ABSTRACT
Background: Emerging evidence has linked childhood maltreatment with cardiovascular disease risk; however, the association between childhood maltreatment and cardiac arrhythmias remains unclear. Moreover, any genetic predispositions to atrial fibrillation (AF), a common cardiac arrhythmia associated with an elevated risk of stroke, heart failure, and mortality, that modify such associations have been undocumented.
Purpose: To examine the associations between childhood maltreatment and incident arrhythmias, and whether a genetic predisposition to arrhythmias modifies these associations.
Methods: This prospective analysis included 151,741 participants from the UK Biobank (mean age 55.8 years, 43.4% male). Childhood maltreatment, including five types, was measured using the Childhood Trauma Screener (CTS). Incident arrhythmias (AF, ventricular arrhythmias [VA], and bradyarrhythmia [BA]) were documented through linked hospital admission and death registry. Weighted AF genetic risk score was calculated. Cox proportional hazard models were conducted to test for associations between childhood maltreatment and incident arrhythmias.
Results: During a median follow-up of 12.21 years (interquartile range, 11.49–12.90 years), 6,588 AF, 2,093 BA, and 742 VA events occurred. Compared with the absence of childhood maltreatment, having 3–5 types of childhood maltreatment was associated with an increased risk of incident AF (HR, 1.23; 95%CI 1.09–1.37), VA (HR, 1.39; 95%CI 1.03–1.89), and BA (HR, 1.32; 95%CI 1.09–1.61) after adjusting demographic, socioeconomic and lifestyle factors. The associations between cumulative type of childhood maltreatment and the risk of AF (Poverall < .001; Pnonlinear = .674) and BA (Poverall = .007; Pnonlinear = .377) demonstrated a linear pattern. There was a gradient association between childhood maltreatment and AF risks across the intermediate and high genetic risk groups (both Ptrend < .05) but not within the low genetic risk group (Ptrend = .378), irrespective of non-significant interaction effect (Pinteraction = .204).
Conclusion: Childhood maltreatment was associated with higher risks of incident arrhythmias, especially AF and BA. Genetic risk of AF did not modify these associations.
HIGHLIGHTS
Previous studies indicate that childhood maltreatment is associated with cardiovascular disease risk.
Childhood maltreatment was associated with an increased risk of incident arrhythmias, particularly atrial fibrillation and bradyarrhythmia. Genetic predisposition to atrial fibrillation did not significantly modify these associations.
Childhood maltreatment could be a new psychological risk factor for cardiac arrhythmias in later life. Inquiries into childhood maltreatment and subsequent referral to psychological services may be helpful.
Antecedentes: La evidencia emergente ha relacionado el maltrato infantil con el riesgo de enfermedad cardiovascular; sin embargo, la asociación entre el maltrato infantil y las arritmias cardíacas sigue sin estar clara. Además, no se ha documentado ninguna predisposición genética a la fibrilación auricular (AF – por sus siglas en inglés), una arritmia cardíaca común asociada con un riesgo elevado de accidente cerebrovascular, insuficiencia cardíaca y mortalidad, que modifique tales asociaciones.
Propósito: Examinar las asociaciones entre el maltrato infantil y las arritmias incidentes y si una predisposición genética a las arritmias modifica estas asociaciones.
Métodos: Este análisis prospectivo incluyó a 151.741 participantes del Biobanco del Reino Unido (edad media 55,8 años, 43,4% hombres). El maltrato infantil, incluidos cinco tipos, se midió mediante el Childhood Trauma Screener (CTS, por sus siglas en inglés). Las arritmias incidentes (AF, arritmias ventriculares [VA, por sus siglas en inglés] y bradiarritmia [BA, por sus siglas en inglés]) se documentaron a través de registros vinculados de admisiones hospitalarias y defunciones. Se calculó la puntuación de riesgo genético de AF ponderada. Se realizaron modelos de riesgo proporcionales de Cox para probar las asociaciones entre el maltrato infantil y las arritmias incidentes.
