ABSTRACT
Introduction: Few studies have examined the psychopathological consequences for parents of children who were survivors of a motor vehicle crash (MVC). This study assessed the impact of dissociation and peritraumatic distress on the severity of PTSD and post-traumatic major depressive episode (MDE) symptoms in mothers during the first years after the MVC and the role that cortisol response might play in this association.
Methods: 125 mothers were included. Peritraumatic distress and dissociation were assessed. Morning salivary cortisol was tested at the baseline. Participants were assessed for a probable diagnosis of PTSD and MDE at 5 weeks, 6 months and 12 months.
Results: At 5 weeks, 12 (13.6%) mothers exhibited probable PTSD. During the first year, the PCL score was higher when the (i) Peritraumatic Distress Inventory (PDI) score increased and (ii) the Peritraumatic Dissociation Experience Questionnaire (PDEQ) score increased. Cortisol levels were lower when the PDI score increased.
Conclusion: This is the first study to assess the mothers of MVC survivors for one year following the trauma. We confirm that peritraumatic responses are useful for predicting the severity of PTSD symptoms. These results could encourage the implementation of follow-up programmes not only for survivors but also for their mothers.
HIGHLIGHTS
Mothers of children involved in motor vehicle accident are at risk for developing PTSD.
Peritraumatic responses (distress and dissociation) are associated to the severity of PTSD symptoms.
Low salivary cortisol levels were associated with high peritraumatic distress.
Antecedentes: Pocos estudios han examinado las consecuencias psicopatológicas para los padres de niños que fueron sobrevivientes de un accidente automovilístico (MVC, por sus siglas en inglés).
Objetivo: Este estudio evaluó el impacto de la disociación y la angustia peritraumática en la gravedad del TEPT y los síntomas del episodio depresivo mayor (EDM) postraumático en las madres durante los primeros años después del MVC y el papel que podría desempeñar la respuesta del cortisol en esta asociación.
Métodos: Se incluyeron 125 madres. Se evaluó la angustia peritraumática y la disociación. El cortisol salival matutino se analizó al inicio del estudio. Los participantes fueron evaluados para un diagnóstico probable de TEPT y EDM a las 5 semanas, 6 meses y 12 meses.
Resultados: A las 5 semanas, 12 (13,6%) madres exhibieron TEPT probable. Durante el primer año, la puntuación PCL (lista de chequeo para TEPT) fue mayor cuando i) aumentó la puntuación del Inventario de angustia peritraumática (PDI, por sus siglas en inglés) y ii) aumentó la puntuación del Cuestionario de experiencias de disociación peritraumática (PDEQ, por sus siglas en inglés). Los niveles de cortisol fueron más bajos cuando aumentó la puntuación PDI.
Conclusión: Este es el primer estudio que evalúa a las madres de sobrevivientes de MVC un año después del trauma. Confirmamos que las respuestas peritraumáticas son útiles para predecir la gravedad de los síntomas del TEPT. Estos resultados podrían incentivar la implementación de programas de seguimiento no solo para las sobrevivientes sino también para sus madres.
背景: 很少有研究考查车祸 (MVC) 幸存者儿童父母的精神病理学结果。
目的: 本研究评估了 MVC 后第一年母亲分离和创伤期精神痛苦对 PTSD 严重程度和创伤后重性抑郁发作 (MDE) 症状的影响,以及皮质醇反应在这种关联中可能的作用。
方法: 纳入了 125 名母亲。评估了创伤期精神痛苦和分离。在基线检测了早晨唾液皮质醇。在第 5 周、第 6 个月和第 12 个月时,对参与者进行了可能的 PTSD 和 MDE 诊断评估。
结果: 在 5 周时,12 名 (13.6%) 母亲表现出可能的 PTSD。在第一年,当 i) 创伤期精神痛苦量表 (PDI) 得分增加和 ii) 创伤期分离经历问卷 (PDEQ) 得分增加时,PCL 得分较高。当 PDI 评分增加时,皮质醇水平较低。
结论: 这是第一项评估 MVC 幸存者母亲在创伤后一年内的研究。我们确认创伤期反应有助于预测 PTSD 症状的严重程度。这些结果可以促进对于不仅幸存者且对其母亲随访计划的实施。
PALABRAS CLAVE:
1. Introduction
Over 50 million people around the world are injured each year in a motor vehicle crash (MVC) (WHO | Global Status Report on Road Safety Citation2015, n.d.). In 2009, more than 2 million Americans were admitted to the emergency room following an MVC (Hruska et al., Citation2014). In 2018, it is estimated that MVCs were responsible for 58,363 physical accidents in France, leaving 3,503 dead and 73,787 injured. The mortality rate was estimated at 49/1,000,000 in the general population and 13/1,000,000 in children (the French Interministerial Road Safety Monitoring Centre (ONISR), Citation2019). From their analysis of the WHO World Mental Health Surveys, Kessler et al. (Citation2017) concluded that globally, the most common trauma exposures were witnessing or discovering death or serious injury (35.7%), followed by traumas involving accidents (34.3%) (Kessler et al., Citation2017). 90% of the patients admitted to accident and emergency (A&E) who do not need to be hospitalised later develop adverse post-traumatic neuropsychiatric sequelae (McLean et al., Citation2020). Among these adverse post-traumatic sequalae that MVC survivors risk developing are post-traumatic stress disorder (PTSD), a major depressive episode (MDE), or the consumption of harmful substances (e.g. alcohol) (O’Donnell et al., Citation2004). Unfortunately, few studies include long-term follow-up of trauma survivors and those that do have typically focused on individuals who directly experienced the traumatic event (compared to those who learned about or witnessed the traumatic event) (Blanchard et al., Citation2004). For the paediatric population, one review highlighted that approximately 13% of children present PTSD at 3–6 months post-MVC (Olofsson et al., Citation2009). Another study showed that 17.6% displayed moderate to severe post-traumatic stress symptoms at 12 months post-MVC (Landolt et al., Citation2005). A recent study found that 7% to 17% of children presented PTSD 3 years after an MVC (Meiser-Stedman et al., Citation2017).
However, only a few studies have assessed post-traumatic psychiatric disorders in the parents or relatives of MVC survivors. Only Allenou et al. have highlighted the clinically significant symptoms of PTSD reported in many mothers whose children were admitted to A&E following an MVC (Allenou et al., Citation2010). This study highlights the preliminary results of a longitudinal study described in full in this article. Only the results for 78 (62%) of the 125 participants, and only PTSD symptoms 5 months post trauma without cortisol analyses were presented. Mental health recovery in children exposed to trauma is largely dependent on their parents’ mental health, and therefore it is important to know how parents cope in such cases (De Young et al., Citation2014; Goddard et al., Citation2019). A recent study highlighted that peritraumatic distress in mothers of severely ill children was correlated with PTSD (mainly intrusion and arousal) and depression (Aftyka et al., Citation2021).
Reactions that occur at the time of the trauma and immediately after have come to be described as ‘peritraumatic’ (Brunet et al., Citation2001). Two main types of peritraumatic reactions can occur after trauma: distress and dissociation. Peritraumatic distress can be considered as an emotional (negative emotions, e.g. helplessness, sadness, anger, fear) and physical (e.g. losing control, sweating, shaking and palpitations) response. Peritraumatic distress mediates the associations between PTSD and both the number of stressors experienced and psychological hardiness (Antičević et al., Citation2021). Peritraumatic dissociation can be considered as a cognitive (e.g. disorientation, amnesia, dissociation) response to trauma (Brunet et al., Citation2001; Marmar et al., Citation1994). Peritraumatic dissociation and its psychophysiological correlates might set the stage for later intrusive memory formation (Danböck et al., Citation2021). Peritraumatic responses have been reported to be predictors of PTSD symptoms in various groups of trauma survivors, including survivors of MVCs (Vance et al., Citation2018).
