Abstract
Lymphedema is caused by dysfunctional lymph vessels or as a complication of cancer treatment leading to edema and adipose tissue deposition. One hypothesis is that adipocyte hypertrophy contributes to the volume increase in lymphedema. The aim of the study was to compare adipocyte size in arm and leg lymphedema and controls. The adipocyte size difference was also compared between the arms and legs. Furthermore, any link between adipocyte size difference and gender, lymphedema onset, duration, previous radio- and chemotherapy was studied, as well as any relationship to total excess volume increase in the extremities, body mass index (BMI) and body weight. Adipose tissue biopsies from the lymphedematous and non-affected extremities were taken from 47 patients. The adipocytes sizes were measured using an Olympus PROVIS microscope, Olympus DP50 camera (Olympus, Tokyo, Japan) and ImageJ program (NIH, Bethesda, MD). Additional information was obtained from the Lymphedema Center database. The data were assembled in Excel and statistics was calculated in SPSS® Statistics 23 (IBM®, Armonk, NY). The adipocyte size (mean ± SEM) in the lymphedematous extremities was significantly larger, 8880 ± 291 μm2, compared to the adipocyte size in the non-affected extremities, where it was 7143 ± 280 μm2, i.e. 24% larger (p < .001). The adipocyte size increase was larger in arm than in leg lymphedema. No correlation was found between adipocyte size and gender or onset. However, a negative correlation was found between adipocyte size difference and duration. No correlation was found between adipocyte size and previous chemo- or radiotherapy. There was a positive correlation between adipocyte size and BMI. Hypertrophy of adipocytes was seen in the lymphedematous extremities versus control and contributes to the excess volume.
Introduction
Lymphedema
Lymphedema is a chronic condition and in developed countries it is a common complication of cancer treatment involving lymph node removal and postoperative irradiation. It is known that around 50% of patients undergoing lymph node dissection followed by irradiation for cancer treatment will develop lymphedema [Citation1,Citation2]. The most common cause of lymphedema globally is infection with parasitic worms, called filarial worms, which are prevalent in tropical and subtropical countries [Citation3].
Lymphedema is a chronic and progressive condition with no curative treatment, and sequels of lymphedema as a complication of cancer treatment are even reported by patients to be more difficult to deal with than the original cancer treatment [Citation4]. Lymphedema leads to cosmetic as well as physical disabilities due to a vastly enlarged extremity. Usual complaints include pain, tension, heaviness and weakness, sensory deficit of the extremity as well as clothing difficulties, anxiety and social stigmatization [Citation5–7]. Lymphedema also weakens the immune system leading to repeated erysipelas attacks, and also causes heavy strain on the shoulder and neck when localized in the upper extremities. Lymphedema causes functional impairment and limits physical mobility and activity which leads to further negative effects on health and quality of life [Citation8].
Lymph accumulation in peripheral tissue
Lymph vessel dysfunction in lymphedema leads to accumulation of lymph in peripheral tissue and results in tissue edema. In secondary lymphedema, the combination of lymphatic vessel damage, removal of lymph nodes and scarring of the surrounding tissues leads to an overload of the remaining lymph vessels. These become dilated and their valves become inefficient, thus unable to transport the lymph fluid. This effect is then spread to the very distal connected lymphatic vessels [Citation8]. The same goes for primary lymphedema.
Fat deposition causing volume excess
In addition to accumulation of lymph fluid, deposition of adipose tissue begins within the first year after the debut of lymphedema [Citation9]. Previous research has shown that the excess volume, that is the difference in volume between the affected and the non-affected extremity, in a non-pitting arm lymphedema comprises 73–81% of fat [Citation6,Citation9]. Lymphatic ablation in the tails of mice resulted in subcutaneous adipose tissue deposition with a twofold increase in fat thickness [Citation10]. The deposition of adipose tissue explains why compression therapy and microsurgical reconstructions are not fully effective alone, and why liposuction is required to remove large excess volume in late-stage non-pitting lymphedema. Liposuction shows complete reduction of the excess volume in patients with post-mastectomy arm lymphedema after a 15-year follow-up. Just as in non-surgical treatment, the use of compression garments is nevertheless mandatory and lifelong to retain the effect of liposuction [Citation8].
