C. albicans efflux pump genes (CDR1, CDR2 and MDR1) were determined. C. albicans with homozygous deletions of CDR1 and of CDR2 were generated using CRISPR-Cas9 system and tested for chitosan susceptibility.
Results
While deleting any individual efflux pump genes had no effect on chitosan susceptibility, simultaneous deletion of multiple pumps (in AD∆) increased sensitivity to both types of chitosan. Interestingly, the overexpression of CDR1, CDR2 or MDR1 in AD∆ barely affected its sensitivity. Moreover, C. albicans with homozygous deletions of CDR1 and/or CDR2 showed similar sensitivity to wildtype.
Conclusion
Thus, C. albicans susceptibility to chitosan was not affected by drug-efflux pumps. Chitosan may be a promising antifungal agent against pump-overexpressing azole-resistant C. albicans.
KEY MESSAGES
1. Neither deletion of efflux pump genes, nor overexpression of major C. albicans efflux pumps in pump-deficient S. cerevisiae, nor deletion of major efflux pumps in C. albicans affects yeast susceptibility to chitosan.
2. Chitosan may be an effective antifungal agent against drug-resistant C. albicans.
Introduction
Candida can cause superficial and invasive infections that affect millions of people worldwide, especially in immunocompromised persons [Citation1]. Candida albicans is the most prevalent species, and invasive infections have up to 40% mortality rate [Citation2]. It is listed as one of the four highest priority fungal pathogens by the World Health Organization (WHO) [Citation3]. The limited repertoire of antifungal drugs coupled with increasing antifungal drug resistance has made treatments even more difficult [Citation4].
Azoles, especially fluconazole, are the most commonly used antifungal drugs for Candida infections, but drug resistance is increasingly prevalent, mainly through overexpression of drug efflux pumps [Citation5]. ATP binding cassette (ABC) and Major facilitator superfamily (MFS) transporter proteins are two main classes of efflux pumps in yeasts [Citation6]. Upregulation of CDR1 and CDR2 of the pleiotropic drug resistance (PDR) subfamily of ABC transporters, and MDR1, an MFS transporter, results in reduced intracellular drug accumulation and antifungal drug resistance [Citation5].
The rise in antifungal drug resistance necessitates the development of novel antifungal agents with low toxicity. Chitosan, a natural polymer from the deacetylation of chitin in crustacean shells, has high biocompatibility and antibacterial and antifungal activities [Citation7]. Many chitosan derivatives exist with different molecular weights (MW), degrees of deacetylation and modifications, which could affect their antimicrobial activity [Citation8]. Several derivatives with various MW were shown to be effective against planktonic cells and biofilm of Candida species [Citation9–11]. Mechanistically, high MW chitosan could disrupt cell membrane functions, while low MW chitosan could enter the cells and interfere with DNA and RNA synthesis [Citation8].
Importantly, it is unknown if chitosan activity is affected by the common antifungal resistance mechanisms, especially drug efflux pumps. Taking advantage of the gene deletion library and multiple efflux pump deletion strains in Saccharomyces cerevisiae, we first employed this model to test the effects of individual vs. multiple efflux pump gene deletion on susceptibility to chitosan. Then, we examined the effects of Candida efflux pump gene overexpression in S. cerevisiae and gene deletions in C. albicans. We hypothesized that high MW chitosan that likely acts on the outer cell membrane would not be affected by efflux pumps, while low MW chitosan may be a pump substrate. If chitosan is not affected by efflux pumps, it may be useful against efflux pump-overexpressing azole-resistant C. albicans.
Materials and methods
Yeast strains and growth condition
Yeast were cultured in Yeast-extract-Peptone-Dextrose (YPD) broth and incubated overnight at 30°C with shaking (180 rpm). The culture was adjusted to an optical density at 600 nm (OD600) of 0.1 and incubated until log phase (OD600 = 0.4–0.6).