Furthermore, PTSD and MDE co-occur frequently following traumatic events (Contractor et al., Citation2015, Citation2017, Citation2018; Rytwinski et al., Citation2013). The peritraumatic dissociation and distress associated with an objective traumatic event attests to a severe stressor that potentially causes an MDE. In MVCs, peritraumatic distress was associated with depressive symptoms (Nishi et al., Citation2009). In a recent study, it was correlated with depression over two weeks (Joormann et al., Citation2020). Moreover, peritraumatic distress in mothers of severely ill children appears to be correlated with PTSD and depression (Aftyka et al., Citation2021).
Moreover, MDE has been shown to be associated with impaired immune response regulation (Dowlati et al., Citation2010; Miller et al., Citation2009). During an MDE, an external stress factor could be a precipitating factor that involves the hypothalamic–pituitary–adrenal (HPA) axis in the stress response (Dowlati et al., Citation2010; Miller et al., Citation2008; Pariante & Miller, Citation2001). In fact, during stress experienced as part of an MDE, there is an activation cascade of the hypothalamus and then the pituitary, with increases in the concentrations of CRH, then ACTH (Dowlati et al., Citation2010; Miller et al., Citation2008; Pariante & Miller, Citation2001). In response, cortisol synthesis in the adrenal cortex increases. In addition, the cortisol awakening response has been shown to prospectively predict MDE (Adam et al., Citation2010; Goodyer et al., Citation2000; Hardeveld et al., Citation2014; Stroud et al., Citation2019). With regard to neurobiological correlates associated with traumatic impact, despite some indications of an association between PTSD and a dysregulated HPA axis functionality as demonstrated by diurnal cortisol output, a recent review revealed mixed findings (Speer et al., Citation2019). Moreover, Inslicht et al. showed that a higher cortisol response to awakening was a pre-exposure risk factor for peritraumatic dissociation and acute stress disorder symptoms (Inslicht et al., Citation2011). In fact, several studies have found evidence of altered cortisol levels in patients with PTSD. Often, there is a decrease in basal cortisol level and an increase in the reactivity of the HPA stimulation tests. Based on these findings, it is assumed that these patients may show signs of cortisol dysregulation after trauma. Therefore, post-trauma cortisol levels are considered to be a potential biomarker of PTSD. However, longitudinal studies using indicators of cortisol secretion are scarce (Sopp et al., Citation2021).
Considering that peritraumatic responses have been associated with PTSD and depressive symptoms in various groups of trauma survivors, including survivors of MVCs (Vance et al., Citation2018), we aimed to replicate and extend this finding in a prospective study involving mothers whose child was an MVC survivor. In our group, levels of peritraumatic distress and peritraumatic dissociation were comparable to those in other trauma survivors and 18% of the mothers were considered to be suffering from probable PTSD at 5 weeks post trauma. Significant positive correlations were found between PTSD symptoms and peritraumatic distress and peritraumatic dissociation. These preliminary results only concerned a limited number of mothers (N = 72) and the acute symptoms (5 weeks post trauma) of PTSD. In addition, further investigation into the relationship between peritraumatic responses, PTSD and diurnal cortisol is warranted. Therefore, we aim to assess these associations. Finally, since PTSD and MDE frequently co-occur following traumatic events, our goal was to assess the prevalence of probable MDE, and the relationship between salivary cortisol levels in the week following the crash and PTSD and probable MDE.
Considering the preliminary results already presented (Allenou et al., Citation2010), the principal objective of this study was to examine the relationship between peritraumatic responses (peritraumatic distress and peritraumatic dissociation) and the severity of PTSD symptoms in a group of mothers whose children had been survivors of an MVC, during the first years following the MVC. We expect that there is an association between peritraumatic responses and PTSD symptom severity. The secondary objectives of the study were (i) to estimate the prevalence of MDE and PTSD symptoms in these mothers after the MVC; (ii) to assess the relationship between peritraumatic psychological responses (peritraumatic distress and peritraumatic dissociation) and salivary cortisol levels in the week following the crash and (iii) to assess the relationship between salivary cortisol levels in the week following the crash and PTSD and probable MDE. We expect that there is (i) a high prevalence of MDE and PTSD symptoms; (ii) an association between peritraumatic responses and cortisol and (iii) that cortisol predicts the occurrence of MDE and PTSD symptoms. Moreover, we expect that there is a positive association between peritraumatic responses and PTSD symptom severity, with higher peritraumatic scores associated with higher levels of PTSD symptoms.
We expect that there is a negative association between peritraumatic responses and cortisol, with lower cortisol levels associated with higher peritraumatic scores.
2. Methods
2.1. Subjects and procedure
Mothers of children aged 8–17 years who were admitted to the paediatric accident and emergency unit (A&E) of Toulouse University Hospital following an MVC were enrolled from January 2006 to 2009. This examination of PTSD symptoms in mothers is part of a larger study that examines the consequences of MVCs for children (Bui et al., Citation2010). The study was approved by the local Ethics Committee (Toulouse II Ethics Committee). Participants gave their written informed consent after receiving a comprehensive explanation of the procedures. Participation in the parent study required that the child complete a number of self-reported measures. Therefore, mothers of children under 8 were excluded. The other exclusion criteria were: mothers under the age of 18, the mother or child living more than an hour’s drive from the assessment centre, or who did not speak fluent French, suffering from intellectual disability, a psychotic disorder, or from a severe medical condition. During this period, 592 children from the ages of 8–15 were admitted to paediatric emergency care at Toulouse University Hospital (CHU). 304 mothers were approached, 125 of whom agreed to participate in the study. Among the reasons given for refusing to participate were a lack of interest in the study (n = 50), the MVC not being serious and the child being uninjured (n = 30), not being available for the first assessment (n = 19), and the child already receiving psychiatric treatment (n = 10). Other families gave different reasons (divorce, holidays, etc.) or no reason at all.
Of the 125 mothers included, 89 (71%) completed the 5-week post-trauma follow-up, 67 (54%) completed the 6-month post-trauma follow-up and 63 (50%) completed the 1-year follow-up. Mothers who agreed to participate had younger children, and children who were more frequently hospitalised after admission to A&E than those mothers who refused ().
Figure 1. PCL score according to PDI and PDEQ score and loess regression for each time-measurement (not adjusted).
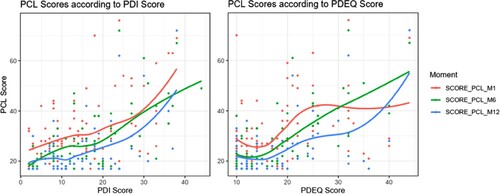
Of the 125 mothers, 121 (97%) filled out the PDI and the PDEQ during the week following the crash. Their mean age was 41.01 years (SD: 5.68).
The sociodemographic data are summarised in .
Table 1. Socio-demographic characteristics.
2.2. Measures
The Peritraumatic Distress Inventory (PDI) is a 13-item self-reporting measure that assesses the level of distress experienced during a traumatic event (e.g. ‘I felt afraid for my safety’, ‘I felt helpless to do more’, ‘I was horrified by what happened’, etc.). Each item is scored on a 5-point Likert scale, from 0 (not true) to 4 (absolutely true). The total sum of the scores obtained for the responses to all items ranges from 0 to 52 with higher scores indicating increased distress. A total score of at least 15 indicates peritraumatic distress (Guardia et al., Citation2013). There is an analysis using two possible sub-factors (dysphoric emotions and threat perception) (Jehel et al., Citation2005). The PDI has been validated in French and was found to demonstrate good test–retest reliability, convergent and divergent validity, as well as good internal consistency (Cronbach’s α of .84 in our sample) (Brunet et al., Citation2001; Jehel et al., Citation2005; Birmes et al., Citation2005). We specified that the traumatic event was the announcement of an MVC. Peritraumatic distress was assessed within the first week post trauma.