Resultados: Durante una mediana de seguimiento de 12,21 años (rango intercuartil, 11,49–12,90 años), ocurrieron 6.588 eventos de AF, 2.093 BA y 742 VA. En comparación con la ausencia de maltrato infantil, tener de 3 a 5 tipos de maltrato infantil se asoció con un mayor riesgo de incidencia de AF (HR, 1,23; IC 95% 1,09–1,37), VA (HR, 1,39; IC 95% 1,03–1,89) y BA (HR, 1,32; IC95% 1,09–1,61) después de ajustar factores demográficos, socioeconómicos y de estilo de vida. Las asociaciones entre el tipo acumulativo de maltrato infantil y el riesgo de AF (P general <0,001; P no lineal = 0,674) y BA (P general = 0,007; P no lineal = 0,377) demostraron un patrón lineal. Hubo una asociación gradual entre el maltrato infantil y los riesgos de FA en los grupos de riesgo genético intermedio y alto (tendencia de P < 0,05) pero no dentro del grupo de riesgo genético bajo (tendencia de P = 0,378), independientemente del efecto de interacción no significativo (P interacción = 0,204).
Conclusión: El maltrato infantil se asoció con mayores riesgos de incidencia de arritmias, especialmente AF y BA. El riesgo genético de AF no modificó estas asociaciones.
1. Introduction
Cardiac arrhythmias have emerged as a severe health threat, with a prevalence of 1–5% among middle-aged and older adults (Khurshid et al., Citation2018). As the most common form of arrhythmia, atrial fibrillation (AF) leads to a series of significant health outcomes, such as stroke and heart failure (Weng et al., Citation2018). Other forms of arrhythmia, ventricular arrhythmias (VA) and bradyarrhythmia (BA), may cause fatal health outcomes, including sudden cardiac death (Zeppenfeld et al., Citation2022). Although the traditional modifiable risk factors of arrhythmia, such as smoking, alcohol consumption, or obesity, have been well-recognised, they have not been managed well and the pandemic of arrhythmia has still not been alleviated (Wei et al., Citation2021). It suggests that additional risk factors should be considered. Psychological stressful factors have recently been reported to be an important risk factor for cardiac arrhythmias (Du et al., Citation2017) and its related complications, such as stroke, heart failure, or other cardiovascular events (Kivimäki & Steptoe, Citation2018). Specifically, childhood maltreatment, one of the most common psychological stressors occurring before the age of 18 years, has recently gained increasing attention in cardiology research (Bhutta et al., Citation2023; Gilbert et al., Citation2009). Therefore, it is intriguing to see whether childhood maltreatment is associated with a greater risk of cardiac arrhythmias.
Several lines of evidence have suggested a potential link between childhood maltreatment and arrhythmia. First, previous studies demonstrated that mental stress, including childhood maltreatment, may induce a series of pathological changes related to the development of arrhythmias, for instance, increased activation of the hypothalamic-pituitary-adrenocortical axis (Uradu et al., Citation2017), the autonomic nervous system (Chen & Tan, Citation2007), inflammation (Staerk et al., Citation2017), or endothelial dysfunction (Corban et al., Citation2021). Second, individuals with childhood maltreatment are more likely to have unhealthy lifestyles and a cluster of cardiovascular risk factors, including smoking,(Chamberlain et al., Citation2011) obesity, (Wang et al., Citation2004) or extreme physical activity levels (La Gerche & Heidbuchel, Citation2014). These pathological and lifestyle changes may lead to structural and electrophysiological alterations of the atrial substrate, which may further trigger the onset of arrhythmias (Suglia et al., Citation2018; Wei et al., Citation2021). Moreover, previous studies indicate that childhood maltreatment increases the risk of the onset of stroke, coronary heart disease, myocardial infarction, and heart failure, which are the major complications of arrhythmias (Chandan et al., Citation2020; Dong et al., Citation2004; Godoy et al., Citation2021). However, there have been no studies investigating the association between childhood maltreatment and incident arrhythmias. Additionally, a recent study indicated that diverse genetic and environmental factors may contribute to the genesis of arrhythmias (Giudicessi et al., Citation2021). However, it is unclear whether genetic susceptibility to arrhythmias modifies the association between childhood maltreatment and arrhythmias.
Therefore, the current study aimed to investigate the association between childhood maltreatment and the incidence of arrhythmias, including AF, VA, and BA, later in life in the UK Biobank. Second, we investigated whether this association was modified by genetic risks.
