ABSTRACT
Background: Experiences of negative social interactions and childhood trauma (CT) can lead to aberrant hypothalamic–pituitary–adrenal functions. Poor theory of mind (ToM) ability is related to increased social stress levels; however, studies on the relationship between ToM and cortisol remain scarce.
Objective: This study aimed to evaluate the relationship between ToM and the hair cortisol concentration (HCC) in healthy young adults considering the moderating role of CT.
Method: A total of 206 healthy young adults were divided into two groups based on an experience of moderate-to-severe childhood trauma (CT+ and CT–). To determine whether CT moderated the relationship between ToM and HCC, moderation analysis was conducted controlling for age, sex, years of education, and scores of perceived stress, depression, and anxiety.
Results: CT+ individuals reported higher subjective stress perception and depressive symptoms than CT– individuals, whereas anxiety-related symptoms, ToM, and HCC were not different between the groups. The experience of CT significantly moderated the relationship between ToM and HCC. The association between poorer ToM ability and higher HCC was significant only in CT+ group.
Conclusion: CT is a moderator of the association between ToM and HCC, indicating the importance of CT in social cognition and the stress response.
HIGHLIGHTS
Impaired social cognition and childhood trauma (CT) is associated with cortisol secretion.
Higher hair cortisol levels and poorer theory of mind (ToM) were associated in adults with CT.
CT is a significant moderator of the link between ToM and hair cortisol levels.
Antecedentes: Las experiencias de interacciones sociales negativas y el trauma infantil (CT por sus siglas en inglés) pueden conducir a funciones hipotalámicas-pituitarias-adrenales aberrantes. La pobre capacidad de teoría de la mente (ToM por sus siglas en inglés) está relacionada con mayores niveles de estrés social; sin embargo, los estudios sobre la relación entre ToM y cortisol siguen siendo escasos.
Objetivo: Este estudio tuvo como objetivo evaluar la relación entre ToM y concentración de cortisol en el cabello (HCC por sus siglas en inglés) en adultos jóvenes sanos considerando el papel moderador del CT.
Método: Un total de 206 adultos jóvenes sanos se dividieron en dos grupos en función de una experiencia de trauma infantil de moderada a severa (CT+ y CT–). Para determinar si el CT moderaba la relación entre ToM y HCC, se realizó un análisis de moderación controlando la edad, sexo, años de educación y las puntuaciones de estrés percibido, depresión y ansiedad.
Resultados: Individuos CT+ informaron una mayor percepción subjetiva de estrés y síntomas depresivos que los individuos CT–, mientras que los síntomas relacionados con ansiedad, ToM y HCC no fueron diferentes entre los grupos. La experiencia de CT moderó significativamente la relación entre ToM y HCC. La asociación entre una capacidad de ToM más pobre y un HCC más alto fue significativa solo en el grupo CT+.
Conclusión: CT es un moderador de la asociación entre ToM y HCC, lo que indica la importancia del CT en la cognición social y la respuesta al estrés.
背景:负性社交互动经历和童年创伤 (CT)可导致下丘脑-垂体-肾上腺功能异常。心智理论(ToM)能力差与社交应激水平增加有关;然而,关于 ToM 和皮质醇之间关系的研究仍然很少。
目的:本研究旨在考虑 CT 的调节作用,评估健康年轻人中 ToM 与头发皮质醇浓度 (HCC) 之间的关系。
方法:根据中度至重度童年创伤经历,将总共206名健康青年分为两组(CT+和CT–)。为了确定 CT 是否调节了 ToM 和 HCC 之间的关系,进行了调节分析,控制了年龄、性别、教育年限以及感知应激、抑郁和焦虑得分。
结果:CT+ 个体报告的主观应激感知和抑郁症状高于 CT– 个体,而焦虑相关症状、ToM 和 HCC 无组间差异。 CT 经历显著调节了 ToM 和 HCC 之间的关系。较差的 ToM 能力与较高的 HCC 之间的关联仅在 CT+ 组中显著。
结论:CT是ToM与HCC关联的调节因素,表明CT在社交认知和应激反应中的重要性。
1. Introduction
Stress is a process of adaptation to the challenges of the external and internal environment (Selye, Citation1950). The hypothalamic–pituitary–adrenal (HPA) axis, which helps to preserve physiological resources needed to maintain homeostasis, is a key system to cope with stress by releasing cortisol (Friedman et al., Citation2012). However, exposure to chronic stress could yield prolonged activation and dysregulation of the HPA axis (Dietz et al., Citation2013). Dysregulation of the HPA axis can manifest as the hypersecretion or hyposecretion of cortisol (Adam et al., Citation2017) and adversely affects metabolic, cardiovascular and immune systems (Dietz et al., Citation2013).
Social stress refers to strain due to perceived negative social interactions, such as conflict, criticism, failure of support and rejection (Lindert et al., Citation2021). Recurrent and prolonged stressful experiences of these negative social interactions can lead to compromised functioning of the HPA axis (Friedman et al., Citation2012). For example, poor diurnal cortisol regulation was found in individuals with cumulative social strain over ten years (Friedman et al., Citation2012). In addition to increased risks for physical illness including coronary heart disease, diabetes and hypertension (Seeman & McEwen, Citation1996), alteration of the HPA axis by long-lasting social stress has been associated with increased vulnerability to mental illness (Lapp et al., Citation2019). Interestingly, not everyone encountering stressful social situations develops mental diseases (Bartolomucci et al., Citation2005). Thus, the various impacts of stress imply the presence of factors contributing to individual susceptibility to social stress.