The Peritraumatic Dissociative Experiences Questionnaire (PDEQ) is a 10-item self-reporting questionnaire that assesses the degree of dissociation experienced during trauma (e.g. ‘I had moments of losing track of what was going on – I blanked out or spaced out or in some way felt I was not part of what was going on’, ‘I felt like I was on autopilot – I ended up doing things that I later realised I hadn’t consciously decided to do’, ‘My sense of time changed – things seemed to be happening in slow motion’, etc.). Each item is scored on a 5-point Likert scale ranging from 1 (not at all true) to 5 (absolutely true). The sum of all items provides a total score that ranges from 10 to 50, with higher scores indicating increased dissociation. A total score of 22 denotes a clinically significant peritraumatic dissociation (Birmes et al., Citation2001). There is an analysis using two possible sub-factors: altered consciousness and derealisation (Sijbrandij et al., Citation2012). The PDEQ also showed moderate to high convergent validity, satisfactory test–retest reliability, and internal consistency (Cronbach’s α of .90 in our sample) (Birmes et al., Citation2001, Citation2005; Marmar et al., Citation1998). We specified that the traumatic event was the announcement of an MVC. The PDEQ was assessed within the first week post trauma.
The Post Traumatic Check List-Specific (PCL-S) is a 17-item self-report that parallels diagnostic Criteria B, C and D for PTSD, in relation to an identified ‘stressful experience’, as delineated in the DSM-IV (17–18) (The study started before publication of the PCL-5). Each item is scored on a 5-point Likert scale (1 = ‘not at all’ to 5 = ‘very often’). The scale provides three subscores that correspond to the three main symptom clusters of the disorder: reexperiencing (items 1–5), avoidance/numbing (items 6–12), and hyperarousal (items 13–17). Total scores range from 17 to 85, with higher scores reflecting increased levels of PTSD symptoms. Scores >44 reflect a condition that warrants clinical attention (Ruggiero et al., Citation2006; Ventureyra et al., Citation2002; Yao et al., Citation2003) and in our study corresponded to a ‘probable PTSD’ status. The French version of the PCL-S demonstrated satisfactory test–retest reliability and internal consistency (Cronbach’s α of .91 in our sample) (Ruggiero et al., Citation2006; Ventureyra et al., Citation2002; Yao et al., Citation2003). We specified that the traumatic event was the announcement of an MVC. The PTSD symptoms were assessed 5 weeks, 6 months and 12 months post trauma.
The Mini International Neuropsychiatric Interview (MINI) is a structured interview that evaluates DSM-IV Axis I psychiatric disorders, with priority given to the current diagnosis. The presence of psychiatric disorders is evaluated with screening items that explore the mandatory criteria. In the event of a positive answer to the screening items for each disorder, additional questions are asked to ensure the diagnosis (the complete interview includes yes/no items). Negative answers to screening items rule out diagnoses (Lecrubier et al., Citation1997; Sheehan et al., Citation1998). The MINI was assessed at 5 weeks and 12 months post trauma.
2.3. Salivary cortisol assays (morning cortisol)
Subjects were instructed not to drink, eat or smoke for at least 30 min before saliva collection and to start the protocol at least 1 h after awakening and twice subsequently. As cortisol levels increase shortly after awakening and to avoid potential interference with the sleep-wake transition, participants were asked to collect the first sample in the morning, 1 h after awakening. The second sample was collected the same day 3–5 min after the first. The average of these two samples was used. Cortisol levels were determined from the saliva sample by direct radioimmunoassay (Diagnostic Systems Laboratories-Webster, Texas). The normal ranges (general population), at least 1 h after awakening, are 0.225–1.38 µg/100 ml according to the manufacturer’s specifications. When an adequate volume could not be provided, subjects were excluded from the analysis. Saliva was collected within the first week post trauma.
2.4. Analysis and statistical methods
The analysis was performed on complete data (no imputation for missing data). Therefore, the population analysed comprised all mothers with a predictor measurement and at least one outcome measurement collected during the year and with no missing data on the adjustment variables for the multivariate models. We tested potential associations between each potential predictor (PDI score, PDEQ score and baseline cortisol level) and the PCL score by linear regression models for repeated measures (mixed linear model with random intercept and interaction with time of measure). In order to evaluate associations between a potential predictor and the PCL score independently of other clinical predictors, models were adjusted for a priori-defined co-variables: age, occupation, place of birth (POB), number of children, type of exposure (unless otherwise stated). We proceeded in the same manner for ‘PTSD’ and ‘MDE’, diagnosed with MINI outcomes but using logistic regression models for repeated measures (mixed logistic regression model). To explore the relationship between baseline cortisol levels and peritraumatic psychological responses (PDI score and PDEQ score), we performed bivariate linear regressions. A p-value of <0.05 was considered to be statistically significant. The analyses were performed with R relapse 3.6.1 (R Core Team. 2019. https://www.R-project.org/)
3. Results
The number of subjects lost to follow-up was 36 mothers (28.8%) at 5 weeks, 57 (45.6%) at 6 months, and 62 (49.6%) at 1 year. At 5 weeks, the mothers lost to follow-up were those with a lower educational level (p = 0.037). There was no difference for the baseline clinical variables between the mothers lost to follow-up at 1 year and the mothers still in the study.
19 (15.2%) () mothers were injured co-survivors, regardless of the injury severity.
59 (49.2%) mothers exhibited peritraumatic distress and 37 (30.8%) peritraumatic dissociation (). At 5 weeks, 12 (13.6%) mothers exhibited probable PTSD.
Table 2. Baseline clinical characteristics
A higher level of peritraumatic distress was associated with higher post-traumatic stress symptom severity during the first year. On average, each 1-point increase in PDI scores was associated with a 0.66 [0.44 to 0.88] point increase in PCL scores. The differences remained significant when adjusting for baseline characteristics (age, profession, POB, number of children and type of exposure) (coefficient = 0.47; p < .01) (). A higher level of peritraumatic dissociation was associated with higher post-traumatic stress symptom severity during the first year. On average, each 1-point increase in PDEQ scores was associated with a 0.6 [0.4 to 0.9] point increase in PCL scores. However, this result did not remain significant when adjusting for baseline characteristics (age, profession, POB, number of children and type of exposure). The differences remained significant only for the ‘Altered consciousness’ subscore when adjusting for baseline characteristics ().
Table 3. PCL score for an augmentation of 1 pt in the PDI Score (and by subscore) – mixed linear models.
Table 4. PCL score for an augmentation of 1 pt in the PDEQ Score (and by subscore) – mixed linear models.
With regard to PTSD diagnosed with the MINI, on average, during the first year the risk of PTSD was significantly higher when the baseline PDI score increased (OR = 1.5 [1.1 to 2.1] for every 1-point increase in PDI) (p = .02). The differences remained significant when adjusting for type of exposure (whether or not a co-survivor) (p = .03). The risk of PTSD was significantly associated with the ‘threat perception’ subscore (unadjusted: OR = 3.1; p = .04, adjusted: OR = 2.92; p = .05), but not with the ‘dysphoric emotions’ subscore (). On average, during the first year, the risk of PTSD was not significantly higher when the baseline PDEQ score (and its subscore) increased [ORadjusted = 1.18; 95%CI [0.88; 1.59], p = .26]. On average, during the first year the risk of MDE was not significantly higher when the baseline PDI score [ORadjusted = 1.01; 95%CI [0.82; 1.25] p = .91] or PDEQ score increased significantly [ORadjusted = 0.98; 95%CI [0.78; 1.25], p = .89].