2. Methods
2.1. Study population
This was a prospective population-based cohort study of over 500,000 adults aged between 38 and 72 years enrolled in the UK Biobank during 2006 and 2010 (Fry et al., Citation2017). More details about the UK Biobank are described on the website (https://www.ukbiobank.ac.uk/). All participants provided written informed consent, and the study protocol was approved by the North West Multi-centre Research Ethics Committee in the UK. A subgroup of individuals (N = 502,505) was invited to complete an online mental health questionnaire in 2016. Of them, 157,331 participants responded to online questionnaires about childhood maltreatment. A total of 153,633 participants provided valid and complete answers about childhood maltreatment. The main analysis eliminated participants with a diagnosis of arrhythmias at baseline (N = 1,892), leaving 151,741 participants in the study investigating the association of childhood maltreatment with the incident arrhythmias including AF, VA, and BA determined by ICD-10 codes. Furthermore, the analyses on genetic risks selected the study sample with valid genome wide association study data, and further excluded those: (1) had outliers in heterozygosity and missing rates (N = 290), (2) were sex mismatch (N = 3,280), (3) had sex chromosome aneuploidy (N = 90), (4) had excessive genetic relatedness (N = 64), (5) were non-European ancestry (N = 4,594). A total of 143,423 participants were included in the final study sample for genetic analysis. The flowchart of the inclusion and exclusion of participants in this study is illustrated in Supplementary material Figure S1.
2.2. Assessment of childhood maltreatment
Participants in the UK Biobank completed a retrospectively reported five-item childhood trauma screen (CTS). This assessment consists of one five-point Likert scale item for each type of childhood maltreatment. Childhood maltreatment was classified as emotional abuse, emotional neglect, physical abuse, physical neglect, and sexual abuse. The cut-offs on this scale used to define the presence or absence of childhood maltreatment obeyed the criteria proposed by a previous validation study with good overall (r = 0.88) and satisfactory type-specific correlations (r = 0.55–0.87) (Glaesmer et al., Citation2013), which was recently adopted in another study concerning childhood maltreatment (Ho et al., Citation2020). The response ‘prefer not to answer’ to any CTS question was considered a missing value. The details of the assessments and cut-offs of the CTS are presented in Supplementary material Text S1.
2.3. Incident arrhythmias
The outcome of the present study was incident arrhythmia, including AF, VA, and BA. In the UK Biobank, the incidence of a clinical endpoint was determined using hospital admission data and death registry records. Participants with AF, VA, or BA were identified as having a primary or secondary diagnosis (hospital admission records), or an underlying or contributory cause of death (death register) according to the tenth revision of the International Statistical Classification of Diseases and Related Health Problems codes and Procedure codes until May 2021 (Supplementary material Text S2).
2.4. Genetic risk score for AF
The detailed methods of genotyping, quality control, and imputation in the UK Biobank have been provided elsewhere (Bycroft et al., Citation2018). Based on previously published genome-wide association studies (GWAS), we selected the 134 independent single-nucleotide polymorphisms (SNPs) that are significantly associated with AF, which were extracted from a recent large-scale GWAS of individuals of European ancestry (Supplementary material Table S1) (Roselli et al., Citation2018). Therefore, our current genetic investigation was limited to participants of white ethnicity. The genetic risk scores were formulated as the sum of the number of alleles (0, 1, or 2) for each individual after multiplication with the effect size between the SNPs and AF. Following this, we constructed a weighted genetic risk score for AF using the formula previously reported (Fan et al., Citation2020): weighted genetic risk score = (β1×SNP1 + β2 × SNP2+ … +βn × SNPn) × (n/sum of the β coefficients), where the effect size (β coefficient) for each SNP was derived from previous genome-wide association study data. A higher weighted genetic risk score indicates a higher genetic predisposition to developing AF. Subsequently, within the sample designated for gene – environment interaction analysis, the genetic risk scores for AF were classified into high (quintile 5), moderate (quintile 2–4), or low (quintile 1) categories based on the distribution of the weighted genetic risk score (Lourida et al., Citation2019).