The mechanism behind fat deposition
There is no cure for lymphedema. Despite previous research, there is still too little clarity on the etiology of lymphedema and the mechanism for the adipose tissue deposition, which continues to hinder the development of new targeted or preventive treatments [Citation11].
A viable hypothesis is that adipose tissue hypertrophy greatly contributes to the extremity enlargement in lymphedema. Research shows a connection of adipose tissue hypertrophy with a slow lymph flow and structural changes in the lymphatic system [Citation12,Citation13]. In patients with lymphedema, a correlation has been found between total excess volume in the lymphedematous arm and the excess fat volume [Citation9]. It is known that adipose tissue also functions as an endocrine active organ, which can be influenced by cytokines, thus chronic inflammation may play an important role in fat deposition [Citation13,Citation14]. The recognition of adipose tissue hypertrophy in various inflammatory conditions has been a subject for research, and a link has been found between prolonged chronic stress and inflammation, with selective hypertrophy of adipose tissue adjacent to lymphoid tissue [Citation15–17]. More recent studies have further indicated that impaired lymphatic flow leads to inflammation and up-regulation of adipocyte differentiating genes and increased expression of adipokines (hormones produced by adipocytes) [Citation18]. This could suggest a possible mechanism for the fat tissue deposition in lymphedema, where the accumulation of lymph fluid leads to activation of adipocyte differentiation and proliferation [Citation11]. Lymphedema in mouse-tail models has indeed shown a significant hypertrophy of subcutaneous fat droplets. However, animal models do not fully replicate lymphedema, but merely function as a model of sustained lymphatic fluid stasis, and thus cannot replace studies on lymphedema in humans [Citation10].
Studies with MR imaging have shown a growth of adipose tissue in lymphedema and an increase of fat lobules causing a volume increase in lymphedema [Citation19]. Studies of female patients with lower extremity lymphedema following gynecological cancer treatment showed larger lobules of adipose tissue and hypertrophic changes, suggesting that long-term impairment of lymphatic flow can result in altered adipose tissue remodeling [Citation20]. There is only one study measuring single adipocyte sizes histologically in lymphedema after breast cancer treatment. This study confirmed larger adipocytes in the lymphedematous arm in one female cadaver studied. The adipocyte size increase was observed before overt lymphedema was seen in the female patient, who had a subjective feeling of arm swelling after cancer treatment [Citation21].
Aims
The purpose of the study was to systematically investigate a potential difference in adipocyte size in non-pitting lymphedema compared to controls to see if adipocyte hypertrophy contributes to the volume increase seen in lymphedema. The adipocyte size difference was also compared between arm and leg lymphedema and considering gender. Furthermore, any potential relationships between adipocyte size difference and lymphedema onset (time from cancer operation to lymphedema), duration, previous radiotherapy and chemotherapy were studied, as well as potential links to total excess volume increase in the lymphedematous extremity, body mass index (BMI) and body weight, respectively.
Materials and methods
Subjects
Forty-seven patients, all with non-pitting stage 2–3 lymphedema, participated in the study: 25 patients with arm lymphedema after breast cancer treatment, 22 patients with leg lymphedema, of which five were primary and 17 were secondary to treatment of gynecologic cancer (n = 12), penis cancer (n = 2), Hodgkin’s lymphoma (n = 2) and melanoma (n = 1). Of the 41 patients who developed lymphedema secondary to cancer treatment, two patients had received chemotherapy only, 15 had received irradiation only and 19 had received both chemotherapy and irradiation after their cancer operation. Five patients had not received chemotherapy or radiotherapy.
All 47 patients underwent liposuction of the lymphedema in the years ranging from 2005 to 2010, when the adipose tissue biopsies were taken for adipocyte size measurement. The age distribution among the patients at the time of the biopsies ranged from 17 to 88 years ().
Table 1. Age (years) at the time of biopsy of all patients and patients with arm and leg lymphedema.
Adipose tissue biopsies
The adipose tissue biopsies were obtained from the lymphedematous extremity and the non-affected extremity in the same patient. For legs, the biopsy was taken via a one-cm incision at the medial aspect of the knee, and in arms it was taken at the medial aspect of the elbow. All biopsies were taken before liposuction. The samples were fixed in paraformaldehyde and later embedded in paraffin and then cut into 5 µm sections, followed by hematoxylin and eosin staining.