Table 5. PTSD risk for an augmentation 1 pt in PDI Score (and by subscore) – mixed logisitc models.
Five weeks after their child’s crash, 13 (15.5%) mothers reported a probable MDE. Mothers with probable PTSD 5 weeks after the crash more frequently exhibited probable MDE than mothers who did not report probable PTSD (41.7% vs 11.4% p = .019 Fisher’s test). Six months after the crash, 7 (10.3%) mothers reported probable PTSD and one year after the crash, 4 (6.3%) mothers reported probable PTSD.
Cortisol levels were significantly lower when the PDI score was positive (p = 0.020) (). No relationship was noted between salivary cortisol levels and a probable MDE 5 weeks after the crash [ORadjusted = 0.63; 95%CI [0.21; 1.88], p = .41] (adjusted for age, profession, POB, number of children and type of exposure).
Table 6. Association between baseline characteristics and baseline cortisol levels (bivariate)
There was no relationship between the salivary cortisol level and a probable PTSD in the first year after the crash.
4. Discussion
To our knowledge, this is the first prospective follow-up study that assesses the development of PTSD symptoms over 1 year in a group of mothers whose child was a survivor of an MVC. Among these mothers, 13.5% had probable PTSD 5 weeks after their child’s crash, 10.3% at 6 months after, and 6.3% at 1 year after. We found a 15.5% rate of MDE 5 weeks after the crash and 10.9% after 1 year. For mothers with MDE 5 weeks after the crash, 33.6% reported a history of MDE. Moreover, we confirmed the relationships between peritraumatic distress and PTSD during the first year following the MVC, as well as those between PTSD and MDE. With regards to peritraumatic dissociation, we only found an association between altered consciousness and PTSD.
For the direct survivors of MVCs, the prevalence of subsequent PTSD is estimated to be 10% to 50% according to the study (Blanchard et al., Citation1996, Citation2004; Blaszczynski et al., Citation1998; Brom et al., Citation1993; Feinstein & Dolan, Citation1991; Goldberg & Gara, Citation1990; Green et al., Citation1993; Hickling et al., Citation1992; Kupchik et al., Citation2007; Matsuoka et al., Citation2008; Mayou et al., Citation1993). Up to 26% of patients with predominantly mild MVC-related injuries report PTSD and reduced quality of life over two years post-MVC (Kenardy et al., Citation2015, Citation2018). Concerning the diagnosis of PTSD, patients were assessed one month (Blanchard et al., Citation1996; Brom et al., Citation1993; Green et al., Citation1993; Matsuoka et al., Citation2008) to one year (Blanchard et al., Citation2004; Goldberg & Gara, Citation1990) after the crash. In our study group, although the mothers were not all co-survivors, we noted PTSD rates comparable to the lower values observed for direct survivors. This attests to the severity of the psychological impact on the mother when her child is a survivor of a crash. In fact, the event ‘your child is a survivor of a crash’ seems to be particularly traumatic for a mother even if she is not involved in the crash or is a witness to it. However, in our study group, the mothers with probable PTSD were more often co-survivors of the same MVC as their child.
With regard to MDE, in this sample of mothers, we found a 15.5% rate of MDE 5 weeks after the crash and 10.9% after 1 year. For mothers with MDE at 5 weeks after the crash, 33.6% reported a history of MDE. Nevertheless, major depressive disorder (MDD) could be a risk factor for PTSD and for the co-occurrence of PTSD/MDE during an additional traumatic experience (Arbisi et al., Citation2012; Britt et al., Citation2007; Dickstein et al., Citation2010; Goodwin et al., Citation2012). Previous studies concerning mental disorders arising after MVCs have reported a rate of MDE at between 15% and 70% (Blanchard et al., Citation1994, Citation2004; Blaszczynski et al., Citation1998; Culpan & Taylor, Citation1973; Goldberg & Gara, Citation1990; Hickling et al., Citation1992; Matsuoka et al., Citation2008; Shalev et al., Citation1998). A recent study using self-reported symptom questionnaires estimates the incidence of depressive symptoms to be 10% (Kenardy et al., Citation2015). MDE assessments were performed one month (Blanchard et al., Citation1994; Matsuoka et al., Citation2008; Shalev et al., Citation1998) to one year (Blanchard et al., Citation2004; Goldberg & Gara, Citation1990) after the crash. In the study sample, the mothers of children involved in MVCs also reported MDE at rates comparable to those observed for direct survivors.
As expected, we confirmed the relationships between peritraumatic distress and PTSD during the first year following the MVC, as well as those between PTSD and MDE (Hoppen & Morina, Citation2019; Vance et al., Citation2018). With regard to peritraumatic dissociation, we only found an association between altered consciousness and PTSD. Considering the peritraumatic responses, from a clinical perspective, assessing peritraumatic distress as a potential risk factor in the development of adverse psychiatric outcomes might be useful in triaging patients into low-risk and high-risk groups after a traumatic event in order to initiate early preventative treatment measures in cases of psychiatric sequelae (Vance et al., Citation2018). When considering the use of the PDI and the PDEQ in this manner, it is important to bear in mind that it cannot be posited that there is a causal link between these peritraumatic responses and PTSD. At best, peritraumatic responses can be considered as risk factors for developing PTSD symptoms (Vance et al., Citation2018) after an MVC. However, dissociation failed to predict PTSD in our study. This could be due a gender effect. In fact, gender differences are well-established in the prevalence and severity of PTSD. There is also growing evidence that men and women develop PTSD symptoms differently. A meta-analysis of 1,182 women and 1,150 men (motor vehicle crash survivors and fire and flood survivors) showed that PTSD development is mediated by peritraumatic factors. This mediation is moderated by gender. Men with a higher level of neuroticism are at particular risk for developing PTSD (Cyniak-Cieciura et al., Citation2022).
The prevalence of MDE reported in studies may or may not be directly associated with the exposure to and consequences of the traumatic event. While exposure to MVC and the consequences could play a causal role in the aetiology of MDE, other influential pre-MVC and post-MVC variables may also lead to the development and maintenance of depression (Hoppen & Morina, Citation2019). However, PTSD and MDE should be prioritised as serious global health issues following exposure to trauma.
Salivary cortisol measured the week after the crash was significantly lower in mothers who reported peritraumatic distress. For those mothers, these values corresponded to the low values found in the general population (according to the manufacturer’s standards). We found no difference regarding peritraumatic dissociation. Inslicht et al. (Citation2011) found that a higher increase in salivary cortisol 0–30 min after awakening, assessed during academic training, was a prospective predictor of greater peritraumatic dissociation, but not of peritraumatic distress and ASD symptoms during the first 3 years of police service (Inslicht et al., Citation2011). The absence of a relationship between reduced basal cortisol and dissociation is also consistent with the studies by Kobayashi and Delahanty (Kobayashi & Delahanty, Citation2014). Their results did not support the existence of subgroups of PTSD patients (dissociators versus non-dissociators) who may have different basal cortisol levels. Moreover, they highlighted that peritraumatic dissociation was a better predictor of PTSD symptom severity than waking cortisol levels.