2.5. Assessment of covariates
The following characteristics were treated as the potential covariates (Supplementary material Text S3): age (continuous), sex (male/female), ethnicity (white/others), assessment centre (England/Scotland/Wales), employment status (employed/unemployed), education level (college or university degree/non-college or university degree), Townsend Deprivation Index (continuous), smoking status (never/current/past), alcohol consumption status (abstainer/light or moderate drinker/heavy or abusive drinker), physical activity (continuous, metabolic equivalent task-summed days), healthy diet score (continuous, 0–5 points), body mass index (BMI, continuous, kg/m2), grip strength (kg), insomnia (never/rarely, sometimes, usually), hypertension (yes/no), hypercholesterolemia (yes/no), diabetes mellitus (yes/no), anxiety or depression (yes/no), structural heart disease (yes/no), ischemic heart disease (yes/no), heart failure (yes/no), and stroke (yes/no) at baseline.
2.6. Statistical analysis
Baseline characteristics of the present study were summarised as percentages for categorical variables and mean (standard deviation, SD) or median (interquartile range, IQR) for continuous variables, as appropriate. Missing values of the potential covariates were imputed by the chained equation using ‘mice’ R packages.
We categorised the participants into three groups according to their cumulative types of childhood maltreatment: no maltreatment, 1–2 types, and 3–5 types. Hazard ratios (HR) and 95% confidence intervals (CI) for the association of childhood maltreatment and its five types with each endpoint were calculated using Cox regression models. We constructed three multivariate-adjusted Cox models: model 1 was adjusted for age and sex; model 2 was additionally adjusted for socioeconomic factors, including ethnicity, assessment centre, employment status, education level, and Townsend Deprivation Index; and model 3 was further adjusted for lifestyle factors, including smoking status, alcohol consumption status, physical activity, and healthy diet score. The cumulative types of childhood maltreatment were treated as continuous variables to perform trends test. We used restricted cubic spline analyses to examine the potential linear or nonlinear patterns of associations among the cumulative types of childhood maltreatment with each endpoint. The population-attributable fractions (PAFs) were estimated, which indicated the proportion of events that would have been prevented if all individuals had been without any childhood maltreatment. Furthermore, we examined the associations of each type of childhood maltreatment with subsequent risk for AF, VA, and BA, respectively.
In the genetic risk interaction analyses, we evaluated the genetic risk-modifying associations of childhood maltreatment in participants with different AF genetic risks. First the joint associations of childhood maltreatment and genetic risks with incident AF were assessed, with the ‘low genetic risks and no childhood maltreatment’ category as the reference group. Stratified analyses were conducted to estimate the associations between childhood maltreatment and incident AF in each genetic risk group. In addition, to determine whether the interaction between childhood maltreatment and the genetic risk score affected AF, we added interaction terms between childhood maltreatment and genetic risk. Furthermore, we investigated the associations of childhood maltreatment with incident AF in each subset of participants with either low, intermediate, or high genetic risk, respectively, using multivariate Cox regressions.
Subsequently, we performed several sensitivity analyses. We first adjusted for physical and mental conditions (including body mass index, grip strength, insomnia, hypertension, hypercholesterolemia, diabetes mellitus, anxiety or depression, structural heart disease, ischemic heart disease, heart failure, and stroke) in the Cox models. Next, we restricted the analytic sample to participants without comorbidities (ischemic heart disease, structural heart disease, heart failure, and stroke) at baseline. Alternatively, we conducted the main analyses in subgroups stratified by several covariates, such as age, sex, smoking status, alcohol consumption status, physical activity, and BMI, and tested for potential interactions of childhood maltreatment with the above covariates using the cross-product terms. Finally, we estimated the sub-distribution HR to account for the competing risk of all-cause mortality using the Fine and Gray competing risks regression model. Proportional hazard assumptions were inspected using Schoenfeld’s tests, and the results indicated no substantial departures. P-values were two-sided with statistical significance set at <.05. All statistical analyses were performed using the R software (version 4.1.2; R Development Core Team).