S. cerevisiae BY4741 and C. albican SC5314 were wild-type controls. S. cerevisiae with single deletion of efflux pump strains were from Mat-a haploid yeast deletion library (Invitrogen, USA). Strains used in this study included S. cerevisiae with double deletion of PDR1 and PDR3, major transcription factors of several pump genes (gift from Prof. L. Jensen) [Citation12], ADΔ strain, with simultaneous deletions of seven pump genes [Citation13] and ADΔ overexpressing C. albicans efflux pump genes (ADΔ/CaCDR1A-HIS, ADΔ/CaCDR1B-HIS, AD/CaCDR2A-HIS and AD/CaMDR1A) [Citation13,Citation14] (gifts from Prof. R. Cannon).
C. albicans with homozygous deletions of CDR1 and CDR2 genes were created using the transient CRISPR-Cas9 system [Citation15,Citation16]. The sequence of sgRNA (GTTTTGGGGAGACCCGGTGC) was chosen from a previously proposed guide RNA sequence, amplified and cloned into pV1093 (gift from Prof. G. Fink) [Citation15]. C. albicans were electroporated with CaCas9, sgRNA and CaSAT1 flipper cassette carrying nourseothricin-resistant marker (amplified from pSFS2AS, gift from Prof. J. Shieh, using primers: CDR1S1_F:CTCTTCTTCAGAAAACCATTCCATTAATGAATACCACGGGTTTGATGCCCATACAAGTGAAAACATTCAGAATTTAGCCAGGAAGCTTCGTACGCTGCAGGTC, CDR1S2_R:CAACAACAATAGTCTAAAAACGTCTATTATATTTTAGACGTTTGAGATACCACCATGTCAAAAAACAAACTGTTTAATTCTGATATCATCGATGAATTCGAG, CDR2S1_F:CTTGAAAAAATTATTTGAATCTGATTCTGATTATTATAAGCCATCGAAATTAGGGGTTGCCTATAGAAATTTAAGAGCTTACGGGAAGCTTCGTACGCTGCAGGTC, CDR2S2_R:CAAACAATCACAAATAACGTATAAATAATAATAAGAAAAAAAAAATATGAATACTAATTGTAAAATAAGACCCCATCCTCTGATATCATCGATGAATTCGAG) [Citation17]. Transformants were selected on YPD containing 400 µg/ml nourseothricin (ClonNAT, Werner bioagents GmbH, Germany) and verified by PCR. Cells were grown on YEP + 2% maltose to induce flippase for CaSAT1-marker removal, and re-transformed with CRISPR-Cas9 fragments to generate homozygous deletion.
Chitosan and antifungal activity measurement
Shrimp oligomer (7–9 kDa) and polymer (900–1000 kDa) chitosan (Taming Enterprises, Samut-Sakhon, Thailand) with >85% degree of deacetylation were dissolved in 1% acetic acid at 30 mg/ml (w/v) and autoclaved.
Minimum Fungicidal Concentration (MFC) of chitosan was determined by broth and agar dilution methods. For broth dilution, log-phase cultures (≈106 cells/ml) was incubated with two-fold serial dilutions of chitosan in YPD at 30°C with shaking for 18 h, and 100 µl aliquot was plated on YPD agar and incubated for 48 h. For agar dilution, 5 µl of log-phase cultures (≈106 cells/ml) were spotted on YPD agar containing various concentrations of chitosan and incubated for 48 h. The lowest concentration of chitosan that showed no growth was recorded as MFC.
