Abstract
Background
Vulvoperineal defects resulting from surgical treatment of (pre)malignancies may result in reconstructive challenges. The vertical rectus abdominis muscle flap and, more recently, the fasciocutaneous lotus petal flap are often used for reconstruction in this area. The goal of this review is to compare the postoperative complications of application of these flaps. Methods: A comprehensive literature search of the PubMed, MEDLINE and Cochrane Library databases was performed until 6 June 2020. Search terms included the lotus petal flap, vertical rectus abdominis muscle flap and the vulvoperineal area. Articles were independently screened by two researchers according to the PRISMA-guidelines. Results: A total of 1074 citations were retrieved and reviewed, of which 55 were included for full text analysis. Following lotus petal flap reconstructions, the complication rate varied from 0.0% to 69.9%, with more complications concerning the recipient site compared with the donor site complications (26.0% versus 4.5%). Following vertical rectus abdominis muscle flap reconstructions the complication rate varied between 0.0% and 85.7% with almost twice the number of recipient site complications compared to donor site complications (37.1% versus 17.8%). Conclusions: Overall, the lotus petal flap has lower complication rates at both the donor and the recipient site compared with the vertical rectus abdominis muscle flap. When both options seem viable, the lotus petal flap procedure may be preferred on the basis of the reported lower complication rates.
Abbreviations: APE: abdominoperineal excision; ELAPE: extra levator abdominoperineal excision; LP flap: lotus petal flap; NIH: National Institute of Health; NR: not reported; RCT: randomized controlled trial; VRAM flap: vertical rectus abdominis myocutaneous flap
Introduction
The surgical treatment of gynecological and colorectal (pre)malignancies may result in vulvoperineal defects that cannot be closed primarily. The ablation leaves a soft tissue defect in an area of the body, where the bacterial count is high. Therefore, it is not surprising that wound infections are often encountered [Citation1]. Besides, most patients receive neo-adjuvant or adjuvant (chemo)radiotherapy which may cause delayed wound healing and increases the risk of developing wound complications [Citation2]. Wound complications occur in up to 22% of the cases with vulvoperineal wound closure without application of flaps [Citation3]. It has been proven that wound closure using a flap reconstruction helps to decrease the rate of wound-healing problems to 16%, by providing healthy, well-vascularized, and nonirradiated tissue [Citation4–6].
There are several reconstructive options for closure of vulvoperineal defects that cannot be closed by simple wound edge adaptation. One of the most commonly used myocutaneous flaps, is the vertical rectus abdominis myocutaneous (VRAM) flap [Citation7]. This flap has a rich vascularization and offers enough bulk to fill pelvic defects; however, in up to 25% of cases, abdominal wall herniation is described [Citation8]. Abdominal wall herniation in itself carries the risk of bowel strangulation and perforation. The most commonly used fasciocutaneous flap for the reconstruction of vulvoperineal defects in our practice is the lotus petal (LP) flap. This flap is based on the internal and external pudendal arteries and is a versatile flap. Flap harvest does not impair the donor site functionally and leaves a relatively inconspicuous scar. Nevertheless, rates of minor wound complications of 30% and risk of perineal herniation of up to 21% are still at hand [Citation2,Citation9]. Ten years ago the VRAM flap was the most commonly used flap for vulvoperineal reconstruction [Citation6]. Nowadays an LP flap also appears a viable option for reconstruction in the area. In our clinic, we have positive experiences with the LP flap. We experienced the LP flap procedure as a quick and easy to perform procedure, which can be performed in either prone or lithotomy position. Also, the donor site is left with just minimal functional compromises and results in an inconspicuous, easy to hide, scar [Citation10]. Earlier reports show an acceptable major complication rate [Citation11]. Therefore, over the past decade, it has become the first option for reconstruction of vulvoperineal defects. However, the advantages and disadvantages of these reconstructions have never been systematically reviewed.
The purpose of this study is to provide a thorough analysis of the literature regarding post-operative complications following reconstruction of vulvar, perineal or vulvoperineal defects with a VRAM or LP flap. Our goal is to review whether or not there are differences in complications in general and differences between the complications occurring at the donor site and recipient site more specific. We aim to identify evidence-based advantages and disadvantages of each reconstruction procedure. This information can help the plastic surgeon during patient counselling, decision making and follow-up.
Methods
Study selection
This systematic review was performed according to the guidelines of the Cochrane Collaboration and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (www.prisma-statement.org). The protocol was developed a priori and registered in the PROSPERO database (CRD42017056537).