3. Results
3.1. Baseline characteristics of the study sample
This study consisted of 151,741 participants. Supplementary material Table S2 displays comparable information regarding the inclusion and exclusion of participants. The characteristics of the analytic sample according to the childhood maltreatment categories are presented in . The mean (SD) age of the study sample at baseline was 55.8 (7.7) years, and 43.4% of participants were male. We documented 6,588 incidents of AF, 742 VA incidents, and 2,093 BA incidents during a median follow-up of 12.21 years (IQR, 11.49–12.90 years) for AF, 12.24 years (11.56–12.92) for VA, and 12.23 years (11.54–12.92) for BA. In total, 42,500 participants (28.0%) had at least one or two types of childhood maltreatment, and 8,049 (5.3%) had three to five types of childhood maltreatment. Generally, participants who had more childhood maltreatment were more likely to be younger, female, and to have a history of hypertension, diabetes mellitus, anxiety, or depression.
Table 1. Characteristics of participants at baseline in the UK Biobank study.
3.2. Associations of childhood maltreatment with arrhythmias
The participants with 3–5 types of childhood maltreatment, compared with the absence of maltreatment, had a greater risk of developing AF (HR, 1.27; 95% CI, 1.13–1.42), VA (HR, 1.47; 95% CI, 1.09–1.99) and BA (HR, 1.38; 95% CI, 1.14–1.68) (, Model 1) in age – and sex-adjusted models. After further adjusting for socioeconomic and lifestyle factors, these associations remained significant (for AF, HR, 1.23; 95% CI, 1.09–1.37; for VA, HR, 1.39; 95% CI, 1.03–1.89; for BA HR, 1.32; 95% CI, 1.09–1.61) (, Model 3). The associations between cumulative type of childhood maltreatment and AF (Poverall < .001; Pnonlinear = .674) and BA (Poverall = .007; Pnonlinear = .377) demonstrated a linear pattern, indicating that a higher burden of childhood maltreatment was associated with a greater risk of incident AF and BA (). In addition, compared with the absence of childhood maltreatment, the presence of any childhood maltreatment was associated with an increased risk of incident AF (HR, 1.10; 95% CI, 1.04–1.15) and BA (HR, 1.09; 95% CI, 1.00–1.20), but not VA (Supplementary material Table S3, Model 3). 3.12% (95% CI, 1.14%−4.81%) of new-onset AF and 2.95% (95% CI, 0.00%−5.94%) of new-onset BA events during follow-up might have been prevented if none of the participants had any childhood maltreatment (Supplementary material Table S3, Model 3). Among all types of childhood maltreatment, physical abuse was the type of maltreatment most prominently associated with risk of AF (HR, 1.20; 95% CI 1.10–1.31) and VA (HR, 1.29; 95% CI 1.02–1.65) that might have been prevented if there was no experience of physical abuse. However, emotional abuse demonstrated a most significant association with risks of incident VA (HR, 1.34; 95% CI 1.06–1.70) and BA (HR, 1.21; 95% CI 1.03–1.41) (Supplementary material Table S4, Model 3).
Figure 1. Relationship between childhood maltreatment and AF (A), VA (B), and BA (C). HRs were adjusted for age, sex, ethnicity, assessment centre, employment status, education level, Townsend Deprivation Index, smoking status, alcohol consumption status, physical activity, and healthy diet score. AF, atrial fibrillation; BA, bradyarrhythmia; HR, hazard ratio; VA, ventricular arrhythmias.
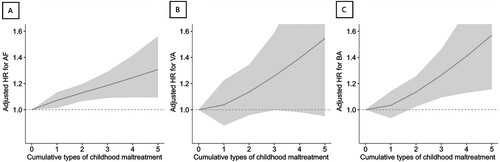
Table 2. Associations of childhood maltreatment with subsequent risk for AF, VA, and BA.
3.3. Associations of childhood maltreatment and genetic risks profile with incident AF
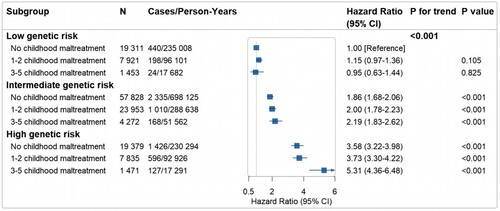
No significant interactions were found between childhood maltreatment and the genetic risk of AF (Pinteraction = .204) after fully adjusting for demographic, socioeconomic, and lifestyle characteristics. In the overall sample, compared with the participants with no childhood maltreatment and low genetic risks, participants with high genetic risks and 3–5 types of childhood maltreatment displayed the highest risk of AF (HR, 5.31; 95% CI, 4.36–6.48) (). In the stratified analyses according to the genetic risk profiles, compared with the absence of childhood maltreatment, having 3–5 types of childhood maltreatment showed a significant increase in the risk of incident AF (HR, 1.49; 95% CI 1.24–1.78) among participants with high genetic risk (Ptrend = .002) (Supplementary material Table S5). Genetic risk scores were independently associated with an increased hazard of incident AF (Supplementary material Table S6).