Adipocyte size measurement
The biopsy sections were viewed in 10× enlargements using an Olympus PROVIS microscope and images were taken with an Olympus DP50 camera (Olympus, Tokyo, Japan). The size of the adipocytes was measured using the ImageJ program (NIH, Bethesda, MD). Analyses were made at ImmuneBiotech AB, Medicon Village (Lund, Sweden) (https://www.immunebiotech.com). The size measurement of the adipocytes was carried out by the same executor using the same high precision microscope, camera and imaging program.
To increase the accuracy of adipocyte size measurement, five images of each biopsy were analyzed and the adipocyte sizes of 10 blindly chosen cells per image were measured and expressed in µm2. Thus, the average size of a total of 50 adipocytes per biopsy was used for expressing the mean adipocyte size.
Past medical history
Past medical history in regard to lymphedema was obtained from the Lymphedema Center database and the medical records of each patient. Data include:
Onset, the time from cancer operation to the debut of lymphedema;
Duration of lymphedema before the biopsy was taken;
Previous radiotherapy (if the lymphedema was secondary to cancer treatment);
Previous chemotherapy (if the lymphedema was secondary to cancer treatment);
Body weight and BMI at the time of the biopsies;
Preoperative volume of lymphedematous and contralateral extremities before liposuction.
Statistics
Statistical analyses were performed in SPSS® Statistics 23 (IBM®, Armonk, NY). The normality of all data points was tested and confirmed using a Shapiro–Wilk test. Since all data on adipocyte size were normally distributed, parametric methods (paired and unpaired Student’s t-test), mean and SEM were used to evaluate the significance of the difference in adipocyte size between lymphedema and controls. The level of significance was set to p < .05. The Pearson correlation coefficient was used for bivariate analysis.
The adipocyte size difference was further compared between males and females as well as between arm and leg lymphedema. A potential link between adipocyte size difference and total excess volume increase in the extremities to BMI and body weight was also investigated.
Data input, chart and table creation was performed using Microsoft Excel 15.30 (Redmond, WA).
Ethics
The study was approved by the Ethics of Human Investigation Committee at Lund University Sweden (503/2006) and all participants gave their informed consent to participate. The procedures followed were in accordance with the Declaration of Helsinki of 1964 as revised in 2013, and the Good Clinical Practice guidelines.
Results
Adipocytes are larger in lymphedema than in controls
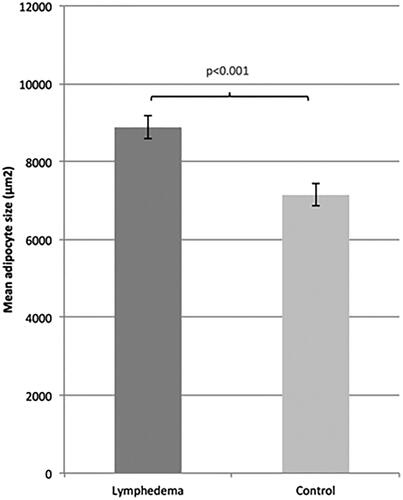
The adipocytes (mean ± SEM) in the tissue samples from the lymphedematous extremities were visually larger than in controls (). The adipocyte size in the lymphedematous extremities was significantly larger, 8880 ± 291 μm2, compared to the adipocyte size in the non-affected extremities, where it was 7143 ± 280 μm2 (), i.e. 24% larger (p < .001). The difference between the size of the adipocytes in the lymphedematous and non-affected extremities was 1737 ± 313 μm2. Out of 47 patients, 38 had larger adipocytes in the affected extremity compared to controls.
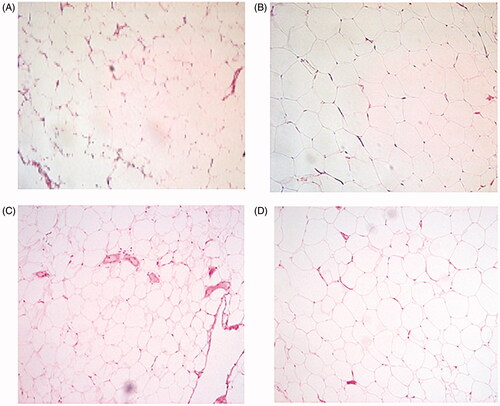
Figure 1. Representative hematoxylin and eosin staining of biopsies from (A) control and (B) patient; cancer-induced lymphedema in left leg. Biopsies from (C) control and (D) patient; cancer-induced lymphedema in right arm.