The search strategy was conducted in collaboration with an information specialist of the University Medical Center Groningen medical library. The search strategy was developed using the PICO method. The participants (‘P’) were patients with vulvar, perineal or vulvoperineal defects, the intervention (‘I’) was either an LP flap or a VRAM flap. The comparison (‘C’) and outcome (‘O’) were left open to assure a wide search result. The search strategies are shown in . An initial literature search was performed on 13 October 2015 in the PubMed and Embase database. The search was updated on 6 June 2020. References of all included studies were screened for eligibility.
Table 1. Search strategies.
The study selection was performed in two rounds: (1) title-abstract round; (2) full-text round. Two authors (J.H. and M.R.) independently assessed all articles retrieved from the search. After each round discrepancies were discussed to reach consensus. In case no consensus was reached, the senior author (M.W.S.) was consulted. If in the first-round inclusion or exclusion criteria could not be assessed from the title and abstract, the study was included for the full-text round. Inclusion- and exclusion criteria are shown in .
Table 2. Inclusion- and exclusion criteria.
Quality assessment
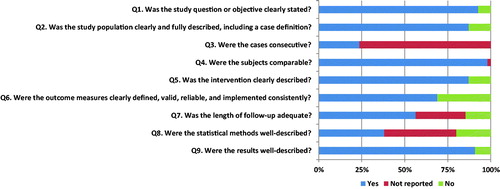
All full-text selected articles were independently scored by two authors (J.H. and M.R.). The articles by Hellinga et al. [10,Citation11] were scored by the second author (M.R.) and an independent epidemiologist to avoid a conflict of interest. We used the ‘Quality Assessment Tool for Case Series Studies’ from the National Institute of Health. (NIH; https://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduction/tools/case-control) This tool is based on nine criteria with binary options (‘Yes’ or ‘No’) (). Follow-up of at least 12 months was regarded adequate, in case of a shorter follow-up, this criterion was answered with ‘no’. We determined the final score based on the number of times ‘yes’ was marked. A final score of less or equal than 4 times ‘yes’ indicated a ‘poor’ quality, 5–7 times ‘yes’ ‘fair’ quality and 8–9 times ‘yes’ ‘good’ quality. Discrepancies were handled as described above for both the title-abstract and full-text round. Cohen’s kappa was determined to measure the agreement of the quality assessment score between the two authors.
Data collection
Data collection was performed by the first author (J.H.) and cross-checked by the second author (M.R.). We extracted the age, sex, length of follow-up, indication for resection, type of resection, complications, and postoperative sexual function. The number of complications was extracted as an absolute number since the number of complications per patient was mostly not reported. In case the number of patients with complications was also reported, this percentage was also collected. Complications were categorized in reconstruction site complications and other complications. All reconstruction site complications were grouped by donor site and recipient site. The complications were defined as minor and major, based on the Clavien-Dindo classification, in which minor complications required no intervention or only pharmacological treatment and major complications required surgical intervention [Citation12].
Analysis
Data on LP flap and VRAM flap reconstruction were analyzed separately. For each complication, a weighted average was calculated.
Results
Literature search
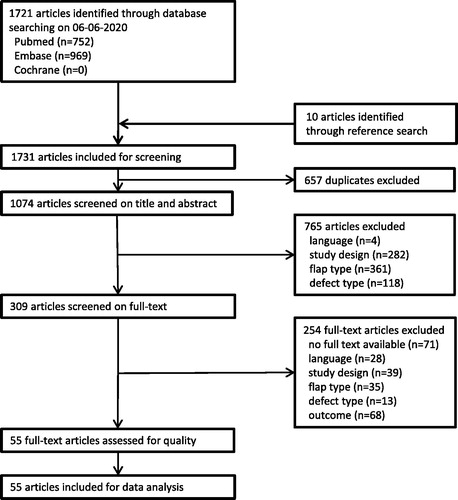
The search yielded 752 citations in Pubmed and 969 citations in Embase. The reference check included ten extra results. In total, 1731 articles were included. After excluding duplicates, 1074 articles remained. No overlapping subjects were found between studies. After title, abstract and full-text selection 55 articles remained for quality assessment. The flowchart of the selection procedure is shown in .
Quality assessment
The interrater agreement on the quality assessment score was substantial with an overall agreement of 87.3% (48 out of 55; κ = 0.758; p = 0.000) [Citation13]. Results of the quality assessment are shown in and the overall score of each article is shown in Appendices I and III. The majority of the studies reached a final score of fair (n = 37; 67.3%). Fourteen studies (25.5%) scored good and four studies (7.3%) scored poor. Overall, most studies clearly stated their study question (n = 51; 92.7%), and results (n = 50; 90.9%). The most frequently not reported item was question 3 ‘Were the cases consecutive?’ (n = 42; 76.4%).