Social cognition is a primary resource for coping with social stress (Ji et al., Citation2021). Theory of mind (ToM), a subset of social cognition, is the ability to be aware of the mental states of others, such as beliefs, desires, thoughts, and intentions (Baron-Cohen et al., Citation1985). Individuals with poor ToM performance have shown misinterpretation of others’ intentions (Brüne, Citation2005), difficulties with social reciprocity (Joseph & Tager-Flusberg, Citation2004), and increased risks of long-term social stress (Dodell-Feder et al., Citation2014). Studies on the relationship between ToM and cortisol are scarce. High levels of mentalizing abilities were associated with increased salivary cortisol reactivity in response to acute social stress in healthy young adults (Tollenaar & Overgaauw, Citation2020), and a lower ToM performance was associated with higher hair cortisol levels in adolescents (Pluck et al., Citation2021). The development of ToM is influenced by several factors, including language abilities (Milligan et al., Citation2007), executive functions (Moses & Tahiroglu, Citation2010), and conversations among family members (Stanzione & Schick, Citation2014). Therefore, to provide clear understanding of the link between ToM and cortisol, assessing moderating factors of the relationship between ToM and cortisol is required.
Exposure to chronic or extreme stress during early childhood, childhood trauma (CT), results in HPA axis dysregulation and leads to pervasive and persistent effects on neurobiological, cognitive, emotional, and social development (Cowell et al., Citation2015). Chronic activation of the HPA axis by CT is associated with increased vulnerability to subsequent life stress and risks for depressive or anxiety disorders (Faravelli et al., Citation2012; Schuler et al., Citation2017). Previous studies on the effects of CT have revealed alterations of cortisol activity [See the meta-analysis by (Khoury et al., Citation2019)], although the results were inconsistent. Some studies have suggested that early adversity leads to high diurnal salivary cortisol levels (Dozier et al., Citation2006) and elevated salivary cortisol reactivity (Harkness et al., Citation2011) while others have shown opposite findings (Alink et al., Citation2012; Ouellet-Morin et al., Citation2011). A recent meta-analysis showed that adults with CT had poor performance in social cognitive abilities (Rokita et al., Citation2018). Furthermore, impaired ToM was the most associated domain of social cognition with experiences of CT (Germine et al., Citation2015b). Therefore, considering the history of CT as a potential moderator of the relationship between ToM and cortisol levels is reasonable.
This study aimed to investigate the relationship between social cognition, particularly ToM ability, and cortisol levels considering the moderating role of CT. Statistically, the moderator must precede the target variable which must precede the outcome variable (Tsuang et al., Citation2011). Because social cognition—including ToM—could be influenced by adverse childhood environments (Germine et al., Citation2015a), we hypothesized that CT moderates the relationship between ToM and HCC to meet the moderation analysis assumption. Recently, the hair cortisol concentration (HCC) has been considered a stable measurement in studies on chronic stress (Russell et al., Citation2012; Stalder et al., Citation2017). Given the long-lasting impact of childhood adversity on the HPA axis, HCC could be a reliable measurement to reflect long-term cumulative cortisol secretion over several months (Russell et al., Citation2012). We previously found an association between stress-related psychological factors and HCC regarding a history of CT (Won Jae Kim et al., Citation2021b). Because psychiatric illnesses (Fiksdal et al., Citation2019) or age (Dettenborn et al., Citation2012) could affect baseline cortisol levels, mentally healthy participants with a limited age to young adulthood would be beneficial to exclude the confounding effects. Therefore, in healthy young adults, we hypothesized that the presence of CT would be a moderating factor that influenced the relationship between ToM and HCC. In addition, we expected that HCC would be higher when the ToM ability was poorer in adults with CT because exposure to CT affected the HPA axis sensitivity to social stress (Heim & Nemeroff, Citation2001) and poor ToM performance increased the risks of long-term social stress (Dodell-Feder et al., Citation2014).
2. Methods
2.1. Participants
This data set was used in a previous study (Won Jae Kim et al., Citation2021b). Briefly, healthy individuals were recruited via advertisement on a job vacancy website. To exclude any lifetime history of psychiatric illness, all the participants were assessed using the structured clinical interview for DSM-IV (First & Gibbon, Citation2004). Exclusion criteria included the following: a medical or surgical history of admission, an admission history in the last six months, a history of head trauma with loss of consciousness and neurological disorder, pregnant or breastfeeding female individuals, and the use of hormonal medication, including steroid or contraceptives. The study was approved by the institutional review boards at Severance Hospital of the Yonsei University Health System, Seoul, Korea (4-2014-0744), and all individuals provided written informed consent.
2.2. Measures
2.2.1. Childhood trauma
Childhood trauma was assessed using the Childhood Trauma Questionnaire (CTQ) (Bernstein et al., Citation2003; Kim et al., Citation2011) and participants were classified into either the group with moderate-to-severe childhood trauma (CT+) or the group without moderate-to-severe childhood trauma (CT–). CTQ is a self-report questionnaire for traumatic experiences during childhood and adolescence and comprises 28 self-report items. Five categorical subscales of childhood trauma were assessed—physical abuse, emotional abuse, sexual abuse, physical neglect, and emotional neglect. The participants were classified as positive for the presence of CT when they reached the moderate-to-severe range of score on at least one of the five subscales (i.e. more than 9 for physical abuse, 12 for emotional abuse, 7 for sexual abuse, 9 for physical neglect, 14 for emotional neglect) (Kim et al., Citation2013).