We found no relationship between the salivary cortisol levels measured in the week following the crash and probable PTSD in the year following the crash. PTSD-associated dysregulated HPA axis functionality was only partially demonstrated. In a recent review including ten studies (Speer et al., Citation2019), two studies indicated an association between PTSD and diurnal cortisol, three studies demonstrated no association, and the five remaining studies observed some, although mostly negative association. A potential explanation for altered cortisol levels in individuals with PTSD may not be the disorder itself but rather the severity of symptoms. Gill et al. (Citation2008) demonstrated that dysregulated HPA axis functioning related to lower morning cortisol levels was specifically correlated with PTSD hyperarousal symptoms (Gill et al., Citation2008). Moreover, early stress in PTSD patients is considered to be a major risk factor, and circulating levels of hormones involved in the flight-or-fight response to stress are altered, including lower baseline cortisol levels (Villain et al., Citation2018). In general, women appear to have a more sensitised HPA axis with lower overall plasma cortisol than men. A meta-analysis indicated that women with PTSD showed lower levels of basal cortisol than female controls (Meewisse et al., Citation2007). Therefore, peritraumatic distress could be a clinical marker of lower baseline cortisol levels for subjects at risk of developing PTSD. Moreover, a recent systematic review and meta-analysis showed that cortisol levels in the acute post-traumatic phase were not associated with higher subsequent PTSD symptoms. However, age moderated the relationship between cortisol and the risk of PTSD symptoms. These findings suggest that secondary preventive actions for PTSD may require different criteria to define high-risk groups based on HPA activity in youth and adults (Morris et al., Citation2016). A second important factor is the timing of cortisol assessment. Consolidation models posit that lower circulating cortisol levels enhance SNS-driven consolidation of traumatic memory, suggesting that cortisol should be measured within 6 h of the event (Joëls et al., Citation2011; Yehuda, Citation2002).
The strength of our study is that we highlighted that simply informing mothers of the child's MVC still resulted in significant distress and was predictive of PTSD and MDE for many.
5. Limits
Measuring PTSD with a self-reporting method rather than assessment by a clinician with a well-established structured clinical interview is a limitation. The MINI was designed as a less resource-intensive option that can be administered by research staff without diagnostic skills, but it may misclassify major depression compared to the SCID (Levis et al., Citation2019), not to mention the intensity of symptoms. Once the diagnosis has been established, examining the intensity of depressive symptoms and their correlation with PTSD symptoms could make it possible to identify any association between peritraumatic distress and dissociation and PTSD and MDE.
The second limitation is that we had no information about the severity of the child’s trauma, and this could influence the results.
Another limitation is the number of subjects lost to follow-up: 36 mothers at 5 weeks, 57 at 6 months, and 62 at 1 year.
In addition, an important factor is the timing of cortisol assessment. Consolidation models posit that lower circulating cortisol levels enhance SNS-driven consolidation of traumatic memory, suggesting that cortisol should be measured within 6 h of the event to have a bearing on symptomatic outcomes (Morris et al., Citation2016). We were unable to follow the same protocol. The lack of an overall relationship between simple and poorly controlled measurements of HPA activity and subsequent PTSD symptoms does not mean that the HPA axis function before, during or after trauma is not relevant to symptomatic outcomes. Moreover, we did not take account of some potential confounding variables that could influence salivary cortisol levels (e.g. medication use, current dx of psychopathology or body mass index). In addition, there is a brisk increase of cortisol levels within 20–30 min after awakening in the morning. This phenomenon is termed the cortisol awakening response (CAR). The CAR is considered a reliable measure for the acute reagibility of the HPA axis. However, by measuring samples 1 h after awakening, we are not sure that a residual from the CAR was captured.
To conclude, our study focused on a population of mothers and the prevalence of PTSD and MDD is higher in the female population than in the male population (Kessler et al., Citation2005). Therefore, the results could not be generalised to all.
6. Conclusion
In a group of mothers whose child was admitted to accident and emergency after an MVC, the prevalence of probable PTSD and probable MDE was significant. This is the first time that the mothers of MVC survivors have been assessed for a year following the incident. If they were reproduced, these results would encourage the implementation of follow-up programmes starting at A&E admission, not only for survivors but also for their mothers. Care could be family-based and offered to the child survivor and also to their mother. Screening for peritraumatic reactions should be done within hours or days following the injury. Mothers with the most severe reactions may benefit from an interview with a psychologist. We confirm that peritraumatic responses such as those measured using the Peritraumatic Distress Inventory and the subscale of altered consciousness in the Peritraumatic Dissociation Experiences Questionnaire are useful in predicting the severity of PTSD symptoms. This quality was verified for the mothers of MVC survivors, even though several of them were also survivors of the same MVC.
Availability of data
The data that support the findings of this study are available.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Adam, E. K., Doane, L. D., Zinbarg, R. E., Mineka, S., Craske, M. G., & Griffith, J. W. (2010). Prospective prediction of major depressive disorder from cortisol awakening responses in adolescence. Psychoneuroendocrinology, 35(6), 921–931. https://doi.org/10.1016/j.psyneuen.2009.12.007
- Aftyka, A., Rozalska, I., Pawlak, A., Goś, A., & Taczała, J. (2021). Peritraumatic distress in mothers of severely ill children: A cross-sectional study. Anaesthesiology Intensive Therapy, 53(3), 232–240. https://doi.org/10.5114/ait.2021.105257
- Allenou, C., Olliac, B., Bourdet-Loubère, S., Brunet, A., David, A.-C., Claudet, I., Lecoules, N., Roullet, P., Bui, E., & Birmes, P. (2010). Symptoms of traumatic stress in mothers of children victims of a motor vehicle accident. Depression and Anxiety, 27(7), 652–657. https://doi.org/10.1002/da.20650
- Antičević, V., Bubić, A., & Britvić, D. (2021). Peritraumatic distress and posttraumatic stress symptoms during the COVID-19 pandemic: The contributions of psychosocial factors and pandemic-related stressors. Journal of Traumatic Stress, 34(4), 691–700. https://doi.org/10.1002/jts.22701