Results
MFC of chitosan in wild-type S. cerevisiae and C. albicans
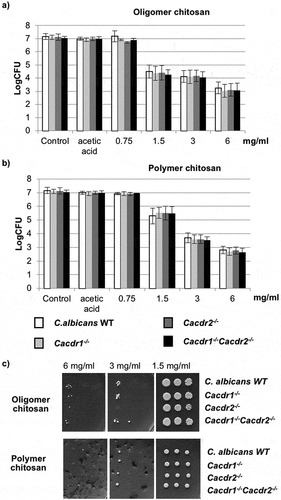
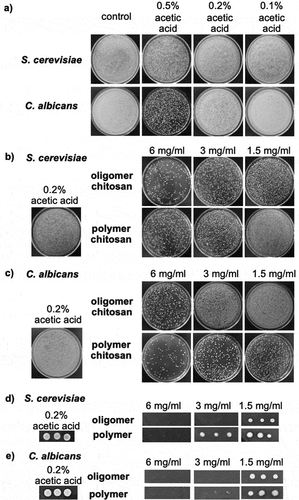
The MFC of two types of chitosan against wild type S. cerevisiae and C. albicans were examined. Since chitosan stock (30 mg/ml) was dissolved in 1% acetic acid, we first determined the highest concentration of acetic acid that does not affect fungal viability to exclude antifungal effects of the acid. shows that 0.2% acetic acid was the highest concentration tolerated by both S. cerevisiae and C. albicans. Thus, the maximum concentration of chitosan tested in this study was 6 mg/ml, which has 0.2% acetic acid. Yeast growth was partially inhibited by oligomer and polymer chitosan at 6 mg/ml (). Using agar dilution, S. cerevisiae was inhibited at 3 mg/ml oligomer and 6 mg/ml polymer, while C. albicans was inhibited at 3 mg/ml of both types of chitosan ().
Figure 1. Minimum Fungicidal Concentration (MFC) of chitosan derivatives. (a) Effect of acetic acid on S. cerevisiae and C. albicans was determined by broth dilution method. MFC of oligomer and polymer chitosan against S. cerevisiae (b, d) and C. albicans (c, e) was determined by broth and agar dilution assays, respectively.
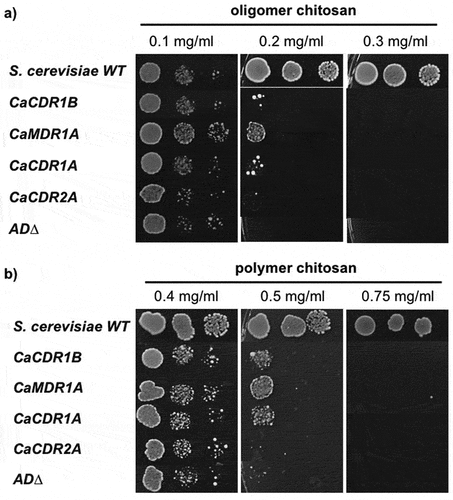
Effects of efflux pump gene deletions and C. albicans efflux pump overexpression
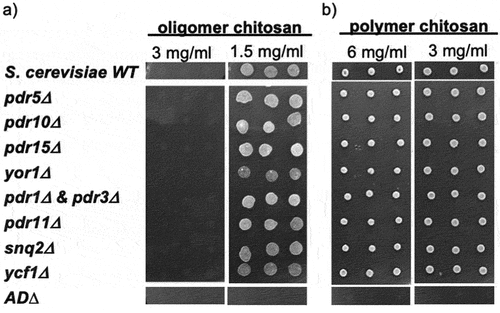
To examine the effects of efflux pumps, we tested chitosan susceptibility of S. cerevisiae with pump gene deletions using agar dilution. At 1.5 mg/ml oligomer, individual efflux pump deletion strains and the double deletion of major transcription factors that regulate the expression of several efflux pumps, pdr1∆pdr3∆ strain, showed similar growth as wild type. In contrast, AD∆ strain, which lacks seven efflux pump genes, showed hypersensitivity to chitosan (). AD∆ strain was also extremely sensitive to 6 mg/ml polymer, while single pump deletions and pdr1∆pdr3∆ strains were inhibited similarly to the wild type ().
Figure 2. Susceptibility of S. cerevisiae strains with indicated efflux pump gene deletion to chitosan, (a) oligomer and (b) polymer, by agar dilution assay.