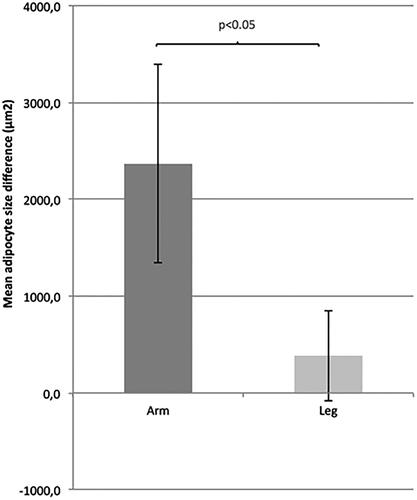
The mean adipocyte size difference in arm lymphedema was around twice as large as the mean adipocyte size difference in leg lymphedema (p < .05) ().
Divergent cell size and leg lymphedema
Nine patients had in fact smaller adipocytes in the lymphedematous extremity compared to the non-affected extremity. For these patients, the adipocyte size (mean ± SEM) in the lymphedematous extremities was 7474 ± 658 μm2, compared to 8675 ± 850 μm2 in the non-affected extremities, thus the mean adipocyte size in the lymphedematous extremities was 14% smaller (p < .2). Seven of these nine patients had leg lymphedema. The average duration of lymphedema in these seven patients was around twice as long compared to the patients who had larger lymphedema adipocytes (on average 19 years and 11 years of duration, respectively) ().
Table 2. Duration and onset (time from cancer operation to debut of lymphedema) of lymphedema, previous radiotherapy and chemotherapy in patients with larger adipocytes in lymphedema than in controls, and patients with smaller adipocytes in lymphedema than in controls (all leg lymphedema).
Adipocyte size increase is larger in arm lymphedema than in leg lymphedema
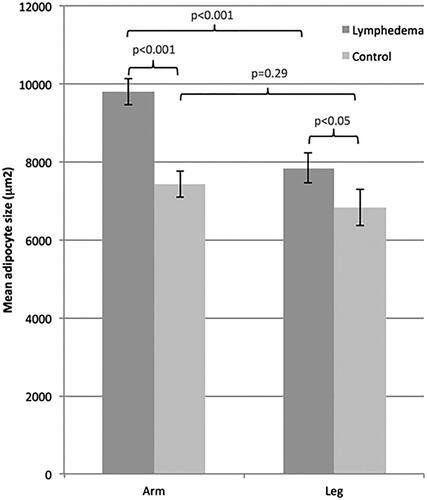
The adipocyte size (mean ± SEM) in the lymphedematous arms, 9793 ± 335 μm2, was 1950 μm2 larger than the adipocyte size in the lymphedematous legs, where it was 7843 ± 392 μm2 (p < .001), while the adipocyte size in the non-affected arms, 7426 ± 340 μm2, was 605 μm2 larger, but not significantly, than the adipocyte size in the non-affected legs, where it was 6822 ± 456 μm2 (p = .29) (). This means that the mean adipocyte size increase is on average 32% in arm lymphedema, but only 15% in leg lymphedema.
No significant adipocyte cell size difference between females and males
The adipocyte size difference in lymphedema and controls did not differ significantly between females and males (p = .96). The mean adipocyte size in the lymphedematous extremities in males was 52 μm2 larger (p = .53), and the mean adipocyte size in control extremities was 41 μm2 larger (p = .97) than in females.
Time aspect and adipocyte size difference
The data showed no correlation between adipocyte size difference and onset of lymphedema after cancer operation (p < .781, Pearson’s correlation coefficient = 0.044). There was a slight but significant negative correlation between adipocyte size difference and duration of lymphedema (p < .02, Pearson’s correlation coefficient= −0.344).