Study characteristics
LP reconstruction
Sixteen out of the 55 articles described the application of the LP flap for reconstructions. Yii et al. [Citation14] were the first in 1996 to report on reconstruction using the LP flap. All but three of these studies are case series [Citation14–26]. Only Negosanti et al. [Citation27], Confalonieri et al. [Citation18] and Thiele et al. [Citation28] reported a case control study in which a reconstruction with LP flaps was compared with other flaps. About half of the studies (56.3%) were small studies (<20 patients), the largest was that of Confalonieri et al. which included 106 patients [Citation14,Citation18,Citation20–22,Citation24,Citation26–28]. In 62.5% of the studies, only females were included [Citation14,Citation15,Citation17–19,Citation21,Citation23,Citation25–27]. Mean age of the subjects varied between 50.3 years and 79 years [Citation21,Citation22]. Length of follow-up was reported in 56.3% of the studies and varied between a mean of 10 and 84 months (details in Appendix I) [Citation17,Citation18].
VRAM reconstruction
A total of 39 articles described the use of the VRAM flap for reconstruction. The first study on VRAM reconstruction was reported in 1989 by Kroll et al. [Citation29]. Most studies were case series (64.1%), 33.3% were case control studies in which the VRAM flap was compared with primary closure or other flaps, and one randomized clinical trial was reported [Citation30]. Most studies (53.8%) were small (<20 subjects), the largest study group consisted of 114 patients [Citation31]. All but one of the studies reported the mean age of the patients, and age varied between 45 and 70.6 years [Citation29,Citation32,Citation33]. Length of follow-up varied between a median of nine months and a mean of 54 months. Seventeen studies (43.6%) did not (completely) report their length of follow-up [Citation5,Citation25,Citation33–47]. (details in Appendix II)
Resection characteristics
LP reconstruction
The main indication for resection in articles were LP reconstruction was performed, were vulvar/vaginal cancer (77.0%) and vulvar dysplasia (10.6). Resection of colorectal or anal cancer was only in 4.6% of the cases indication for reconstruction (; details in Appendix I). In most cases a total (40.8%) or partial (13.5%) vulvectomy was performed. Kim et al. [Citation22] did not report the exact type of resection and Argenta et al. [Citation15] only reported that extirpative surgery was performed (; details in Appendix I).
Table 3. Summary of indication for resection – LP studies.
Table 4. Summary of type of resection – LP studies.
VRAM reconstruction
The main indication for resection preceding VRAM reconstruction, was colorectal cancer (54.3%). Anal cancer was the indication for resection in 15.5% of the cases, uterine or cervical cancer in 9.6% and vulvar or vaginal cancer in 7.0% of the cases (; details in Appendix II). Three studies did not report the indication for resection [Citation29,Citation48,Citation49]. The main types of resection performed were an abdominoperineal excision (APE) (52.7%) or total exenteration (13.4%) (; details in Appendix II). Four studies did not report the type of resection performed [Citation38,Citation39,Citation49,Citation50].
Table 5. Summary of indication for resection – VRAM studies.
Table 6. Summary of type of resection – VRAM studies.
Complications of reconstruction site
LP reconstruction
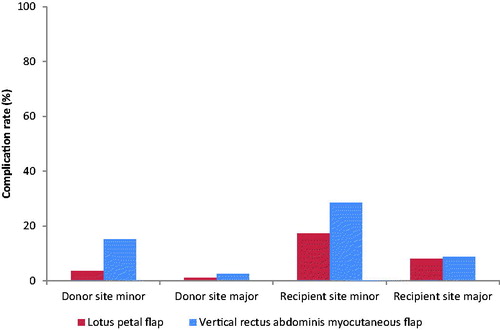
The number of patients with any reconstruction site complication following reconstruction with the LP flap varies from 0.0% to 69.9% [Citation11,Citation14,Citation16,Citation22]. All studies on reconstruction with the LP flap reported whether there were complications in either the donor or recipient site, or not. Most complications occurred at the recipient site (26.0% versus 4.5%). The donor site showed mostly minor complications (3.5% versus 1.0% major complications). Main indication for intervention at the donor site was wound dehiscence (60%). For the recipient site also more minor than major complications were reported (17.8% versus 8.2%). Three studies reported no recipient site complications [Citation14,Citation16,Citation22]. Partial flap loss and partial wound dehiscence accounted for most of the reported complications (5.0% resp. 3.5%). Complete flap loss only occurred in 0.4% of the cases (; details Appendix III). shows the minor and major complication rates of both the donor and recipient site.
Table 7. Summary of reconstruction site complications – LP studies.