- Arbisi, P. A., Kaler, M. E., Kehle-Forbes, S. M., Erbes, C. R., Polusny, M. A., & Thuras, P. (2012). The predictive validity of the PTSD Checklist in a nonclinical sample of combat-exposed National Guard troops. Psychological Assessment, 24(4), 1034–1040. https://doi.org/10.1037/a0028014
- Birmes, P., Brunet, A., Benoit, M., Defer, S., Hatton, L., Sztulman, H., & Schmitt, L. (2005). Validation of the Peritraumatic Dissociative Experiences Questionnaire self-report version in two samples of French-speaking individuals exposed to trauma. European Psychiatry, 20(2), 145–151. https://doi.org/10.1016/j.eurpsy.2004.06.033
- Birmes, P., Carreras, D., Charlet, J. P., Warner, B. A., Lauque, D., & Schmitt, L. (2001). Peritraumatic dissociation and posttraumatic stress disorder in victims of violent assault. The Journal of Nervous and Mental Disease, 189(11), 796–798. https://doi.org/10.1097/00005053-200111000-00011
- Birmes, P. J., Brunet, A., Coppin-Calmes, D., Arbus, C., Coppin, D., Charlet, J.-P., Vinnemann, N., Juchet, H., Lauque, D., & Schmitt, L. (2005). Brief reports: Symptoms of peritraumatic and acute traumatic stress Among victims of an industrial disaster. Psychiatric Services, 56(1), 93–95. https://doi.org/10.1176/appi.ps.56.1.93
- Blanchard, E. B., Hickling, E. J., Freidenberg, B. M., Malta, L. S., Kuhn, E., & Sykes, M. A. (2004). Two studies of psychiatric morbidity among motor vehicle accident survivors 1 year after the crash. Behaviour Research and Therapy, 42(5), 569–583. https://doi.org/10.1016/S0005-7967(03)00162-1
- Blanchard, E. B., Hickling, E. J., Taylor, A. E., Loos, W. R., Forneris, C. A., & Jaccard, J. (1996). Who develops PTSD from motor vehicle accidents? Behaviour Research and Therapy, 34(1), 1–10. https://doi.org/10.1016/0005-7967(95)00058-6
- Blanchard, E. B., Hickling, E. J., Taylor, A. E., Loos, W. R., & Gerardi, R. J. (1994). Psychological morbidity associated with motor vehicle accidents. Behaviour Research and Therapy, 32(3), 283–290. https://doi.org/10.1016/0005-7967(94)90123-6
- Blaszczynski, A., Gordon, K., Silove, D., Sloane, D., Hillman, K., & Panasetis, P. (1998). Psychiatric morbidity following motor vehicle accidents: A review of methodological issues. Comprehensive Psychiatry, 39(3), 111–121. https://doi.org/10.1016/S0010-440X(98)90069-4
- Britt, T. W., Dickinson, J. M., Moore, D., Castro, C. A., & Adler, A. B. (2007). Correlates and consequences of morale versus depression under stressful conditions. Journal of Occupational Health Psychology, 12(1), 34–47. https://doi.org/10.1037/1076-8998.12.1.34
- Brom, D., Kleber, R. J., & Hofman, M. C. (1993). Victims of traffic accidents: Incidence and prevention of post-traumatic stress disorder. Journal of Clinical Psychology, 49(2), 131–140. https://doi.org/10.1002/1097-4679(199303)49:2<131::AID-JCLP2270490202>3.0.CO;2-2
- Brunet, A., Weiss, D. S., Metzler, T. J., Best, S. R., Neylan, T. C., Rogers, C., Fagan, J., & Marmar, C. R. (2001). The Peritraumatic Distress Inventory: A proposed measure of PTSD criterion A2. American Journal of Psychiatry, 158(9), 1480–1485. https://doi.org/10.1176/appi.ajp.158.9.1480
- Bui, E., Brunet, A., Allenou, C., Camassel, C., Raynaud, J.-P., Claudet, I., Fries, F., Cahuzac, J.-P., Grandjean, H., Schmitt, L., & Birmes, P. (2010). Peritraumatic reactions and posttraumatic stress symptoms in school-aged children victims of road traffic accident. General Hospital Psychiatry, 32(3), 330–333. https://doi.org/10.1016/j.genhosppsych.2010.01.014
- Contractor, A. A., Elhai, J. D., Fine, T. H., Tamburrino, M. B., Cohen, G., Shirley, E., Chan, P. K., Liberzon, I., Galea, S., & Calabrese, J. R. (2015). Latent profile analyses of posttraumatic stress disorder, depression and generalized anxiety disorder symptoms in trauma-exposed soldiers. Journal of Psychiatric Research, 68, 19–26. https://doi.org/10.1016/j.jpsychires.2015.05.014
- Contractor, A. A., Greene, T., Dolan, M., & Elhai, J. D. (2018). Relations between PTSD and depression symptom clusters in samples differentiated by PTSD diagnostic status. Journal of Anxiety Disorders, 59, 17–26. https://doi.org/10.1016/j.janxdis.2018.08.004
- Contractor, A. A., Roley-Roberts, M. E., Lagdon, S., & Armour, C. (2017). Heterogeneity in patterns of DSM-5 posttraumatic stress disorder and depression symptoms: Latent profile analyses. Journal of Affective Disorders, 212, 17–24. https://doi.org/10.1016/j.jad.2017.01.029
- Culpan, R., & Taylor, C. (1973). Psychiatric disorders following road traffic and industrial injuries. Australian & New Zealand Journal of Psychiatry, 7(1), 32–39. https://doi.org/10.3109/00048677309161475
- Cyniak-Cieciura, M., Popiel, A., Kendall-Tackett, K., & Zawadzki, B. (2022). Neuroticism and PTSD symptoms: Gender moderates the mediating effect of peritraumatic emotions and dissociation. Psychological Trauma: Theory, Research, Practice, and Policy, 14(3), 462–470. https://doi.org/10.1037/tra0001065
- Danböck, S. K., Rattel, J. A., Franke, L. K., Liedlgruber, M., Miedl, S. F., & Wilhelm, F. H. (2021). Peritraumatic dissociation revisited: Associations with autonomic activation, facial movements, staring, and intrusion formation. European Journal of Psychotraumatology, 12(1), 1991609. https://doi.org/10.1080/20008198.2021.1991609
- De Young, A. C., Hendrikz, J., Kenardy, J. A., Cobham, V. E., & Kimble, R. M. (2014). Prospective evaluation of parent distress following pediatric burns and identification of risk factors for young child and parent posttraumatic stress disorder. Journal of Child and Adolescent Psychopharmacology, 24(1), 9–17. https://doi.org/10.1089/cap.2013.0066
- Dickstein, B. D., Suvak, M., Litz, B. T., & Adler, A. B. (2010). Heterogeneity in the course of posttraumatic stress disorder: Trajectories of symptomatology. Journal of Traumatic Stress, 23(3), 331–339. https://doi.org/10.1002/jts.20523
- Dowlati, Y., Herrmann, N., Swardfager, W., Liu, H., Sham, L., Reim, E. K., & Lanctôt, K. L. (2010). A meta-analysis of cytokines in major depression. Biological Psychiatry, 67(5), 446–457. https://doi.org/10.1016/j.biopsych.2009.09.033
- Feinstein, A., & Dolan, R. (1991). Predictors of post-traumatic stress disorder following physical trauma: An examination of the stressor criterion. Psychological Medicine, 21(1), 85–91. https://doi.org/10.1017/S0033291700014689
- Gill, J., Vythilingam, M., & Page, G. G. (2008). Low cortisol, high DHEA, and high levels of stimulated TNF-α, and IL-6 in women with PTSD. Journal of Traumatic Stress, 21(6), 530–539. https://doi.org/10.1002/jts.20372
- Goddard, E., Onwumere, J., Meiser-Stedman, R., Sutherland, E., & Smith, P. (2019). Relationship between posttraumatic stress symptoms, caregiving response, and parent mental health in youth exposed to single incident trauma. Journal of Affective Disorders, 251, 15–22. https://doi.org/10.1016/j.jad.2019.03.016
- Goldberg, L., & Gara, M. A. (1990). A typology of psychiatric reactions to motor vehicle accidents. Psychopathology, 23(1), 15–20. https://doi.org/10.1159/000284632