Previous therapy and adipocyte size
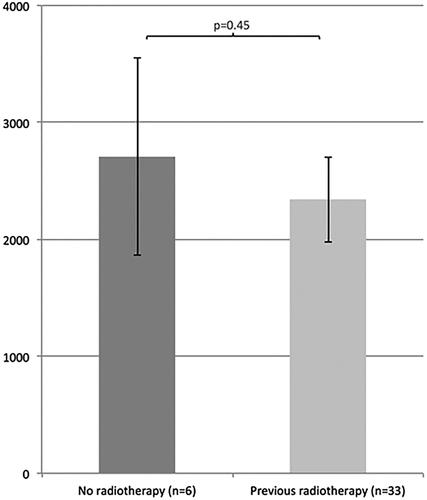
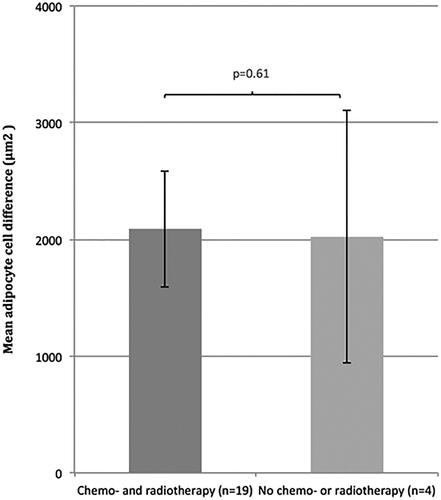
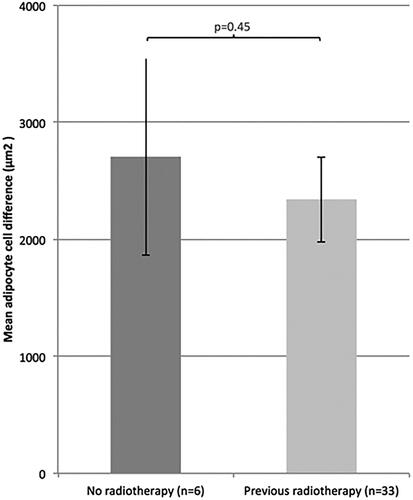
Data showed no correlation between adipocyte size difference and previous radiotherapy (p = .45) () or chemotherapy (p = .54) (). The adipocyte size difference for patients who had received both chemo- and radiotherapy did not differ from patients who did not receive any chemo- or radiotherapy at all in addition to cancer operation (p = .61) ().
Figure 5. Mean adipocyte size difference (mean ± SEM) between patients who had and who had not received previous radiotherapy.
Adipocyte size and total excess volume of lymphedematous arm
The mean preoperative excess volume (mean ± SEM) of the lymphedematous arms was 1333 ± 114 ml. The data showed no significant correlation (p = .996, Pearson’s correlation coefficient= −0.001) between the adipocyte size difference and the preoperative total excess volume of the lymphedematous arm.
Adipocyte size and total excess volume of lymphedematous legs
The mean preoperative excess volume of the lymphedematous legs was 4978 ml (SEM ± 819). The data showed no significant correlation (p = .218, Pearson’s correlation coefficient = 0.274) between the adipocyte size difference and the preoperative total excess volume of the lymphedematous leg.
Adipocyte size difference and BMI, body weight
The data showed a significant correlation between adipocyte size difference and BMI (p < .037, Pearson’s correlation coefficient = 0.308) but no correlation with weight (p = .182, Pearson’s correlation coefficient = 0.200).
Discussion
The study is, to our knowledge, the first to systematically and comprehensively measure the adipocyte size in adipose tissue in lymphedema in humans. The study is therefore the first to histologically confirm that hypertrophy of adipocytes contributes to the volume excess in non-pitting lymphedema. A previous study in an animal lymphedema mouse-tail model has shown both visual enlargement and an increase in the number of adipocytes. However, the mouse-tail model used was not a model of human lymphedema, but a model of sustained stasis of lymphatic fluid and cannot replace clinical studies of lymphedema in humans including the disease debut and progress over time [Citation10].
One possible risk when taking adipose tissue biopsies is that the adipocytes’ cell size may vary in different regions along the extremity. Therefore, in this study, biopsies were taken at the same corresponding locations in all patients. In addition, it has been shown, by repeated biopsies from the same site, that the variation of size of adipocytes within the same subcutaneous region is very limited [Citation22].