Figure 2. Joint associations of cumulative types of childhood maltreatment and genetic risk for AF with subsequent risk of incident AF. HRs were adjusted for age, sex, ethnicity, assessment centre, employment status, education level, Townsend Deprivation Index, smoking status, alcohol consumption status, physical activity, and healthy diet score. AF, atrial fibrillation; CI, confidence interval; HR, hazard ratio.
3.4. Sensitivity analysis
Sensitivity analyses with additional adjustment for physical and mental conditions (body mass index, grip strength, insomnia, hypertension, hypercholesterolemia, diabetes mellitus, anxiety or depression, structural heart disease, ischemic heart disease, heart failure, and stroke) attenuated the association of childhood maltreatment with each endpoint (Supplementary material Table S7), remained a significant positive trend between cumulative types of childhood maltreatment and incident AF (Ptrend = .036) (data not shown). Furthermore, when restricting participants without comorbidities (structural heart disease, ischemic heart disease, heart failure, and stroke) (Supplementary material Tables S8, S9, and S10) and when using the Fine and Gray regression models (Supplementary material Table S11), the results remained consistent.
Most covariates such as age, sex, assessment centre, and body mass index did not significantly modify the associations of childhood maltreatment with incident AF, VA, and BA (Supplementary material Table S12). The association between childhood maltreatment and incident AF was more prominent in participants who were heavy or abusive drinkers (Pinteraction = .018); for incident VA, the association was more evident in participants who were current smokers (Pinteraction = .045).
4. Discussion
To the best of our knowledge, this was the first cohort study of over 150,000 adults to document a significant association between childhood maltreatment and incident arrhythmias, including AF, VA, and BA. We found that a higher accumulative type of childhood maltreatment was associated with a greater risk of incident arrhythmias, especially AF and BA, independent of the traditional risk factors. Among the five types of childhood maltreatment, the association between physical abuse and incident arrhythmia was the strongest. These associations were independent of genetic risk for AF, despite the appearance of a more prominent gradient association between childhood maltreatment and incident AF in participants with intermediate and high genetic risks.
Our main findings agree with the literature showing that stressful experience (Liu, Citation2023), especially maltreatment, are associated with a greater risk of developing various cardiometabolic diseases in adulthood (Bengtsson et al., Citation2023; Liang et al., Citation2022; Soares et al., Citation2020; Suglia et al., Citation2018; Urquijo et al., Citation2023). To our knowledge, this was the first study to establish an independent association between childhood maltreatment and the risk of incident cardiac arrhythmias. This finding supports several previous studies in this area that have suggested that psychological stress is an important risk factor for the development of arrhythmias, particularly AF. For example, several stressors, such as the death of a child (Wei et al., Citation2021) or long working hours (Kivimaki et al., Citation2017) were reported to significantly accelerate the risk of incident AF. We extended these studies by including BA and VA as the outcomes. We noted significant risks of AF and BA, but not VA, associated with childhood maltreatment, possibly due to a small proportion of VA events, which may limit the statistical power to detect an association. Moreover, unrecorded cases and potential misclassification of outcomes might contribute to the absence of findings. Additionally, the involvement of risk factors could confound the direct association. Notably, behaviours such as smoking, obesity, and insomnia were recognised as independent risk factors for arrhythmias (D'Alessandro et al., Citation2012; Godoy et al., Citation2021; Li et al., Citation2021; Patel et al., Citation2022). Moreover, consistent with existing studies focusing on the association between childhood maltreatment and other types of or overall cardiovascular diseases (Bengtsson et al., Citation2023; Dong et al., Citation2004; Suglia et al., Citation2018), we also demonstrated participants with a higher accumulative type of childhood maltreatment experienced an increased risk for AF, BA, and VA. Our findings validated childhood maltreatment as an independent risk factor for the onset of AF and BA. This implies that the early identification of childhood maltreatment could have aided in anticipating subsequent arrhythmia events, particularly in the cases of AF and BA.