VRAM reconstruction
The reported percentage of patients with any reconstruction site complication showed a wide variance from 0.0% to 85.7% [Citation51,Citation52]. Most (59.0%) studies only reported the total amount of reconstruction site complications, and not the complications per patient. Twice as many recipient site complications, following reconstruction with the VRAM flap, were reported compared to donor site complications (39.4% versus 19.3%). In eight (20.5%) studies, no donor site complications occurred [Citation41,Citation49,Citation52–56]. Eight (20.5%) studies did not distinguish between minor and major complications for the donor site [Citation5,Citation7,Citation30,Citation31,Citation39,Citation57–59]. In the remaining studies, minor complications were reported more often than major complications (15.2% versus 2.6%). Partial wound dehiscence and wound infection were the most commonly reported complications of the donor site (7.7% resp. 4.0%). In the study of Sheckter et al. [Citation49] no recipient site complications occurred. Nine (23.1%) studies did not make the distinction between minor and major recipient site complications [Citation30,Citation31,Citation39,Citation42,Citation47,Citation54,Citation57–59]. Minor complications were reported in 28.4% of the cases and major complications in 8.7% of the cases. The most common complication of the recipient site was partial wound dehiscence (10.1%). Also abscess formation (4.5%) and partial flap loss (4.3%) occurred relatively often. Complete flap loss was reported in 2.2% of the cases (; details in Appendix IV). shows the minor and major complication rates of both the donor and recipient site.
Table 8. Summary of reconstruction site complications – VRAM studies.
General complications
LP reconstruction
Two studies reported that no general complications occurred [Citation14,Citation28]. Complications reported in other studies were urinary tract infection (1.3%), deep venous thrombosis (1.3%) and cerebrovascular incident (1.3%) (details in Appendix III).
VRAM reconstruction
The most common general complications following VRAM flap reconstruction were urinary tract infection (3.8%), small bowel obstruction (3.2%), parastomal herniation (1.9%) and deep venous thrombosis (1.7%) (details in Appendix IV).
Sexual function
LP reconstruction
Two studies reported the sexual outcome following LP flap reconstruction [Citation17,Citation25]. The study by Ragoowansi et al. [Citation25] reported that 17% of the patients had returned to sexual activity in 6–9 months following the reconstruction. The other study reported that all ‘sexual active patients’ did not report any problems, however they did not mention the rate of sexual active patients [Citation17].
VRAM reconstruction
Eight studies reported details on postoperative sexual function [Citation36,Citation39,Citation44,Citation47,Citation55,Citation60–62]. Three of the studies used a questionnaire and one study performed a postoperative interview [Citation44,Citation47,Citation61,Citation62]. Rates of return to sexual activity ranged from 26.7–50.0%. However, Casey et al. [Citation39] reported that 18 of the 35 patients were sexually active preoperative and 17 of them were remained active postoperative. The trend showed that younger patients returned more often to sexual activity, however most patients reported a lower quality of their sexual activity. However, Cortinovis et al. [Citation62] reported a higher satisfaction with their sexual activity postoperative.
Discussion
Summary of evidence
Vulvoperineal defects have often been closed with VRAM flaps, however over the last years the LP flap is gaining popularity. Therefore, the aim of this systematic review was to compare the postoperative complications of the LP flap procedure and the VRAM flap procedure for vulvoperineal reconstruction by performing a thorough analysis of literature. Our analysis suggest that patients following the LP flap procedure experience a relative lower number of postoperative reconstruction site complications compared to VRAM flap procedure.
While the LP flap was only described in 1996 by Yii and Niranjan, the VRAM flap was first described for the reconstruction of perineal wounds in 1984 by Shukla et al. [Citation14,Citation63] However, the first articles included in this review on the VRAM flap originate from 1989 [Citation29]. The resulting shorter period of use of LP reconstructions compared to VRAM reconstructions is a clear confounder in our search results. The search included 14 articles on LP flap reconstruction, mainly case series and small study populations, whereas it yielded 39 VRAM flap reconstruction articles, both case series and case controls, and one randomized clinical trial. Our quality assessment scores also showed a lower quality of the LP flap articles compared to the VRAM flap articles. The difference in included articles for both groups and as a result the difference in quality of the articles, may have affected the results of our review.