2.2.2. Theory of mind task
ToM ability was assessed using a six-cartoon picture stories (Brüne, Citation2005). Two scenarios dealt with the cooperation of two characters; two stories depicted one character deceiving another; and two cartoon sequences showed that two characters cooperated to deceive a third one. Each story included four picture cards that were presented face-down in the same order (4-1-2-3). The participants were asked to turn over the cards and sequence the four pictures in logical order. Two points were obtained from sequencing the first and last cards correctly, and one point was given when the second and third cards were sequenced correctly; the maximum sequencing score (ToMs) was 36 points. The questionnaire score (ToMq), ranging from 0 to 23 points, was obtained from the mental state questions of the given characters.
2.2.3. Other psychological measures
The participants’ symptoms of depression and anxiety were assessed using the Beck Depression Inventory (BDI) (Beck et al., Citation1996; Hahn HM et al., Citation1986) and the state anxiety subscale of the State-Trait Anxiety Inventory (SAI) (Hahn DW & Chon, Citation1996; Spielberger et al., Citation1983), although no participant had a psychiatric illnesses. The Perceived Stress Scale (PSS) (Cohen et al., Citation1983; Lee et al., Citation2012) was used to measure an individual’s subjective feelings or thoughts after a stressful event.
2.2.4. Hair cortisol measurement
Hair samples were collected, approximately 10 strands or 10 mg. Considering the relatively invariable growth rate, the hair samples were cut from the posterior vertex of the scalp (Pragst & Balikova, Citation2006). Assuming a mean growth rate of 1 cm of hair per month, the proximal 3 cm of the hair segment representing the cumulative cortisol concentrations of the last three months was used for extraction. The hair strands were stored in a plastic bag for 12 months at room temperature, and then the hair protein was extracted using a MinuteTM Protein extraction kit (Invent Biotechnologies, Plymouth, MN, USA). To correct for the total amount of protein, the Pierce™ BCA Protein Assay Kit (Thermo Fisher Scientific, Waltham, MA, USA) was used. The limit of detection of the enzyme-linked immunosorbent assay was 1.14 ng/mL of cortisol at the 95% confidence limit. The values were expressed as ng of hair cortisol/µg of hair protein, logarithmically transformed using log (base 10) to normalize the data. The mean intra-assay coefficient of variation was 3.47%.
2.3. Statistical analysis
Independent two-sample t-test and chi-square test were performed using SPSS version 25 (IBM Corp., Armonk, NY, USA) to compare the two groups regarding the baseline and clinical characteristics. Moderation analysis was used to evaluate the interaction effect of ToM and CT on HCC. Moderation analyses were performed using PROCESS macro v3.5 (Hayes, Citation2013). Because moderation analysis in PROCESS macro conducts listwise deletion, non-complete cases were completely dropped. To give more prominence of missing ToM data, we performed an additional analysis to compare participants with missing data and participants with complete data (Supplementary Table 1). All continuous variables were mean-centered in moderation analysis. The multivariable regression analysis was conducted to calculate variance inflation factor (VIF). The moderation analysis without sex variable was performed to evaluate a possible suppressor effect of sex. Regarding the possibility of ceiling effect in respect of the average score of ToMq showing near maximum score in both CT− and CT+ groups, we conducted additional sensitivity analysis for the moderation model which dichotomized ToMq score based on the median value. To adjust for potential confounders, we considered age, sex, and SAI as covariates based on a previous report of the association between HCC and these variables (Stalder et al., Citation2017). As depression (Vreeburg et al., Citation2009) and perceived stress (Faresjö et al., Citation2014) were known to be associated with cortisol concentrations, we also considered BDI and PSS as covariates. We also conducted univariable regression to evaluate the association between sex and HCC. For the coding of sex in analyses, 0 refers to men and 1 refers to women. The significance threshold for p-value was 0.05 for all analyses.
3. Results
3.1. Participant characteristics
The baseline demographic and clinical characteristics of the study population are shown in . A total of 206 healthy young adults were included, and the mean age was 23.0 years (range: 19–30). Based on an experience of CT, the CT+ group comprised 80 participants and the CT– group comprised 126 participants. No significant group differences were found in HCC. There was no significant difference in HCC regarding sex which confirms the findings in previous studies (Manenschijn et al., Citation2011; Raul et al., Citation2004; Thomson et al., Citation2010; Won Jae Kim et al., Citation2021a). The CT+ group showed significantly higher scores of BDI and PSS than the CT– group. The other demographic and clinical values did not significantly differ between the groups. No significant differences were found between participants with any missing clinical data and those with complete data (Supplementary Table 1).
Table 1. Baseline characteristics of the study participants.
3.2. Moderating effect of childhood trauma on the relationship between ToM and HCC
For the moderation analysis between ToMq and HCC without any covariates, both the overall model (R2 = 0.05, p = .048) and the moderating effect of CT (B = −0.093, p = .050) were significant. After adjusted for age, sex, educational level, and the scores of the psychological measures, the overall model (R² = 0.13, p = .01) and the moderating effect of CT (B = −0.11, p = .02, f2 = 0.032) became more significant (, ), whereas the moderating effect of CT on the relationship between ToMs and HCC was not significant (B = −0.01, p = .45; Supplementary Table 2). Only the CT+ group demonstrated an association between a poor ToMq score and high HCC. The multivariable regression analysis showed that all the VIF values were less than 2. In sensitivity analysis for the moderation model, ToMq dichotomized based on the median score of 22, and the interaction effect of ToMq and CT was still significant (B = −0.234, p = .036; Supplementary Table 3). The moderation analysis also showed the significant effect of sex on HCC (B = 0.17, p < .01). The univariable regression to evaluate the relationship between sex and HCC found that sex was associated with HCC with a trend to statistical significance (B = −0.045, p = .085). The interaction effect of ToMq and CT remained significant (B = −0.102, p = .037) in the moderation analysis without sex variable.