The patients in the study were mostly females (42 females out of 47 patients in total). This is due to the fact that lymphedema in western countries most commonly appears as a complication of lymph node removal followed by irradiation as part of breast and gynecological cancer treatment. The adipocyte size in lymphedema and control extremities did not differ significantly between females and males, thus the findings in this study could be presumed to be similarly applicable despite gender.
The study has investigated possible hypertrophy of adipocytes in lymphedema and not any potential proliferation, i.e. hyperplasia. There is a possibility that proliferation of adipocytes in lymphedema results in an adipocyte population of different ages and thus a higher variation of cell size dependent on the length of cell lifetime. It has been established that the size of adipocytes correlates to the life cycle of the cell in the way that adipocytes accumulate triacylglycerols up to a threshold when proliferation is initiated. Thus, adipocytes later in the life cycle are larger than adipocytes at earlier stages in the life cycle [Citation23]. In adipose tissue increase in obesity, it is also implied that hypertrophy of adipocytes is the major cause early in the process, whereas an increased number of adipocytes is the major cause for adipose tissue increase in severe obesity [Citation22]. All of this could possibly explain why the average size of adipocytes in some patients were in fact smaller in the lymphedematous extremity. This was seen in nine patients where the adipocytes were smaller in the lymphedematous extremities as compared to controls. One possible explanation is that both hypertrophy and hyperplasia of adipocytes contribute to the volume enlargement in lymphedema and that the ratio of these two might vary across patients.
It would be expected, since hypertrophy of adipocytes in lymphedema was confirmed in this study, that the magnitude of adipocyte size increase could correlate to the increase of the total excess volume of the extremities. Our findings, however, did not indicate this, but could indicate an increased number of proliferated adipocytes leading to the increase of the excess volume in the lymphedematous extremity.
Difference of adipocyte size increase in the leg and arm
The adipocyte size increase differs significantly between arm and leg lymphedema, with an average adipocyte size increase of only 15% in leg lymphedema compared to 32% in arm lymphedema (). The reason for this differentiation between arms and legs is not clear.
Some common characteristics among the patients with smaller adipocytes in lymphedema were noted. The majority of patients (seven out of nine patients) with smaller adipocytes in the lymphedematous extremity compared to controls had leg lymphedema. The average duration of lymphedema was almost twice as long in these patients compared to the patients who had larger lymphedema adipocytes (). The longer duration and the location (leg) of the lymphedema, which are common factors for these patients, could indicate two possibilities. One is that hypertrophy of adipocytes in lymphedema later declines with longer duration of the condition. The results from this study also show a negative correlation between adipocyte size difference in lymphedema and the duration of lymphedema, which means that the mean adipocyte size is smaller with longer duration of the disease. The other is that maybe the mechanism of, or the environment for, lymphedema in the arm and leg differ in some critical aspects.
Data did not confirm any significant correlation between previous radio- and/or chemotherapy and adipocyte size difference in lymphedema (). In a larger sample than in this study it might be possible to investigate how different types of radiotherapy and chemotherapy might influence the difference in cell size, the rationale being that different types of treatments can affect the magnitude of size increase differently.
Another factor to consider is that about half of the patients (10 of 21 patients with arm lymphedema and seven of 13 patients with leg lymphedema) had received a combination of chemotherapy and radiotherapy in addition to the cancer operation. In these 17 patients, it is difficult to determine what the true effect of chemotherapy and radiotherapy, respectively, would independently have on the adipose tissue deposition and adipocyte hypertrophy.
A previous study has shown that super obese (BMI 45–50) individuals can develop primary lymphedema. Moreover, less obese patients have a higher risk of developing lymphedema after lymph node removal and irradiation following cancer treatment than patients with a normal BMI. This supports the existence of a potential reciprocal relationship between obesity and lymphedema in the way that obesity impairs lymphatic transport by overloading the transport capacity and dysfunctional lymph drainage promotes deposition of adipose tissue [Citation11]. The data in the current study also confirm that the BMI of the 47 patients correlated with the adipocyte cell size difference.
A previous study supports the view that adipocyte hypertrophy occurs early, at stages before clinical lymphedema occurs [Citation21]. This would suggest, as later shown by Brorson et al. [Citation8,Citation9] that adipose tissue deposition is initiated very early in the development of lymphedema, within the first year, and could potentially be targeted to prevent the condition at the time of diagnosis. Of course, more research is needed to further support this since the previous study was based on one patient only.