The effect of C. albicans efflux pump overexpression on chitosan susceptibility was examined in AD∆ that overexpressed CDR1, CDR2 or MDR1. Comparing to AD∆ strain (MFC = 0.2 mg/ml oligomer), overexpression of MDR1, and to a lesser extent CDR1, in AD∆ strain showed a small number of colonies at 0.2 mg/ml, but no growth at 0.3 mg/ml oligomer (). Likewise, at the MFC of AD∆ strain (0.5 mg/ml polymer), overexpression of CDR1 or MDR1 allowed only minimal growth ().
Effect of efflux pump deletions in Candida albicans
We next analysed the roles of efflux pumps directly in C. albicans by generating homozygous deletions of single or double CDR1 and CDR2 genes. Using broth dilution, the MFC of C. albicans with single (cdr1-/- or cdr2-/-) or double (cdr1-/-cdr2-/-) homozygous deletion strains was similar to that of wild-type for both types of chitosan (). Similar results were observed using agar dilution ().
Discussion
Here, we showed that while simultaneous deletion of multiple efflux pumps in S. cerevisiae led to increased sensitivity to both oligomer and polymer chitosan, overexpression of Candida efflux pumps, Cdr1, Cdr2 or Mdr1 had minimal effects on increasing chitosan resistance. Furthermore, the deletion of the CDR1 and/or CDR2 in C. albicans did not affect the susceptibility to both types of chitosan. Thus, our results suggest that chitosan could serve as an alternative or adjunctive antifungal agent against azole-resistant C. albicans that overexpress efflux pumps.
Numerous chitosan derivatives exist with various MW, which is an important determinant of their antimicrobial mechanism. It has been proposed that high MW chitosan binds to the cell membrane, thereby blocking nutrient transports and causing leakage, whereas low MW chitosan could enter the cells and disrupt DNA, RNA and protein metabolism [Citation8]. MW could also determine if chitosan would be substrates of efflux pumps. Thus, we examined the roles of efflux pumps on two types of chitosan with different MW, oligomer and polymer. We first employed S. cerevisiae since its efflux pump genes were similar to C. albicans and pump-deletion strains were available [Citation13]. We hypothesized that if any efflux pump contributed to chitosan resistance, the pump-deletion strain would be hypersensitive to chitosan. Our result showed that all single pump deletions showed similar sensitivity to wild-type, suggesting that no single pump plays a predominant role in transporting chitosan. Chitosan susceptibility was unaltered even in the pdr1∆pdr3∆ strain, which has downregulation of multiple efflux pumps. Meanwhile, AD∆ strain with multiple pump deletion was hypersensitive to both types of chitosan. This implies that multiple pumps may play redundant roles in chitosan susceptibility, but it is also possible that simultaneous deletions of multiple pumps may disrupt membrane functions and cause cellular stress that enhances the effect of chitosan. Because ABC pumps play important roles in sterol transport and regulation of asymmetric lipid distribution across the plasma membrane lipid bilayer, multiple pump deletion may interfere with regulation of membrane traffic and cell division [Citation18]. In addition, AD∆ strain was shown to be hypersensitive to a wide range of xenobiotics, which may be direct effects of the pumps or secondary consequences of membrane disturbance [Citation19].
Interestingly, overexpression of C. albicans efflux pump genes important in clinical drug resistance (CDR1, CDR2 or MDR1) only minimally increased the resistance to chitosan in AD∆ strains. This is in contrast to the case of fluconazole susceptibility, where the MIC was increased by 400–600 folds, from 0.5 µg/ml in AD∆ to 200–300 µg/ml or to 75–150 µg/ml, when CDR1 or CDR2 was overexpressed, respectively [Citation13]. Although CDRs show substrate promiscuity and their overexpression in pump-deficient yeasts increased resistance to various structurally unrelated compounds [Citation6], our results showed that their overexpression affects neither oligomer nor polymer chitosan antifungal activity.