Figure 1. Conceptual (A) and statistical (B) model of the association between ToM and HCC moderated by the presence of childhood trauma. The regression coefficients in (B) are calculated in a moderation analysis model including age, sex, education, perceived stress (PSS), depression (BDI) and anxiety (SAI) as covariates.
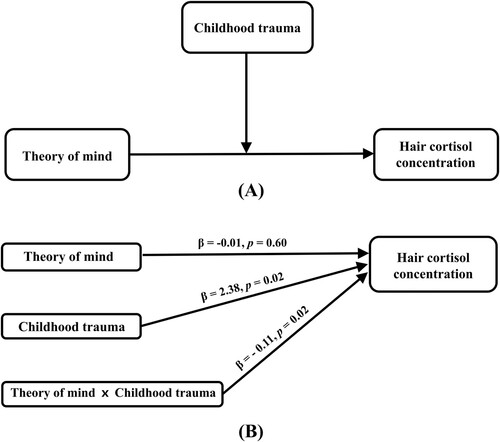
Table 2. Moderation analysis for the association between ToM and HCC according to the presence of childhood trauma.
4. Discussion
This study investigated the impact of CT on the association between ToM and HCC in healthy young adults. Our findings showed that poorer ToMq performance was associated with higher HCC only in the CT+ group, suggesting that CT moderates this relationship. To our best knowledge, this study is the first to identify the moderating effect of CT on the relationship between ToMq and HCC in healthy young adults.
Exposure to CT increases the vulnerability to later stress exposure (McLaughlin et al., Citation2010). Considering the role of social cognition, which helps decode social cues and facilitate healthy social interactions (Joseph & Tager-Flusberg, Citation2004), individuals with poor ToM abilities will likely experience high levels of stress in social situations. Because the moderating effect of CT between ToMq and HCC refers to the interaction between ToMq and CT on HCC, the association between poorer ToM ability and higher HCC only in the CT+ group implies that aberrant HPA axis function is demonstrated by the effect of CT on increasing an individual’s sensitivity to stress (Stroud et al., Citation2018) in conjunction with poor social cognition. Regarding the measure of ToM ability, the mean ToM scores in our healthy adults (mean [SD], ToMs: 34.11 [2.98], ToMq: 21.97 [1.21]) are similar to those from previous studies in Korea (Jeon et al. (Citation2013): ToMs: 33.0 [3.7], ToMq: 21.4 [2.0]; Park et al. (Citation2021): ToMs: 34.0 [3.0], ToMq: 22.0 [1.2]) although direct comparison is not possible. Regarding the ToM scores, there were no significant differences in both ToMq and ToMs between CT+ and CT– groups. These results may suggest that ToM ability itself was not impared in individuals with moderate-to-severe childhood trauma. However, another possible reason for non-significant differences of ToM scores should be considered such as a ceiling effect. Although the ToM task in our study has been widely used in nonclinical groups (Faísca et al., Citation2016; Jeon et al., Citation2013; Lee et al., Citation2014), the average score of ToMq showing near maximum score suggested the possibility of ceiling effect. However, the additional sensitivity analysis for moderation model was consistent with the significant interaction effect of ToM and CT. Nevertheless, the result of non-significant difference between two groups should be interpreted with caution when a ceiling effect was observed in the ToM task. The moderating effect of CT was not significant in interacting with ToMs, though it was for ToMq. ToMq measures the ability to understand false beliefs and intentions of given characters, whereas ToMs measures comprehension of depicted social interactions (Majorek et al., Citation2009; Wolf et al., Citation2010). Therefore, the fact that the only significant moderating effect of CT was noted in interaction with ToMq may suggest that sophisticated ToM abilities rather than basic comprehension of social interactions are important in interacting with CT to associate with HCC.
The results of the association between low ToM ability and elevated HCC in the CT+ group indicate the need for interventions focusing on improving ToM ability to normalize dysregulated cortisol secretion. Recent sociocognitive-based mental training has been shown to reduce HCC in mentally healthy adults (Puhlmann et al., Citation2021). Therefore, ToM training including the practice of perspective-taking and mentalization may have health benefits in individuals with CT by preventing physical or mental illnesses related to dysregulated cortisol secretion.
Regarding HCC in the CT+ group, our results were consistent with recent study findings showing no significant differences in HCC in individuals with CT (Iob et al., Citation2020; Oresta et al., Citation2021). However, several studies have reported aberrant cortisol levels in CT+ groups (Dozier et al., Citation2006; Harkness et al., Citation2011). One explanation could be that our participants did not have mental illness despite the history of moderate-to-severe CT. Although several adults with CT have various psychiatric diseases (Faravelli et al., Citation2012; Schuler et al., Citation2017), some maltreated children become competent adults (Kinard, Citation1998). Because the severity of CT was associated with maladaptive outcomes (Manly et al., Citation2001), an intact HCC in the CT+ group without mental illnesses may imply their resilience. Otherwise, considering the association between childhood adversity and a blunted cortisol response to social stress (Bunea et al., Citation2017), further studies investigating cortisol reactivity by acute stressors and basal cortisol levels might also reveal dysregulation of the cortisol response to chronic social stress in individuals with CT. Regarding the previous studies which reported no difference in HCC between sexes (Kim et al., Citation2021a; Raul et al., Citation2004), we conducted univariable analysis to evaluate the relationship between sex and HCC. The univariable analysis showed that sex was associated with HCC at a trend level. Furthermore, in the moderation analysis, adjusting covariates including age, anxiety, depression and perceived stress, the effect of sex on HCC was significant. Therefore, the significance of the effect of sex on HCC is reasonable.