The indication for resection and type of resection preceding LP flap reconstruction is mainly a gynecological tumor (87.6%) that needed total or partial vulvectomy (54.3%) Colorectal or anal tumors are preceding LP reconstruction only in 4.6% of the patients. In patients of the VRAM flap group the picture is different; 69.8% had a colorectal or anal tumor and 16.6% a gynecological tumor. APE or any type of exenteration was performed in 85.2% of the cases. The difference in indication for and type of resection varies between both groups of patients. This may have caused a selection bias in the application of the flaps. This systematic review revealed that the VRAM flap is more often used following resection of extensive colorectal tumors. This may give the impression that an LP reconstruction is not always feasible for such defects and that the comparison we performed in this review is not a fair one. However, we think that a more appropriate explanation for this difference is that the LP flap was initially only described for vulvar reconstruction. Our group however has very positive experience with the application of LP flaps for reconstruction in the perineal area following APE/ELAPE [Citation10,Citation64]. Each flap of course has its own specific donor site complications. Therefore, when comparing different flaps, only the comparison of generic complications between both reconstruction techniques is relevant for clinical decision making. We are of the opinion that the difference in recipient sites following gynecological respectively anorectal tumor resections does not greatly influence generic donor site complication or flap complication rates. This makes comparison of those techniques, despite the different anatomical reconstruction sites.
The variance in number of patients with one or more reconstruction site complications is smaller following LP flap reconstruction compared to VRAM flap reconstruction (0.0–69.9% versus 0.0%–85.7%). Both reconstruction types show higher number of recipient site complications than donor site complications. However, the percentage of recipient site complications following the LP flap procedure is less than half of that following a VRAM flap procedure. This difference is even larger for reported donor site complications. Both reconstruction procedures show more minor recipient site complications compared to major recipient site complications. The minor/major ratio is a little higher following the LP flap procedure. The types of complications are comparable, but no large wound dehiscences were reported following LP flap reconstruction. Also the total percentage of partial and complete flap loss were lower following the LP flap procedure.
Complications other than those of the reconstruction site were rare, but more frequent following VRAM flap reconstructions. These complications were mostly related to the long operation time of the resection and reconstruction (e.g. urinary tract infection, deep venous thrombosis and small bowel obstruction). No parastomal herniations were reported following LP flap reconstruction which could be explained by the low rate of colorectal resections in this group.
Sexual dysfunction was rarely reported following LP flap (12.%) as well as VRAM flap application (20.5%) [17,Citation25,Citation36,Citation39,Citation44,Citation47,Citation55,Citation60–62]. We find it surprising that there is so little research on the topic of sexual dysfunction, especially since it concerns surgery in the vulvoperineal area. We suppose that postoperative sexual activity rates could also be influence by area of resection, either vulvar or perineal.
Limitations
As in every systematic review the quality of the available evidence greatly influenced the strength of our results. Especially the LP flap group contained only few high evidence studies with poor scores on the quality assessment. The large variability in indication for resection and low uniformity in presenting the reconstruction site complications also undermined the quality of the results. Also the poor levels of presenting data (e.g. other complications) affected the results presented. Unfortunately, there is a lack of prospective studies. Also, a randomized controlled trial is unethical. At best a properly powered multicenter propensity matched control study may be able to reveal the differences more clearly. Therefore our conclusion should be drawn with care.
We tried to give insight in possible publication bias by drawing a funnel plot. However, more than half of the studies did not report the number of patients with one or more complications. In these studies, the complication rate as percentage of included patients cannot be determined. As a consequence, studies with a complication rate of zero would be overrepresented in a funnel plot. Since the complication rate might depend on reconstruction type, a funnel plot would provide an incorrect representation of publication bias, or even introduce selection bias. Therefore, we decided not to include it in this paper.
We aimed to reduce the language bias by also including Dutch and German language articles, besides English language articles. It is thought that positive results will mainly be reported in English language journals. However, the evidence to support this small effect is weak [Citation65]. Unfortunately, there were no studies available that compared both reconstruction procedures in one study and therefore we were not able to perform a meta-analysis.
Conclusions
This systematic review demonstrated lower complication rates in both the donor site and the recipient site, following the LP flap procedure compared to the VRAM flap procedure. This knowledge could guide the plastic surgeon during counselling and to take the decision for either reconstruction technique. In case in which both reconstruction procedures can be applied, the LP flap procedure should be considered owing to the relatively low complication rates.
Disclosure statement
The authors have nothing to disclose.
References
- Althumairi AA, Canner JK, Gearhart SL, et al. Risk factors for wound complications after abdominoperineal excision: analysis of the ACS NSQIP database. Colorectal Dis. 2016;18(7):O260–6.
- Winterton RI, Lambe GF, Ekwobi C, et al. Gluteal fold flaps for perineal reconstruction. J Plast Reconstr Aesthet Surg. 2013;66(3):397–405.
- Bertucci Zoccali M, Biondi A, Krane M, et al. Risk factors for wound complications in patients undergoing primary closure of the perineal defect after total proctectomy. Int J Colorectal Dis. 2015;30(1):87–95.
- Althumairi AA, Canner JK, Gearhart SL, et al. Predictors of perineal wound complications and prolonged time to perineal wound healing after abdominoperineal resection. World J Surg. 2016;40(7):1755–1762.