Among multiple C. albicans CDR genes, CDR1 plays predominant roles in drug resistance, while CDR2 have less effects [Citation6]. The deletion of CDR1 and CDR2 in C. albicans drastically increased azole sensitivity in both laboratory strains and clinically drug-resistant isolates [Citation15]. However, single or double CDR1 and CDR2 deletions had no effect on C. albicans sensitivity to chitosan. This supports the hypothesis that these major efflux pumps do not affect chitosan’s activity and that neither oligomer nor polymer is substrates of these pumps.
Chitosan has broad applications in medical and cosmetics industries because of its high biocompatibility, biodegradability and antibacterial and antifungal activities [Citation7]. We previously reported chitosan derivatives that are effective against common oral Candida species, including clinical isolates and biofilms [Citation9,Citation10,Citation20]. Importantly, oligomer and polymer chitosan were effective against most fluconazole-resistant isolates from HIV-infected individuals, emphasizing their potential as a promising antifungal agent against azole-resistant Candida [Citation10]. Furthermore, chitosan can serve as an active carrier to deliver and enhance the activity of antimicrobial drugs, and its mucoadhesive property can provide retention on the mucosa or under denture to prevent or treat denture stomatitis [Citation7,Citation9]. Therefore, future research into chitosan applications as alternative and/or adjunctive antifungal agent is warranted.
Conclusion
In conclusion, our results showed that S. cerevisiae and C. albicans were susceptible to both oligomer and polymer chitosan with minimal effects from drug efflux pumps. Thus, chitosan may be an effective alternative or adjunctive antifungal agent against drug-resistant C. albicans that overexpress drug efflux pumps.
Author contributions statement
S.M. participated in study design, conducted experiments, data analysis and interpretation and drafted the initial manuscript; P.C., P.S. and J.S. participated in study design, conducted experiments, data analysis and critically revised the manuscript; P.T. participated in study design, data interpretation and critically revised the manuscript; O.M. conceived the idea, designed the study, supervised data collection and analysis, interpreted the data, drafted and critically revised the manuscript. All the authors have read and approved the final manuscript.
Acknowledgments
This work was supported by grants from Asia Research Center (to OM and PT), Dental Research Grant from Faculty of Dentistry and Ratchadapiseksomphot endowment fund, Chulalongkorn University (to Center of Excellence on Oral Microbiology and Immunology). The authors are grateful to Profs. Gerald R. Fink (Whitehead Institute for Biomedical Research, USA), Richard Cannon (University of Otago, New Zealand), Laran T Jensen (Mahidol University, Thailand) and Jia-Ching Shieh (Chung Shan Medical University, Republic of China) for strains and plasmids. We also thank Ms. Sarocha Choochuay for technical assistance.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data generated in the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Bongomin F, Gago S, Oladele R, et al. Global and multi-national prevalence of fungal diseases—estimate precision. JoF. 2017;3(4):57. doi: 10.3390/jof3040057
- Pfaller MA, Diekema DJ, Turnidge JD, et al. Twenty years of the sentry antifungal surveillance program: results for Candida species from 1997–2016. Open Forum Infect Dis. 2019;6(Suppl 1):S79–S94. doi: 10.1093/ofid/ofy358
- Fisher MC, Denning DW. The WHO fungal priority pathogens list as a game-changer. Nat Rev Microbiol. 2023;21(4):211–8. doi: 10.1038/s41579-023-00861-x