The current study has a few limitations. First, the cross-sectional data limit our understanding of causal relationships. Therefore, prospective longitudinal studies are warranted to determine the causality of association. Second, although the bootstrapping method, which was utilized in the moderation analysis by the PROCESS macro, is one of the advanced methods for addressing non-normality (Pek et al., Citation2018), negatively skewed ToM data may show a ceiling effect which might limit our understanding. Since ceiling effects have been often found in most of ToM tasks in healthy participants as well as patient groups (Dodell-Feder et al., Citation2013), developing ToM tasks with increased difficulty would be warranted for a more sophisticated analysis on ToM ability. Third, the results only pertain to mentally healthy individuals, whom could possibly be resilient to negative consequences of moderate-to-severe childhood trauma. Fourth, further studies may exclude potential confounding effects by considering the detailed medical status although we have verified that our young participants did not have severe medical illnesses. Fifth, because several hair characteristics, such as hair washing frequency, hair products, and hair colour (Stalder et al., Citation2017), might be adjusted in analysis using HCC, including more comprehensive features of participants’ hair may further enhance our understanding. Finally, five subtypes of CT might differently affect the relationship between ToM and HCC; therefore, analyses investigating potential moderation effects of each type of CT are warranted.
In conclusion, this study showed the impact of CT on the association between ToM and HCC in healthy adults. By demonstrating that poorer ToM ability was significantly related to higher levels of hair cortisol only in individuals with CT, this study substantiates the importance of CT in social cognition and the stress response.
Supplemental Material
Download MS Word (19.5 KB)Data availability
All data used during this study are available on request from the corresponding author. The data are not publicly available due to privacy restrictions.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Adam, E. K., Quinn, M. E., Tavernier, R., McQuillan, M. T., Dahlke, K. A., & Gilbert, K. E. (2017). Diurnal cortisol slopes and mental and physical health outcomes: A systematic review and meta-analysis. Psychoneuroendocrinology, 83, 25–41. https://doi.org/10.1016/j.psyneuen.2017.05.018
- Alink, L. R., Cicchetti, D., Kim, J., & Rogosch, F. A. (2012). Longitudinal associations among child maltreatment, social functioning, and cortisol regulation. Developmental Psychology, 48(1), 224–236. https://doi.org/10.1037/a0024892
- Baron-Cohen, S., Leslie, A. M., & Frith, U. (1985). Does the autistic child have a “theory of mind” ?? Cognition, 21(1), 37–46. https://doi.org/10.1016/0010-0277(85)90022-8
- Bartolomucci, A., Palanza, P., Sacerdote, P., Panerai, A. E., Sgoifo, A., Dantzer, R., & Parmigiani, S. (2005). Social factors and individual vulnerability to chronic stress exposure. Neuroscience & Biobehavioral Reviews, 29(1), 67–81. https://doi.org/10.1016/j.neubiorev.2004.06.009
- Beck, A. T., Steer, R. A., & Brown, G. (1996). Beck depression inventory–II. Psychological Assessment, 1(82), 10–1037. https://doi.org/10.1037/t00742-000
- Bernstein, D. P., Stein, J. A., Newcomb, M. D., Walker, E., Pogge, D., Ahluvalia, T., Stokes, J., Handelsman, L., Medrano, M., Desmond, D., & Zule, W. (2003). Development and validation of a brief screening version of the childhood trauma questionnaire. Child Abuse & Neglect, 27(2), 169–190. https://doi.org/10.1016/S0145-2134(02)00541-0
- Brüne, M. (2005). Emotion recognition, ‘theory of mind,’ and social behavior in schizophrenia. Psychiatry Research, 133(2), 135–147. https://doi.org/10.1016/j.psychres.2004.10.007
- Bunea, I. M., Szentágotai-Tătar, A., & Miu, A. C. (2017). Early-life adversity and cortisol response to social stress: A meta-analysis. Translational Psychiatry, 7(12), 1274. https://doi.org/10.1038/s41398-017-0032-3
- Cohen, S., Kamarck, T., & Mermelstein, R. (1983). A global measure of perceived stress. Journal of Health and Social Behavior, 24(4), 385–396. https://doi.org/10.2307/2136404
- Cowell, R. A., Cicchetti, D., Rogosch, F. A., & Toth, S. L. (2015). Childhood maltreatment and its effect on neurocognitive functioning: Timing and chronicity matter. Development and Psychopathology, 27(2), 521–533. https://doi.org/10.1017/S0954579415000139
- Dettenborn, L., Tietze, A., Kirschbaum, C., & Stalder, T. (2012). The assessment of cortisol in human hair: Associations with sociodemographic variables and potential confounders. Stress, 15(6), 578–588. https://doi.org/10.3109/10253890.2012.654479
- Dietz, L. J., Stoyak, S., Melhem, N., Porta, G., Matthews, K. A., Walker Payne, M., & Brent, D. A. (2013). Cortisol response to social stress in parentally bereaved youth. Biological Psychiatry, 73(4), 379–387. https://doi.org/10.1016/j.biopsych.2012.08.016