- Goodwin, L., Jones, M., Rona, R. J., Sundin, J., Wessely, S., & Fear, N. T. (2012). Prevalence of delayed-onset posttraumatic stress disorder in military personnel: Is there evidence for this disorder? Results of a prospective UK cohort study. Journal of Nervous & Mental Disease, 200(5), 429–437. https://doi.org/10.1097/NMD.0b013e31825322fe
- Goodyer, I. M., Herbert, J., Tamplin, A., & Altham, P. M. (2000). Recent life events, cortisol, dehydroepiandrosterone and the onset of major depression in high-risk adolescents. British Journal of Psychiatry, 177(6), 499–504. https://doi.org/10.1192/bjp.177.6.499
- Green, M. M., McFarlane, A. C., Hunter, C. E., & Griggs, W. M. (1993). Undiagnosed post-traumatic stress disorder following motor vehicle accidents. Medical Journal of Australia, 159(8), 529–534. https://doi.org/10.5694/j.1326-5377.1993.tb138006.x
- Guardia, D., Brunet, A., Duhamel, A., Ducrocq, F., Demarty, A.-L., & Vaiva, G. (2013). Prediction of trauma-related disorders. The Primary Care Companion for CNS Disorders, 15(1), https://doi.org/10.4088/PCC.12l01406
- Hardeveld, F., Spijker, J., Vreeburg, S. A., Graaf, R. D., Hendriks, S. M., Licht, C. M. M., Nolen, W. A., Penninx, B. W. J. H., & Beekman, A. T. F. (2014). Increased cortisol awakening response was associated with time to recurrence of major depressive disorder. Psychoneuroendocrinology, 50, 62–71. https://doi.org/10.1016/j.psyneuen.2014.07.027
- Hickling, E. J., Blanchard, E. B., Silverman, D. J., & Schwarz, S. P. (1992). Motor vehicle accidents, headaches and post-traumatic stress disorder: Assessment findings in a consecutive series. Headache: The Journal of Head and Face Pain, 32(3), 147–151. https://doi.org/10.1111/j.1526-4610.1992.hed3203147.x
- Hoppen, T. H., & Morina, N. (2019). The prevalence of PTSD and major depression in the global population of adult war survivors: A meta-analytically informed estimate in absolute numbers. European Journal of Psychotraumatology, 10(1), 1578637. https://doi.org/10.1080/20008198.2019.1578637
- Hruska, B., Irish, L. A., Pacella, M. L., Sledjeski, E. M., & Delahanty, D. L. (2014). PTSD symptom severity and psychiatric comorbidity in recent motor vehicle accident victims: A latent class analysis. Journal of Anxiety Disorders, 28(7), 644–649. https://doi.org/10.1016/j.janxdis.2014.06.009
- Inslicht, S. S., Otte, C., McCaslin, S. E., Apfel, B. A., Henn-Haase, C., Metzler, T., Yehuda, R., Neylan, T. C., & Marmar, C. R. (2011). Cortisol awakening response prospectively predicts peritraumatic and acute stress reactions in police officers. Biological Psychiatry, 70(11), 1055–1062. https://doi.org/10.1016/j.biopsych.2011.06.030
- Jehel, L., Brunet, A., Paterniti, S., & Guelfi, J. D. (2005). Validation de la version française de l'inventaire de détresse péritraumatique. The Canadian Journal of Psychiatry, 50(1), 67–71. https://doi.org/10.1177/070674370505000112
- Joëls, M., Fernandez, G., & Roozendaal, B. (2011). Stress and emotional memory: A matter of timing. Trends in Cognitive Sciences, 15(6), 280–288. https://doi.org/10.1016/j.tics.2011.04.004
- Joormann, J., McLean, S. A., Beaudoin, F. L., An, X., Stevens, J. S., Zeng, D., Neylan, T. C., Clifford, G., Linnstaedt, S. D., Germine, L. T., Rauch, S. L., Musey, P. I., Hendry, P. L., Sheikh, S., Jones, C. W., Punches, B. E., Fermann, G., Hudak, L. A., Mohiuddin, K., … Kessler, R. C. (2020). Socio-demographic and trauma-related predictors of depression within eight weeks of motor vehicle collision in the AURORA study. Psychological Medicine, 52(10), 1934–1947. https://doi.org/10.1017/S0033291720003773
- Kenardy, J., Edmed, S. L., Shourie, S., Warren, J., Crothers, A., Brown, E. A., Cameron, C. M., & Heron-Delaney, M. (2018). Changing patterns in the prevalence of posttraumatic stress disorder, major depressive episode and generalized anxiety disorder over 24 months following a road traffic crash: Results from the UQ SuPPORT study. Journal of Affective Disorders, 236, 172–179. https://doi.org/10.1016/j.jad.2018.04.090
- Kenardy, J., Heron-Delaney, M., Warren, J., & Brown, E. (2015). The effect of mental health on long-term health-related quality of life following a road traffic crash: Results from the UQ SuPPORT study. Injury, 46(5), 883–890. https://doi.org/10.1016/j.injury.2014.11.006
- Kessler, R. C., Aguilar-Gaxiola, S., Alonso, J., Benjet, C., Bromet, E. J., Cardoso, G., Degenhardt, L., de Girolamo, G., Dinolova, R. V., Ferry, F., Florescu, S., Gureje, O., Haro, J. M., Huang, Y., Karam, E. G., Kawakami, N., Lee, S., Lepine, J.-P., Levinson, D., … Koenen, K. C. (2017). Trauma and PTSD in the WHO World Mental Health surveys. European Journal of Psychotraumatology, 8(sup5), 1353383. https://doi.org/10.1080/20008198.2017.1353383
- Kessler, R. C., Berglund, P., Demler, O., Jin, R., Merikangas, K. R., & Walters, E. E. (2005). Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry, 62(6), 593–602. https://doi.org/10.1001/archpsyc.62.6.593
- Kobayashi, I., & Delahanty, D. L. (2014). Awake/sleep cortisol levels and the development of posttraumatic stress disorder in injury patients with peritraumatic dissociation. Psychological Trauma: Theory, Research, Practice, and Policy, 6(5), 449–456. https://doi.org/10.1037/a0033013
- Kupchik, M., Strous, R. D., Erez, R., Gonen, N., Weizman, A., & Spivak, B. (2007). Demographic and clinical characteristics of motor vehicle accident victims in the community general health outpatient clinic: A comparison of PTSD and non-PTSD subjects. Depression and Anxiety, 24(4), 244–250. https://doi.org/10.1002/da.20189
- Landolt, M. A., Vollrath, M., Timm, K., Gnehm, H. E., & Sennhauser, F. H. (2005). Predicting posttraumatic stress symptoms in children after road traffic accidents. Journal of the American Academy of Child & Adolescent Psychiatry, 44(12), 1276–1283. https://doi.org/10.1097/01.chi.0000181045.13960.67
- Lecrubier, Y., Sheehan, D., Weiller, E., Amorim, P., Bonora, I., Harnett Sheehan, K., Janavs, J., & Dunbar, G. (1997). The Mini International Neuropsychiatric Interview (MINI). A short diagnostic structured interview: Reliability and validity according to the CIDI. European Psychiatry, 12(5), 224–231. https://doi.org/10.1016/S0924-9338(97)83296-8
- Levis, B., McMillan, D., Sun, Y., He, C., Rice, D. B., Krishnan, A., Wu, Y., Azar, M., Sanchez, T. A., Chiovitti, M. J., Bhandari, P. M., Neupane, D., Saadat, N., Riehm, K. E., Imran, M., Boruff, J. T., Cuijpers, P., Gilbody, S., Ioannidis, J. P. A., … Thombs, B. D. (2019). Comparison of major depression diagnostic classification probability using the SCID, CIDI, and MINI diagnostic interviews among women in pregnancy or postpartum: An individual participant data meta-analysis. International Journal of Methods in Psychiatric Research, 28(4), e1803. https://doi.org/10.1002/mpr.1803
- Marmar, C. R., Weiss, D. S., & Metzler, T. (1998). Peritraumatic dissociation and posttraumatic stress disorder. In J. D. Bremner & C. R. Marmar (Eds.), Trauma, memory, and dissociation (pp. 229–247). American Psychiatric Association.