The previous study has suggested that the association between some environmental factors and health outcomes could be modified by genetic susceptibility (Qi et al., Citation2014). However, we found no statistically significant interaction between childhood maltreatment and genetic susceptibility to AF. Consistently, a previous analysis of UK Biobank revealed a lack of modification effects from genetic risks regarding the association of sleep behaviours with outcomes of incident AF (Li et al., Citation2021). Of note, despite the lack of significant interaction, participants with more cumulative types of childhood maltreatment were accompanied by a higher risk of developing AF, especially among participants with higher genetic risks. This result suggests the necessity for early identification of childhood maltreatment among adults with higher genetic risks.
Several potential mechanisms underlying the association between childhood maltreatment and incident arrhythmias have been speculated. First, participants who suffered from childhood maltreatment were more vulnerable to mental health problems such as symptoms of depression and anxiety or posttraumatic stress disorder (Anda et al., Citation2006; Chapman et al., Citation2004), which in turn may result in cardiac structural remodelling and electrical changes (Huxley et al., Citation2011; Staerk et al., Citation2017). Second, we found that the association between childhood maltreatment and incident arrhythmias was attenuated after adjusting for known cardiovascular risk factors such as obesity, physical inactivity, alcohol consumption, smoking, and hypertension (Chung et al., Citation2020). This finding indicates that these factors may be moderators or mediators of the observed associations. In agreement with this, the other study has also suggested that the abovementioned risk factors may have a role in the etiology of AF (Elliott et al., Citation2023). Third, emerging evidence has suggested that childhood maltreatment is related to multiple pathological changes, including autonomic dysfunction (McLaughlin et al., Citation2014), inflammation (Baumeister et al., Citation2016), and dysfunction of the hypothalamus-pituitary-adrenocortical axis (Tarullo & Gunnar, Citation2006), which may lead to cardiac electrical instability, myocardial ischemia, and increased concentrations of circulating catecholamines, ultimately resulting in arrhythmias (Kivimäki & Steptoe, Citation2018; Tyrka et al., Citation2009). Further experimental studies are needed to further clarify the underlying mechanisms and causal pathways linking childhood maltreatment to arrhythmias. Furthermore, among the 5 types of childhood maltreatment, our findings indicated that physical abuse was significantly associated with risks of incident AF and VA, while emotional abuse demonstrated a notable association with risks of incident VA and BA.
The major strengths of this study include the large sample size and prospective design. However, this study had several limitations. First, childhood maltreatment was retrospectively assessed using a self-reported questionnaire rather than being captured prospectively, which may have caused recall bias and misclassifications. This was a common limitation of the related studies focusing on childhood maltreatment (Reuben et al., Citation2016). One approach to mitigate this bias was to cross-validate self-reported data with records from social services or other child protection agencies (Martin et al., Citation2019). However, it was important to recognise that only a fraction of maltreatment cases was reported, which might lead to underestimation of the true prevalence. Future studies could consider implementing prospective cohort designs starting from infancy or early childhood to establish longitudinal cohorts for monitoring individuals’ experiences of childhood maltreatment and their health outcomes over time. Second, unmeasured residual confounders are inevitable due to the observational nature of this study, although we had adjusted for comprehensive confounders. Third, some subclinical or asymptomatic events of AF, VA, and BA may have been underestimated since the present study ascertained the outcomes based on linked medical records. This may have biased the estimated association with the null hypothesis. Fourth, the UK Biobank cohort is relatively healthy, resulting in a ‘healthy volunteer bias’ (Fry et al., Citation2017), which may limit the generalizability of the current findings to other populations. The association between childhood maltreatment and the risk of incident arrhythmias could have been underestimated. Finally, the gene-environment interaction analysis was also confined to a study sample of European ancestry; therefore, the findings might not be generalisable to other ethnic groups. Future studies should aim to address this limitation by future studies should consider incorporating other cohorts based on diverse populations for external validation to enhance the robustness and applicability of the findings.