- Dodell-Feder, D., Lincoln, S. H., Coulson, J. P., & Hooker, C. I. (2013). Using fiction to assess mental state understanding: A new task for assessing theory of mind in adults. Plos One, 8(11), e81279. https://doi.org/10.1371/journal.pone.0081279
- Dodell-Feder, D., Tully, L. M., Lincoln, S. H., & Hooker, C. I. (2014). The neural basis of theory of mind and its relationship to social functioning and social anhedonia in individuals with schizophrenia. NeuroImage: Clinical, 4, 154–163. https://doi.org/10.1016/j.nicl.2013.11.006
- Dozier, M., Manni, M., Gordon, M. K., Peloso, E., Gunnar, M. R., Stovall-McClough, K. C., Eldreth, D., & Levine, S. (2006). Foster children's diurnal production of cortisol: An exploratory study. Child Maltreatment, 11(2), 189–197. https://doi.org/10.1177/1077559505285779
- Faísca, L., Afonseca, S., Brüne, M., Gonçalves, G., Gomes, A., & Martins, A. T. (2016). Portuguese adaptation of a faux Pas test and a theory of mind picture stories task. Psychopathology, 49(3), 143–152. https://doi.org/10.1159/000444689
- Faravelli, C., Lo Sauro, C., Godini, L., Lelli, L., Benni, L., Pietrini, F., Lazzeretti, L., Talamba, G. A., Fioravanti, G., & Ricca, V. (2012). Childhood stressful events, HPA axis and anxiety disorders. World Journal of Psychiatry, 2(1), 13–25. https://doi.org/10.5498/wjp.v2.i1.13
- Faresjö, Å, Jullander, M., Götmalm, S., & Theodorsson, E. (2014). Higher perceived stress and poorer health reflected in elevated cortisol concentrations measured in extracts of hair from middle-aged healthy women. BMC Psychology, 2(1), 30. https://doi.org/10.1186/s40359-014-0030-7
- Fiksdal, A., Hanlin, L., Kuras, Y., Gianferante, D., Chen, X., Thoma, M. V., & Rohleder, N. (2019). Associations between symptoms of depression and anxiety and cortisol responses to and recovery from acute stress. Psychoneuroendocrinology, 102, 44–52. https://doi.org/10.1016/j.psyneuen.2018.11.035
- First, M. B., & Gibbon, M. (2004). The Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) and the Structured Clinical Interview for DSM-IV Axis II Disorders (SCID-II). In M. J. Hilsenroth & D. L. Segal (Eds.), Comprehensive Handbook of Psychological Assessment, Vol. 2: Personality Assessment. (pp. 134–143). John Wiley & Sons, Inc.
- Friedman, E. M., Karlamangla, A. S., Almeida, D. M., & Seeman, T. E. (2012). Social strain and cortisol regulation in midlife in the US. Social Science & Medicine, 74(4), 607–615. https://doi.org/10.1016/j.socscimed.2011.11.003
- Germine, L., Dunn, E. C., McLaughlin, K. A., & Smoller, J. W. (2015a). Childhood adversity is associated with adult theory of mind and social affiliation, but not face processing. PLoS ONE, 10(6), e0129612. https://doi.org/10.1371/journal.pone.0129612
- Germine, L., Dunn, E. C., McLaughlin, K. A., & Smoller, J. W. (2015b). Childhood adversity is associated with adult theory of mind and social affiliation, but not face processing. PLOS ONE, 10(6), e0129612–e0129612. https://doi.org/10.1371/journal.pone.0129612
- Hahn DW, L. C., & Chon, K. K. (1996). Korean adaptation of Spielberger's STAI (K-STAI). Korean J Health Psycho, 1(1), 1–14.
- Hahn HM, Y. T., Shin, Y. W., Kim, K. H., Yoon, D. J., & Chung, K. J. (1986). A standardization study of beck depression inventory in Korea. J Korean Neuropsychiatr Assoc, 25(3), 487–500.
- Harkness, K. L., Stewart, J. G., & Wynne-Edwards, K. E. (2011). Cortisol reactivity to social stress in adolescents: Role of depression severity and child maltreatment. Psychoneuroendocrinology, 36(2), 173–181. https://doi.org/10.1016/j.psyneuen.2010.07.006
- Hayes, A. F. (2013). Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. Guilford publications.
- Heim, C., & Nemeroff, C. B. (2001). The role of childhood trauma in the neurobiology of mood and anxiety disorders: Preclinical and clinical studies. Biological Psychiatry, 49(12), 1023–1039. https://doi.org/10.1016/S0006-3223(01)01157-X
- Iob, E., Lacey, R., & Steptoe, A. (2020). The long-term association of adverse childhood experiences with C-reactive protein and hair cortisol: Cumulative risk versus dimensions of adversity. Brain, Behavior, and Immunity, 87, 318–328. https://doi.org/10.1016/j.bbi.2019.12.019
- Jeon, I. H., Kim, K. R., Kim, H. H., Park, J. Y., Lee, M., Jo, H. H., Koo, S. J., Jeong, Y. J., Song, Y. Y., Kang, J. I., Lee, S. Y., Lee, E., & An, S. K. (2013). Attributional style in healthy persons: Its association with 'theory of mind' skills. Psychiatry Investigation, 10(1), 34–40. https://doi.org/10.4306/pi.2013.10.1.34
- Ji, D., Flouri, E., & Papachristou, E. (2021). Social cognition and cortisol in the general population: A systematic review and meta-analysis. Stress and Health, 37(3), 415–430. https://doi.org/10.1002/smi.3013