Recent studies have indicated that inflammation plays an important role in lymphedema and adipose tissue deposition, including up-regulation of adipocyte differentiating genes and increased expression of adipokines [Citation11–18]. Therefore, one possibility for adipose tissue deposition is that the degree of inflammation could affect the magnitude of hypertrophy of adipocytes in lymphedema. However, since these findings were not known at the time the biopsies were collected, this study has not included an investigation of a potential difference in any degree of inflammation in the tissue samples to compare them with the adipocyte size difference. Future research on tissue samples with immunohistochemistry will be performed to estimate the degree of inflammation.
However, the proven hypertrophy and enlargement of adipocytes in lymphedema in this study support the findings of a previous study, i.e. that adipose differentiation regulating proteins, such as cytosine–cytosine–adenosine–adenosine–thymidine (CCAAT) enhancer binding protein-alpha and adiponectin, are significantly upregulated due to lymphatic fluid stasis [Citation17,Citation18].
A study on intraorbital tissue of patients with active Graves’ ophthalmopathy, where adipogenesis is one of the main processes, showed an overexpression of the adipose tissue marker stearoyl-coenzyme A desaturase as well as the pro-inflammatory gene cyclooxygenase-2 (COX-2). Nonsteroidal anti-inflammatory drugs (NSAIDs), such as diclofenac, were further shown to reduce the number of mature adipocytes by approximately 50% in active Graves’ ophthalmopathy [Citation24]. This finding suggests that anti-inflammatory drugs could prevent the differentiation of adipocytes. It would be of clinical importance if future research, studying the effect of anti-inflammatory drugs on fat-transformation in lymphedema, led to a method to prevent the adipose tissue deposition.
Recently, teprotumumab, a human monoclonal antibody inhibitor of insulin-like growth factor I receptor (IGF-IR), has been used in patients with Graves’ ophthalmopathy with promising results [Citation25]. Inhibition of IGF-IR results in reduction in the volume of orbital fat, muscle or both by possibly decreasing inflammation in orbital tissues. So far data from studies to determine the mechanism underlying the drug action are lacking. Thus, future studies of the effect of teprotumumab on adipose tissue enlargement in patients with lymphedema would be very interesting.
The strengths of this study are the extensive number of subjects included (47 patients with lymphedema), the systematic and precise measurement of adipocytes with the same high precision microscope, camera and imaging program, carried out by the same person, which measured a high number (50) of adipocytes of each biopsy to express the mean adipocyte size. Moreover, the main result in this study is supported by a p value <.001 and thus the result is of very high significance.
The main limitation of this study is the difficulty in separating the effects of some of the factors analyzed, such as various chemo- and radiotherapies, location of lymphedema, duration and onset of lymphedema.
Conclusions
There was a significant enlargement of the adipocytes in lymphedematous extremities compared to controls, thus hypertrophy of the adipocytes is indeed an important mechanism leading to volume excess due to fat deposition in lymphedema.
This study also indicated that location (arm versus leg) and duration of lymphedema influence adipocyte size increase and potentially the progress of lymphedema.
In summary, looking at the current study and other recent studies on the underlying mechanisms of lymphedema, there seems to be a fairly strong relationship between lymphedema, chronic low-grade inflammation, adipose tissue deposition and obesity. Further clarity of the molecular mechanism underlying the development of lymphedema could promote development of treatment methods that prevent adipose tissue deposition as a complication of cancer treatment.
Acknowledgements
The authors thank the late Professor Unne Stenram – Department of Clinical Pathology, Skåne University Hospital, Lund, Department of Clinical Sciences, Lund University, Lund, Sweden – for his advice and great support when initiating the study.
Disclosure statement
None of the authors have any conflicts of interest to disclose.
Additional information
Funding
References
- Petrek JA, Senie RT, Peters M, et al. Lymphedema in a cohort of breast carcinoma survivors 20 years after diagnosis. Cancer. 2001;92(6):1368–1377.
- Rockson SG. Lymphedema. Curr Treat Options Cardiovasc Med. 2006;8(2):129–136.
- Cabral S, Bonfim C, Oliveira R, et al. Knowledge, attitudes and perceptions regarding lymphatic filariasis: study on systematic noncompliance with mass drug administration. Rev Inst Med Trop Sao Paulo. 2017;59:e23.