- Chessin DB, Hartley J, Cohen AM, et al. Rectus flap reconstruction decreases perineal wound complications after pelvic chemoradiation and surgery: a cohort study. Ann Surg Oncol. 2005;12(2):104–110.
- Devulapalli C, Tong Jia Wei A, DiBiagio JR, et al. Primary versus flap closure of perineal defects following oncologic resection: a systematic review and meta-analysis. Plast Reconstr Surg. 2016;137(5):1602–1613.
- Butler CE, Gündeslioglu AÖ, Rodriguez-Bigas MA. Outcomes of immediate vertical rectus abdominis myocutaneous flap reconstruction for irradiated abdominoperineal resection defects. J Am Coll Surg. 2008;206(4):694–703.
- O'Dowd V, Burke JP, Condon E, et al. Vertical rectus abdominis myocutaneous flap and quality of life following abdominoperineal excision for rectal cancer: a multi-institutional study. Tech Coloproctol. 2014;18(10):901–906.
- Christensen HK, Nerstrøm P, Tei T, et al. Perineal repair after extralevator abdominoperineal excision for low rectal cancer. Dis Colon Rectum. 2011;54:711–717.
- Hellinga J, Khoe PCKH, Van Etten B, et al. Fasciocutaneous lotus petal flap for perineal wound reconstruction after extralevator abdominoperineal excision: application for reconstruction of the pelvic floor and creation of a neovagina. Ann Surg Oncol. 2016;23(12):4073–4079.
- Hellinga J, Khoe PCKH, Stenekes MW, et al. Complications after vulvar and perineal reconstruction with a lotus petal flap. Ann Plast Surg. 2018;80(3):268–271.
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213.
- Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174.
- Yii NW, Niranjan NS. Lotus petal flaps in vulvo-vaginal reconstruction. Br J Plast Surg. 1996;49(8):547–554.
- Argenta PA, Lindsay R, Aldridge RB, et al. Vulvar reconstruction using the “lotus petal” fascio-cutaneous flap. Gynecol Oncol. 2013;131(3):726–729.
- Bodin F, Dissaux C, Seigle-Murandi F, et al. Posterior perineal reconstructions with “supra-fascial” lotus petal flaps. J Plast Reconstr Aesthet Surg. 2015;68(1):e7.
- Buda A, Confalonieri PL, Rovati LCV, et al. Better anatomical and cosmetic results using tunneled lotus petal flap for plastic reconstruction after demolitive surgery for vulvar malignancy. Int J Gynecol Cancer. 2012;22(5):860–864.
- Confalonieri PL, Gilardi R, Rovati LC, et al. Comparison of V-Y advancement flap versus lotus petal flap for plastic reconstruction after surgery in case of vulvar malignancies: a retrospective single center experience. Ann Plast Surg. 2017;79(2):186–191.
- Franchelli S, Leone MS, Bruzzone M, et al. The gluteal fold fascio-cutaneous flap for reconstruction after radical excision of primary vulvar cancers. Gynecol Oncol. 2009;113(2):245–248.
- Hashimoto I, Nakanishi H, Nagae H, et al. The gluteal-fold flap for vulvar and buttock reconstruction: anatomic study and adjustment of flap volume. Plast Reconstr Surg. 2001;108(7):1998–2005.
- Herraiz Roda JL, Llueca Abella JA, Maazouzi Y, et al. Vulvar reconstruction in vulvar cancer: “lotus petal” suprafascial flap. Gynecol Surg. 2016;13(1):51–55.
- Kim JT, Ho SY, Hwang JH, et al. Perineal perforator-based island flaps: the next frontier in perineal reconstruction. Plast Reconstr Surg. 2014;133(5):683e
- Misani M, Rovati LC, Confalonieri P, et al. Modified lotus petal flap for vulvo-vaginal reconstruction after resection for vulvar cancer: a single institution experience . Handchir Mikrochir Plast Chir. 2011;43(4):250–254.
- Pantelides NM, Davies RJ, Fearnhead NS, et al. The gluteal fold flap: a versatile option for perineal reconstruction following anorectal cancer resection. J Plast Reconstr Aesthet Surg. 2013;66(6):812–820.
- Ragoowansi R, Yii N, Niranjan N. Immediate vulvar and vaginal reconstruction using the gluteal-fold flap: long-term results. Br J Plast Surg. 2004;57(5):406–410.
- Sawada M, Kimata Y, Kasamatsu T, et al. Versatile lotus petal flap for vulvoperineal reconstruction after gynecological ablative surgery. Gynecol Oncol. 2004;95(2):330–335.