- Joseph, R. M., & Tager-Flusberg, H. (2004). The relationship of theory of mind and executive functions to symptom type and severity in children with autism. Development and Psychopathology, 16(1), 137–155. https://doi.org/10.1017/S095457940404444X
- Khoury, J. E., Bosquet Enlow, M., Plamondon, A., & Lyons-Ruth, K. (2019). The association between adversity and hair cortisol levels in humans: A meta-analysis. Psychoneuroendocrinology, 103, 104–117. https://doi.org/10.1016/j.psyneuen.2019.01.009
- Kim, D., Bae, H., Han, C., Oh, H. Y., & Macdonald, K. (2013). Psychometric properties of the childhood trauma questionnaire-short form (CTQ-SF) in Korean patients with schizophrenia. Schizophrenia Research, 144(1-3), 93–98. https://doi.org/10.1016/j.schres.2012.12.020
- Kim, D., Park, S.-C., Yang, H., & Oh, D. H. (2011). Reliability and validity of the Korean version of the childhood trauma questionnaire-short form for psychiatric outpatients. PSYCHIATRY INVESTIGATION, 8(4), 305–311. https://doi.org/10.4306/pi.2011.8.4.305
- Kim, W. J., Park, K. M., Park, J. T., Seo, E., An, S. K., Park, H. Y., & Lee, E. (2021a). Sex-specific association of hair cortisol concentration with stress-related psychological factors in healthy young adults. Biology of Sex Differences, 12(1), 56. https://doi.org/10.1186/s13293-021-00399-8
- Kim, W. J., Park, J. T., Park, H. Y., An, S. K., & Lee, E. (2021b). Effect of childhood trauma on the association between stress-related psychological factors and hair cortisol level in young adults. Psychiatry Investigation, 18(11), 1131–1136. https://doi.org/10.30773/pi.2021.0256
- Kinard, E. M. (1998). Methodological issues in assessing resilience in maltreated children. Child Abuse & Neglect, 22(7), 669–680. https://doi.org/10.1016/S0145-2134(98)00048-9
- Lapp, H. E., Ahmed, S., Moore, C. L., & Hunter, R. G. (2019). Toxic stress history and hypothalamic-pituitary-adrenal axis function in a social stress task: Genetic and epigenetic factors. Neurotoxicology and Teratology, 71, 41–49. https://doi.org/10.1016/j.ntt.2018.01.011
- Lee, J., Shin, C., Ko, Y. H., Lim, J., Joe, S. H., Kim, S., Jung, I. K., & Han, C. (2012). The reliability and validity studies of the Korean version of the perceived stress scale. Korean Journal of Psychosomatic Medicine, 2, 127–134.
- Lee, S. B., Koo, S. J., Song, Y. Y., Lee, M. K., Jeong, Y. J., Kwon, C., Park, K. R., Park, J. Y., Kang, J. I., Lee, E., & An, S. K. (2014). Theory of mind as a mediator of reasoning and facial emotion recognition: Findings from 200 healthy people. Psychiatry Investigation, 11(2), 105–111. https://doi.org/10.4306/pi.2014.11.2.105
- Lindert, J., Paul, K. C., Lachman Margie, E., Ritz, B., & Seeman, T. (2021). Social stress and risk of declining cognition: A longitudinal study of men and women in the United States. Social Psychiatry and Psychiatric Epidemiology, 57, 1875–1884. https://doi.org/10.1007/s00127-021-02089-7
- Majorek, K., Wolfkühler, W., Küper, C., Saimeh, N., Juckel, G., & Brüne, M. (2009). “Theory of mind” and executive functioning in forensic patients with schizophrenia. Journal of Forensic Sciences, 54(2), 469–473. https://doi.org/10.1111/j.1556-4029.2008.00966.x
- Manenschijn, L., Koper, J. W., Lamberts, S. W., & van Rossum, E. F. (2011). Evaluation of a method to measure long term cortisol levels. Steroids, 76(10-11), 1032–1036. https://doi.org/10.1016/j.steroids.2011.04.005
- Manly, J. T., Kim, J. E., Rogosch, F. A., & Cicchetti, D. (2001). Dimensions of child maltreatment and children's adjustment: Contributions ofdevelopmental timing and subtype. Development and Psychopathology, 13(4), 759–782. https://doi.org/10.1017/S0954579401004023
- McLaughlin, K. A., Conron, K. J., Koenen, K. C., & Gilman, S. E. (2010). Childhood adversity, adult stressful life events, and risk of past-year psychiatric disorder: A test of the stress sensitization hypothesis in a population-based sample of adults. Psychological Medicine, 40(10), 1647–1658. https://doi.org/10.1017/S0033291709992121
- Milligan, K., Astington, J. W., & Dack, L. A. (2007). Language and theory of mind: Meta-analysis of the relation between language ability and false-belief understanding. Child Development, 78(2), 622–646. https://doi.org/10.1111/j.1467-8624.2007.01018.x
- Moses, L. J., & Tahiroglu, D. (2010). Clarifying the relation between executive function and children’s theories of mind. Self and Social Regulation: Social Interaction and the Development of Social Understanding and Executive Functions, 218–233. https://doi.org/10.1093/acprof:oso/9780195327694.003.0009
- Oresta, S., Vinkers, C. H., van Rossum, E. F. C., Penninx, B., & Nawijn, L. (2021). How childhood trauma and recent adverse events are related to hair cortisol levels in a large adult cohort. Psychoneuroendocrinology, 126, 105150. https://doi.org/10.1016/j.psyneuen.2021.105150