- Chang DW. Lymphaticovenular bypass for lymphedema management in breast cancer patients: a prospective study. Plast Reconstr Surg. 2010;126(3):752–758.
- Piller NB, Thelander A. Treatment of chronic postmastectomy lymphedema with low level laser therapy: a 2.5 year follow-up. Lymphology. 1998;31(2):74–86.
- Brorson H, Ohlin K, Olsson G, et al. Adipose tissue dominates chronic arm lymphedema following breast cancer: an analysis using volume rendered CT images. Lymphat Res Biol. 2006;4(4):199–210.
- Hoffner M, Bagheri S, Hansson E, et al. SF-36 shows increased quality of life following complete reduction of postmastectomy lymphedema with liposuction. Lymphat Res Biol. 2017;15(1):87–98.
- Brorson H. Liposuction in lymphedema treatment. J Reconstr Microsurg. 2016;32(1):56–65.
- Brorson H, Ohlin K, Olsson G, et al. Breast cancer-related chronic arm lymphedema is associated with excess adipose and muscle tissue. Lymphat Res Biol. 2009;7(1):3–10.
- Zampell JC, Aschen S, Weitman ES, et al. Regulation of adipogenesis by lymphatic fluid stasis: part I. Adipogenesis, fibrosis, and inflammation. Plast Reconstr Surg. 2012;129(4):825–834.
- Mehrara BJ, Greene AK. Lymphedema and obesity: is there a link? Plast Reconstr Surg. 2014;134(1):154e–160e.
- Harvey NL, Srinivasan RS, Dillard ME, et al. Lymphatic vascular defects promoted by Prox1 haploinsufficiency cause adult-onset obesity. Nat Genet. 2005;37(10):1072–1081.
- Schneider M, Conway EM, Carmeliet P. Lymph makes you fat. Nat Genet. 2005;37(10):1023–1024.
- Pond CM. Adipose tissue and the immune system. Prostaglandins Leukot Essent Fatty Acids. 2005;73(1):17–30.
- Borley NR, Mortensen NJ, Jewell DP, et al. The relationship between inflammatory and serosal connective tissue changes in ileal Crohn's disease: evidence for a possible causative link. J Pathol. 2000;190(2):196–202.
- Sadler D, Mattacks CA, Pond CM. Changes in adipocytes and dendritic cells in lymph node containing adipose depots during and after many weeks of mild inflammation. J Anat. 2005;207(6):769–781.
- Rockson SG. The elusive adipose connection. Lymphat Res Biol. 2004;2(3):105–106.
- Aschen S, Zampell JC, Elhadad S, et al. Regulation of adipogenesis by lymphatic fluid stasis: part II. Expression of adipose differentiation genes. Plast Reconstr Surg. 2012;129(4):838–847.
- Idy-Peretti I, Bittoun J, Alliot FA, et al. Lymphedematous skin and subcutis: in vivo high resolution magnetic resonance imaging evaluation. J Invest Dermatol. 1998;110(5):782–787.
- Tashiro K, Feng J, Wu SH, et al. Pathological changes of adipose tissue in secondary lymphoedema. Br J Dermatol. 2017;177(1):158–167.
- Tassenoy A, Vermeiren K, van der Veen P, et al. Demonstration of tissue alterations by ultrasonography, magnetic resonance imaging and spectroscopy, and histology in breast cancer patients without lymphedema after axillary node dissection. Lymphology. 2006;39(3):118–126.
- Bjorntorp P. Size, number and function of adipose tissue cells in human obesity. Horm Metab Res. 1974;1974(Suppl. 4):77–83.
- Smith J, Al-Amri M, Dorairaj P, et al. The adipocyte life cycle hypothesis. Clin Sci. 2006;110(1):1–9.
- Vondrichova T, de Capretz A, Parikh H, et al. COX-2 and SCD, markers of inflammation and adipogenesis, are related to disease activity in Graves' ophthalmopathy. Thyroid. 2007;17(6):511–517.
- Smith TJ, Kahaly GJ, Ezra DG, et al. Teprotumumab for thyroid-associated ophthalmopathy. N Engl J Med. 2017;376(18):1748–1761.