- Negosanti L, Sgarzani R, Fabbri E, et al. Vulvar reconstruction by perforator flaps: algorithm for flap choice based on the topography of the defect. Int J Gynecol Cancer. 2015;25(7):1322–1327.
- Thiele JR, Weber J, Neeff HP, et al. Reconstruction of perineal defects: a comparison of the myocutaneous gracilis and the gluteal fold flap in interdisciplinary anorectal tumor resection. Front Oncol. 2020;10:668
- Kroll SS, Pollock R, Jessup JM, et al. Transpelvic rectus abdominis flap reconstruction of defects following abdominal-perineal resection. Am Surg. 1989;55(10):632–637.
- Touny A, Othman H, Maamoon S, et al. Perineal reconstruction using pedicled vertical rectus abdominis myocutaneous flap (VRAM). J Surg Oncol. 2014;110(6):752–757.
- Nelson RA, Butler CE. Surgical outcomes of VRAM versus thigh flaps for immediate reconstruction of pelvic and perineal cancer resection defects. Plast Reconstr Surg. 2009;123(1):175–183.
- Jain AK, Defranzo AJ, Marks MW, et al. Reconstruction of pelvic exenterative wounds with transpelvic rectus abdominis flaps: A case series. Ann Plast Surg. 1997;38(2):115–123.
- Kouraklis G. Reconstruction of the pelvic floor using the rectus abdominis muscles after radical pelvic surgery. Dis Colon Rectum. 2002;45(6):836–839.
- Barker JA, Blackmore AE, Owen RP, et al. Prone cylindrical abdominoperineal resection with subsequent rectus abdominis myocutaneous flap reconstruction performed by a colorectal surgeon. Int J Colorectal Dis. 2013;28(6):801–806.
- Barker T, Branagan G, Wright E, et al. Vertical rectus abdominis myocutaneous flap reconstruction of the perineal defect after abdominoperineal excision is associated with low morbidity. Colorectal Dis. 2013;15:1177–83.
- Bell SW, Dehni N, Chaouat M, et al. Primary rectus abdominis myocutaneous flap for repair of perineal and vaginal defects after extended abdominoperineal resection. Br J Surg. 2005;92(4):482–486.
- Berger JL, Westin SN, Fellman B, et al. Modified vertical rectus abdominis myocutaneous flap vaginal reconstruction: an analysis of surgical outcomes. Gynecol Oncol. 2012;125(1):252–255.
- Buchel EW, Finical S, Johnson C. Pelvic reconstruction using vertical rectus abdominis musculocutaneous flaps. Ann Plast Surg. 2004;52(1):22–26.
- Casey WJ, Tran NV, Petty PM, et al. A comparison of 99 consecutive vaginal reconstructions: an outcome study. Ann Plast Surg. 2004;52(1):27–30.
- Creagh TA, Dixon L, Frizelle FA. Reconstruction with vertical rectus abdominus myocutaneous flap in advanced pelvic malignancy. J Plast Reconstr Aesthet Surg. 2012;65(6):791–797.
- Erdmann MWH, Waterhouse N. The transpelvic rectus abdominis flap: its use in the reconstructin of extensive perineal defects. Ann R Coll Surg Engl. 1995;77:229–232.
- De Haas WG, Miller MJ, Temple WJ, et al. Perineal wound closure with the rectus abdominis musculocutaneous flap after tumor ablation. Ann Surg Oncol. 1995;2(5):400–406.
- Hinojosa MW, Parikh DA, Menon R, et al. Recent experience with abdominal perineal resection with vertical rectus abdominis myocutaneous flap reconstruction after preoperative pelvic radiation. Am Surg. 2009;75(10):995–999.
- Holman FA, Martijnse IS, Traa MJ, et al. Dynamic article: Vaginal and perineal reconstruction using rectus abdominis myocutaneous flap in surgery for locally advanced rectum carcinoma and locally recurrent rectum carcinoma. Dis Colon Rectum. 2013;56(2):175–185.
- Houvenaeghel G, Ghouti L, Moutardier V, et al. Rectus abdominis myocutaneous flap in radical oncopelvic surgery: a safe and useful procedure. Eur J Surg Oncol. 2005;31(10):1185–1190.
- Petrie N, Branagan G, McGuiness C, et al. Reconstruction of the perineum following anorectal cancer excision. Int J Colorectal Dis. 2009;24(1):97–104.
- Smith HO, Genesen MC, Runowicz CD, et al. The rectus abdominis myocutaneous flap: modifications, complications, and sexual function. Cancer. 1998;83(3):510–520.
- Chokshi RJ, Kuhrt MP, Arrese D, et al. Reconstruction of total pelvic exenteration defects with rectus abdominus myocutaneous flaps versus primary closure. Am J Surg. 2013;205(1):64–70.