- Ouellet-Morin, I., Odgers, C. L., Danese, A., Bowes, L., Shakoor, S., Papadopoulos, A. S., Caspi, A., Moffitt, T. E., & Arseneault, L. (2011). Blunted cortisol responses to stress signal social and behavioral problems among maltreated/bullied 12-year-old children. Biological Psychiatry, 70(11), 1016–1023. https://doi.org/10.1016/j.biopsych.2011.06.017
- Park, H. Y., Seo, E., Park, K. M., Koo, S. J., Lee, E., & An, S. K. (2021). Shame and guilt in youth at ultra-high risk for psychosis. Comprehensive Psychiatry, 108, 152241. https://doi.org/10.1016/j.comppsych.2021.152241
- Pek, J., Wong, O., & Wong, A. C. M. (2018). How to address non-normality: A taxonomy of approaches, reviewed, and illustrated. Frontiers in Psychology, 9), https://doi.org/10.3389/fpsyg.2018.02104
- Pluck, G., Córdova, M. A., Bock, C., Chalen, I., & Trueba, A. F. (2021). Socio-economic status, executive functions, and theory of mind ability in adolescents: Relationships with language ability and cortisol. British Journal of Developmental Psychology, 39(1), 19–38. https://doi.org/10.1111/bjdp.12354
- Pragst, F., & Balikova, M. A. (2006). State of the art in hair analysis for detection of drug and alcohol abuse. Clinica Chimica Acta, 370(1), 17–49. https://doi.org/10.1016/j.cca.2006.02.019
- Puhlmann, L. M. C., Vrticka, P., Linz, R., Stalder, T., Kirschbaum, C., Engert, V., & Singer, T. (2021). Contemplative mental training reduces hair glucocorticoid levels in a randomized clinical trial. Psychosomatic Medicine, 83(8), 894–905. https://doi.org/10.1097/PSY.0000000000000970
- Raul, J. S., Cirimele, V., Ludes, B., & Kintz, P. (2004). Detection of physiological concentrations of cortisol and cortisone in human hair. Clinical Biochemistry, 37(12), 1105–1111. https://doi.org/10.1016/j.clinbiochem.2004.02.010
- Rokita, K. I., Dauvermann, M. R., & Donohoe, G. (2018). Early life experiences and social cognition in major psychiatric disorders: A systematic review. European Psychiatry, 53, 123–133. https://doi.org/10.1016/j.eurpsy.2018.06.006
- Russell, E., Koren, G., Rieder, M., & Van Uum, S. (2012). Hair cortisol as a biological marker of chronic stress: Current status, future directions and unanswered questions. Psychoneuroendocrinology, 37(5), 589–601. https://doi.org/10.1016/j.psyneuen.2011.09.009
- Schuler, K. L., Ruggero, C. J., Goldstein, B. L., Perlman, G., Klein, D. N., & Kotov, R. (2017). Diurnal cortisol interacts with stressful events to prospectively predict depressive symptoms in adolescent girls. Journal of Adolescent Health, 61(6), 767–772. https://doi.org/10.1016/j.jadohealth.2017.06.005
- Seeman, T. E., & McEwen, B. S. (1996). Impact of social environment characteristics on neuroendocrine regulation. Psychosomatic Medicine, 58(5), 459–471. https://doi.org/10.1097/00006842-199609000-00008
- Selye, H. (1950). Stress and the general adaptation syndrome. BMJ, 1(4667), 1383–1392. https://doi.org/10.1136/bmj.1.4667.1383
- Spielberger, C., Gorsuch, R., & Lushene, R. (1983). State-trait anxiety inventory STAI (Form Y). Redw City Mind Gard.
- Stalder, T., Steudte-Schmiedgen, S., Alexander, N., Klucken, T., Vater, A., Wichmann, S., Kirschbaum, C., & Miller, R. (2017). Stress-related and basic determinants of hair cortisol in humans: A meta-analysis. Psychoneuroendocrinology, 77, 261–274. https://doi.org/10.1016/j.psyneuen.2016.12.017
- Stanzione, C., & Schick, B. (2014). Environmental language factors in theory of mind development: Evidence from children who are deaf/hard-of-hearing or who have specific language impairment. Topics in Language Disorders, 34(4), 296–312. https://journals.lww.com/topicsinlanguagedisorders/Fulltext/2014/10000/Environmental_Language_Factors_in_Theory_of_Mind.4.aspx https://doi.org/10.1097/TLD.0000000000000038
- Stroud, C. B., Harkness, K., & Hayden, E. (2018). The stress sensitization model. In The oxford handbook of stress and mental health.
- Thomson, S., Koren, G., Fraser, L. A., Rieder, M., Friedman, T. C., & Van Uum, S. H. (2010). Hair analysis provides a historical record of cortisol levels in Cushing's syndrome. Experimental and Clinical Endocrinology & Diabetes, 118(2), 133–138. https://doi.org/10.1055/s-0029-1220771
- Tollenaar, M. S., & Overgaauw, S. (2020). Empathy and mentalizing abilities in relation to psychosocial stress in healthy adult men and women. Heliyon, 6(8), e04488. https://doi.org/10.1016/j.heliyon.2020.e04488
- Tsuang, M. T., Tohen, M., & Jones, P. (2011). Textbook of Psychiatric Epidemiology. John Wiley & Sons.
- Vreeburg, S. A., Hoogendijk, W. J. G., van Pelt, J., DeRijk, R. H., Verhagen, J. C. M., van Dyck, R., Smit, J. H., Zitman, F. G., & Penninx, B. W. J. H. (2009). Major depressive disorder and hypothalamic-pituitary-adrenal axis activity: Results from a large cohort study. Archives of General Psychiatry, 66(6), 617–626. https://doi.org/10.1001/archgenpsychiatry.2009.50
- Wolf, F., Brüne, M., & Assion, H. J. (2010). Theory of mind and neurocognitive functioning in patients with bipolar disorder. Bipolar Disorders, 12(6), 657–666. https://doi.org/10.1111/j.1399-5618.2010.00854.x