- Marmar, C. R., Weiss, D. S., Schlenger, W. E., Fairbank, J. A., Jordan, B. K., Kulka, R. A., & Hough, R. L. (1994). Peritraumatic dissociation and posttraumatic stress in male Vietnam theater veterans. American Journal of Psychiatry, 151(6), 902–907. https://doi.org/10.1176/ajp.151.6.902
- Matsuoka, Y., Nishi, D., Nakajima, S., Kim, Y., Homma, M., & Otomo, Y. (2008). Incidence and prediction of psychiatric morbidity after a motor vehicle accident in Japan: The Tachikawa Cohort of Motor Vehicle Accident Study. Critical Care Medicine, 36(1), 74–80. https://doi.org/10.1097/01.CCM.0000291650.70816.D6
- Mayou, R., Bryant, B., & Duthie, R. (1993). Psychiatric consequences of road traffic accidents. BMJ, 307(6905), 647–651. https://doi.org/10.1136/bmj.307.6905.647
- McLean, S. A., Ressler, K., Koenen, K. C., Neylan, T., Germine, L., Jovanovic, T., Clifford, G. D., Zeng, D., An, X., Linnstaedt, S., Beaudoin, F., House, S., Bollen, K. A., Musey, P., Hendry, P., Jones, C. W., Lewandowski, C., Swor, R., Datner, E., … Kessler, R. (2020). The AURORA Study: A longitudinal, multimodal library of brain biology and function after traumatic stress exposure. Molecular Psychiatry, 25(2), 283–296. https://doi.org/10.1038/s41380-019-0581-3
- Meewisse, M.-L., Reitsma, J. B., de Vries, G.-J., Gersons, B. P. R., & Olff, M. (2007). Cortisol and post-traumatic stress disorder in adults: Systematic review and meta-analysis. British Journal of Psychiatry, 191(5), 387–392. https://doi.org/10.1192/bjp.bp.106.024877
- Meiser-Stedman, R., Smith, P., Yule, W., Glucksman, E., & Dalgleish, T. (2017). Posttraumatic stress disorder in young children 3 years posttrauma: Prevalence and longitudinal predictors. The Journal of Clinical Psychiatry, 78(3), 334–339. https://doi.org/10.4088/JCP.15m10002
- Miller, A. H., Maletic, V., & Raison, C. L. (2009). Inflammation and its discontents: The role of cytokines in the pathophysiology of major depression. Biological Psychiatry, 65(9), 732–741. https://doi.org/10.1016/j.biopsych.2008.11.029
- Miller, G. E., Chen, E., Sze, J., Marin, T., Arevalo, J. M. G., Doll, R., Ma, R., & Cole, S. W. (2008). A functional genomic fingerprint of chronic stress in humans: Blunted glucocorticoid and increased NF-κB signaling. Biological Psychiatry, 64(4), 266–272. https://doi.org/10.1016/j.biopsych.2008.03.017
- Morris, M. C., Hellman, N., Abelson, J. L., & Rao, U. (2016). Cortisol, heart rate, and blood pressure as early markers of PTSD risk: A systematic review and meta-analysis. Clinical Psychology Review, 49, 79–91. https://doi.org/10.1016/j.cpr.2016.09.001
- Nishi, D., Matsuoka, Y., Noguchi, H., Sakuma, K., Yonemoto, N., Yanagita, T., Homma, M., Kanba, S., & Kim, Y. (2009). Reliability and validity of the Japanese version of the Peritraumatic Distress Inventory. General Hospital Psychiatry, 31(1), 75–79. https://doi.org/10.1016/j.genhosppsych.2008.09.002
- Observatoire national Interministériel de la sécurité routière (ONISR). (2019). Accidentalité routière 2018.
- O’Donnell, M. L., Creamer, M., Pattison, P., & Atkin, C. (2004). Psychiatric morbidity following injury. American Journal of Psychiatry, 161(3), 507–514. https://doi.org/10.1176/appi.ajp.161.3.507
- Olofsson, E., Bunketorp, O., & Andersson, A.-L. (2009). Children and adolescents injured in traffic–associated psychological consequences: A literature review. Acta Paediatrica, 98(1), 17–22. https://doi.org/10.1111/j.1651-2227.2008.00998.x
- Pariante, C. M., & Miller, A. H. (2001). Glucocorticoid receptors in major depression: Relevance to pathophysiology and treatment. Biological Psychiatry, 49(5), 391–404. https://doi.org/10.1016/S0006-3223(00)01088-X
- Ruggiero, K. J., Rheingold, A. A., Resnick, H. S., Kilpatrick, D. G., & Galea, S. (2006). Comparison of two widely used PTSD-screening instruments: Implications for public mental health planning. Journal of Traumatic Stress, 19(5), 699–707. https://doi.org/10.1002/jts.20141
- Rytwinski, N. K., Scur, M. D., Feeny, N. C., & Youngstrom, E. A. (2013). The co-occurrence of major depressive disorder among individuals with posttraumatic stress disorder: A meta-analysis. Journal of Traumatic Stress, 26(3), 299–309. https://doi.org/10.1002/jts.21814
- Shalev, A. Y., Freedman, S., Peri, T., Brandes, D., Sahar, T., Orr, S. P., & Pitman, R. K. (1998). Prospective study of posttraumatic stress disorder and depression following trauma. American Journal of Psychiatry, 155(5), 630–637. https://doi.org/10.1176/ajp.155.5.630
- Sheehan, D. V., Lecrubier, Y., Sheehan, K. H., Amorim, P., Janavs, J., Weiller, E., Hergueta, T., Baker, R., & Dunbar, G. C. (1998). The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. The Journal of Clinical Psychiatry, 59(Suppl 20), 22–33. quiz 34-57.
- Sijbrandij, M., Engelhard, I. M., Opmeer, B. C., van de Schoot, R., Carlier, I. V. E., Gersons, B. P. R., & Olff, M. (2012). The structure of peritraumatic dissociation: A cross validation in clinical and nonclinical samples. Journal of Traumatic Stress, 25(4), 475–479. https://doi.org/10.1002/jts.21716
- Sopp, M. R., Michael, T., Lass-Hennemann, J., Haim-Nachum, S., & Lommen, M. J. J. (2021). Longitudinal associations between hair cortisol, PTSD symptoms, and sleep disturbances in a sample of firefighters with duty-related trauma exposure. Psychoneuroendocrinology, 134, 105449. https://doi.org/10.1016/j.psyneuen.2021.105449
- Speer, K. E., Semple, S., Naumovski, N., D’Cunha, N. M., & McKune, A. J. (2019). HPA axis function and diurnal cortisol in post-traumatic stress disorder: A systematic review. Neurobiology of Stress, 11, 100180. https://doi.org/10.1016/j.ynstr.2019.100180
- Stroud, C. B., Vrshek-Shallhorn, S., Norkett, E. M., & Doane, L. D. (2019). The cortisol awakening response (CAR) interacts with acute interpersonal stress to prospectively predict depressive symptoms among early adolescent girls. Psychoneuroendocrinology, 107, 9–18. https://doi.org/10.1016/j.psyneuen.2019.04.017
- Vance, M. C., Kovachy, B., Dong, M., & Bui, E. (2018). Peritraumatic distress: A review and synthesis of 15 years of research. Journal of Clinical Psychology, 74(9), 1457–1484. https://doi.org/10.1002/jclp.22612
- Ventureyra, V. A. G., Yao, S.-N., Cottraux, J., Note, I., & De Mey-Guillard, C. (2002). The validation of the Posttraumatic Stress Disorder Checklist Scale in posttraumatic stress disorder and nonclinical subjects. Psychotherapy and Psychosomatics, 71(1), 47–53. https://doi.org/10.1159/000049343
- Villain, H., Benkahoul, A., Birmes, P., Ferry, B., & Roullet, P. (2018). Influence of early stress on memory reconsolidation: Implications for post-traumatic stress disorder treatment. PloS One, 13(1), e0191563. https://doi.org/10.1371/journal.pone.0191563
- WHO | Global Status Report on Road Safety 2015. (n.d.). WHO. Retrieved August 2, 2016, from http://www.who.int/violence_injury_prevention/road_safety_status/2015/en/
- Yao, S.-N., Cottraux, J., Note, I., De Mey-Guillard, C., Mollard, E., & Ventureyra, V. (2003). [Evaluation of Post-traumatic Stress Disorder: Validation of a measure, the PCLS]. L’Encéphale, 29(3 Pt 1), 232–238.
- Yehuda, R. (2002). Current status of cortisol findings in post-traumatic stress disorder. Psychiatric Clinics of North America, 25(2), 341–368, vii. https://doi.org/10.1016/S0193-953X(02)00002-3