- Sheckter CC, Shakir A, Vo H, et al. Reconstruction following abdominoperineal resection (APR): indications and complications from a single institution experience. J Plast Reconstr Aesthet Surg. 2016;69(11):1506–1512.
- Loessin SJ, Meland NB, Devine RM, et al. Management of sacral and perineal defects following abdominoperineal resection and radiation with transpelvic muscle flaps. Dis Colon Rectum. 1995;38(9):940–945.
- Espinosa-de-Los-Monteros A, Arista-de la Torre L, Vergara-Fernandez O, et al. Contralateral component separation technique for abdominal wall closure in patients undergoing vertical rectus abdominis myocutaneous flap transposition for pelvic exenteration reconstruction. Ann Plast Surg. 2016;77(1):90–92.
- Hardt J, Mai S, Weiß C, et al. Abdominoperineal resection and perineal wound healing in recurrent, persistent, or primary anal carcinoma. Int J Colorectal Dis. 2016;31(6):1197–1203.
- Nuñez JE, Alvarado NC, Pereira AS, et al. Abdominoperineal resection in anal cancer: reconstruction of the perineum with a myocutaneous flap from the anterior rectus abdominis muscle. Cir Esp. 2011;89:31–36.
- Peacock O, Pandya H, Sharp T, et al. Biological mesh reconstruction of perineal wounds following enhanced abdominoperineal excision of rectum (APER). Int J Colorectal Dis. 2012;27(4):475–482.
- Shepherd JH, Van Dam PA, Jobling TW, et al. The use of rectus abdominis myocutaneous flaps following excision of vulvar cancer. Br J Obstet Gynaecol. 1990;97(11):1020–1025.
- Haverland R, Rebecca AM, Hammond J, et alJ. A case series of robot-assisted rectus abdominis flap harvest for pelvic reconstruction: a single institution experience. J Minim Invasive Gynecol. 2020.
- Horch RE, Hohenberger W, Eweida A, et al. A hundred patients with vertical rectus abdominis myocutaneous (VRAM) flap for pelvic reconstruction after total pelvic exenteration. Int J Colorectal Dis. 2014;29(7):813–823.
- Gupta V, Lennox GK, Covens A. The rectus abdominus myoperitoneal flap for vaginal reconstruction. Gynecol Oncol Rep. 2020;32:100567.
- Stein MJ, Karir A, Ramji M, et al. Surgical outcomes of VRAM versus gracilis flaps for the reconstruction of pelvic defects following oncologic resection. J Plast Reconstr Aesthet Surg. 2019;72(4):565–571.
- Pursell SH, Day TG, Jr, Tobin GR. Distally based rectus abdominis flap for reconstruction in radical gynecologic procedures. Gynecol Oncol. 1990;37(2):234–238.
- Tei TM, Stolzenburg T, Buntzen S, et al. Use of transpelvic rectus abdominis musculocutaneous flap for anal cancer salvage surgery. Br J Surg. 2003;90(5):575–580.
- Cortinovis U, Sala L, Bonomi S, et al. Rectus abdominis myofascial flap for vaginal reconstruction after pelvic exenteration. Ann Plast Surg. 2018;81(5):576–583.
- Shukla HS, Hughes LE. The rectus abdominis flap for perineal wounds. Ann R Coll Surg Engl. 1984;66(5):337–339.
- Hellinga J, Stenekes MW, Werker PMN, et al. Quality of life, sexual functioning, and physical functioning following perineal reconstruction with the lotus petal flap. Ann Surg Oncol. 2020.
- Jüni P, Holenstein F, Sterne J, et al. Direction and impact of language bias in meta-analyses of controlled trials: empirical study. Int J Epidemiol. 2002;31(1):115–123.
- Bakx R, van Lanschot JJ, Zoetmulder FA. Inferiorly based rectus abdominis myocutaneous flaps in surgical oncology: indications, technique, and experience in 37 patients. J Surg Oncol. 2004;85(2):93–97.
- Combs PD, Sousa JD, Louie O, et al. Comparison of vertical and oblique rectus abdominis myocutaneous flaps for pelvic, perineal, and groin reconstruction. Plast Reconstr Surg. 2014;134(2):315–323.
- Pang J, Broyles JM, Berli J, et al. Abdominal- versus thigh-based reconstruction of perineal defects in patients with cancer. Dis Colon Rectum. 2014;57(6):725–732.
Appendix 1
Appendix A. Characteristics of included studies with LP flaps
Appendix 2
Appendix B. Complications of included studies with LP flaps.
Appendix 3
Appendix C. Characteristics of included studies with VRAM flaps