- Kontoyiannis DP. Antifungal resistance: an emerging reality and a global challenge. J Infect Dis. 2017;216(suppl_3):S431–S435. doi: 10.1093/infdis/jix179
- Lee Y, Robbins N, Cowen LE. Molecular mechanisms governing antifungal drug resistanceNpj Antimicrob Resist. 2023;1(1):5. doi: 10.1038/s44259-023-00007-2
- Prasad R, Balzi E, Banerjee A, et al. All about CDR transporters: Past, present, and future. Yeast. 2019;36(4):223–233. doi: 10.1002/yea.3356
- Ke CL, Deng F-S, Chuang C-Y, et al. Antimicrobial actions and applications of chitosan. Polymers. 2021;13(6):904. doi: 10.3390/polym13060904
- Hosseinnejad M, Jafari SM. Evaluation of different factors affecting antimicrobial properties of chitosan. Int J Biol Macromol. 2016;85:467–475. doi: 10.1016/j.ijbiomac.2016.01.022
- Namangkalakul W, Benjavongkulchai S, Pochana T, et al. Activity of chitosan antifungal denture adhesive against common Candida species and Candida albicans adherence on denture base acrylic resin. J Prosthet Dent. 2020;123(1):e181 1–e181 7. doi: 10.1016/j.prosdent.2019.09.026
- Thanyasrisung P, Satitviboon W, Howattanapanich S, et al. Antifungal drug resistance in oral Candida isolates from HIV-infected and healthy individuals and efficacy of chitosan as an alternative antifungal agent. Arch Oral Biol. 2023;147:105628. doi: 10.1016/j.archoralbio.2023.105628
- Srimaneepong V, Thanamee T, Wattanasirmkit K, et al. Efficacy of low-molecular weight chitosan against Candida albicans biofilm on polymethyl methacrylate resin. Aust Dent J. 2021;66(3):262–269. doi: 10.1111/adj.12826
- Jensen AN, Chindaudomsate W, Thitiananpakorn K, et al. Improper protein trafficking contributes to artemisinin sensitivity in cells lacking the KDAC Rpd3p. FEBS Lett. 2014;588(21):4018–4025. doi: 10.1016/j.febslet.2014.09.021
- Lamping E, Monk BC, Niimi K, et al. Characterization of three classes of membrane proteins involved in fungal azole resistance by functional hyperexpression in Saccharomyces cerevisiae. Eukaryot Cell. 2007;6(7):1150–1165. doi: 10.1128/EC.00091-07
- Holmes AR, Tsao S, Lamping E, et al. Amino acid residues affecting drug pump function in Candida albicans–C. albicans drug pump function. Nippon Ishinkin Gakkai Zasshi. 2006;47(4):275–281. doi: 10.3314/jjmm.47.275
- Vyas VK, Barrasa MI, Fink GR. A Candida albicans CRISPR system permits genetic engineering of essential genes and gene families. Sci Adv. 2015;1(3):e1500248. doi: 10.1126/sciadv.1500248
- Min K, Ichikawa Y, Woolford CA, et al. Candida albicans gene deletion with a transient CRISPR-Cas9 system. mSphere. 2016;1(3). doi: 10.1128/mSphere.00130-16
- Lai WC, Sun H-F, Lin P-H, et al. A new rapid and efficient system with dominant selection developed to inactivate and conditionally express genes in Candida albicans. Curr Genet. 2016;62(1):213–235. doi: 10.1007/s00294-015-0526-6
- Rizzo J, Stanchev LD, da Silva VKA, et al. Role of lipid transporters in fungal physiology and pathogenicity. Comput Struct Biotechnol J. 2019;17:1278–1289. doi: 10.1016/j.csbj.2019.09.001
- Nakamura K, Niimi M, Niimi K, et al. Functional expression of Candida albicans drug efflux pump Cdr1p in a Saccharomyces cerevisiae strain deficient in membrane transporters. Antimicrob Agents Chemother. 2001;45(12):3366–3374. doi: 10.1128/AAC.45.12.3366-3374.2001
- Thienngern P, Panichuttra A, Ratisoontorn C, et al. Efficacy of chitosan paste as intracanal medication against Enterococcus faecalis and Candida albicans biofilm compared with calcium hydroxide in an in vitro root canal infection model. BMC Oral Health. 2022;22(1):354. doi: 10.1186/s12903-022-02385-x