5. Conclusion
This cohort study provides evidence that, irrespective of the genetic risk for arrhythmias, participants with a higher burden of childhood maltreatment had a greater risk of incident cardiac arrhythmia, including AF and BA, but not VA. Our findings add to the growing body of research indicating that psychological stressful factors could be important for the development of arrhythmias. Future studies are warranted to address whether intervention for the subsequent adverse effects arising from childhood maltreatment may help reduce the risk of developing cardiac arrhythmias.
Contributions
YYL conceived of the study design. YC contributed to the statistical analyses; HX, JJZ, SA, and HF helped with the software and modified the methods. YC, HX and JJZ were primarily responsible for writing the manuscript, with the help of XS and ZH. The manuscript was revised by YYL, YZ and YHL. In addition, YYL had full access to all the data in the study. JHZ, YYL, YZ and YHL were responsible for the integrity and accuracy of the data analysis. All authors gave their final approval and agreed to be accountable for all aspects of the work. YZ and YHL supervised this work and were the corresponding authors.
Ethics approval
The study protocol was approved by the North West Multi-centre Research Ethics Committee in the UK (16/NW/0274).
Supplemental Material.docx
Download MS Word (615.6 KB)Acknowledgements
This research was conducted using the UK Biobank Resource under Project (Application Number 59117).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Individual-level data from the UK Biobank are not publicly available due to their policy, but the data will be made available after the application of the UK Biobank (https://www.ukbiobank.ac.uk/).
Additional information
Funding
References
- Anda, R. F., Felitti, V. J., Bremner, J. D., Walker, J. D., Whitfield, C., Perry, B. D., Dube, S. R., & Giles, W. H. (2006). The enduring effects of abuse and related adverse experiences in childhood. A convergence of evidence from neurobiology and epidemiology. European Archives of Psychiatry and Clinical Neuroscience, 256(3), 174–186. https://doi.org/10.1007/s00406-005-0624-4
- Baumeister, D., Akhtar, R., Ciufolini, S., Pariante, C. M., & Mondelli, V. (2016). Childhood trauma and adulthood inflammation: a meta-analysis of peripheral C-reactive protein, interleukin-6 and tumour necrosis factor-α. Molecular Psychiatry, 21(5), 642–649. https://doi.org/10.1038/mp.2015.67
- Bengtsson, J., Elsenburg, L. K., Andersen, G. S., Larsen, M. L., Rieckmann, A., & Rod, N. H. (2023). Childhood adversity and cardiovascular disease in early adulthood: A Danish cohort study. European Heart Journal, 44(7), 586–593. https://doi.org/10.1093/eurheartj/ehac607
- Bhutta, Z. A., Bhavnani, S., Betancourt, T. S., Tomlinson, M., & Patel, V. (2023). Adverse childhood experiences and lifelong health. Nature Medicine, 29(7), 1639–1648. https://doi.org/10.1038/s41591-023-02426-0
- Bycroft, C., Freeman, C., Petkova, D., Band, G., Elliott, L. T., Sharp, K., Motyer, A., Vukcevic, D., Delaneau, O., O’Connell, J., Cortes, A., Welsh, S., Young, A., Effingham, M., McVean, G., Leslie, S., Allen, N., Donnelly, P., & Marchini, J. (2018). The UK Biobank resource with deep phenotyping and genomic data. Nature, 562(7726), 203–209. https://doi.org/10.1038/s41586-018-0579-z
- Chamberlain, A. M., Agarwal, S. K., Folsom, A. R., Duval, S., Soliman, E. Z., Ambrose, M., Eberly, L. E., & Alonso, A. (2011). Smoking and incidence of atrial fibrillation: Results from the Atherosclerosis Risk in Communities (ARIC) study. Heart Rhythm, 8(8), 1160–1166. https://doi.org/10.1016/j.hrthm.2011.03.038
- Chandan, J. S., Okoth, K., Gokhale, K. M., Bandyopadhyay, S., Taylor, J., & Nirantharakumar, K. (2020). Increased cardiometabolic and mortality risk following childhood maltreatment in the United Kingdom. Journal of the American Heart Association, 9(10), e015855. https://doi.org/10.1161/JAHA.119.015855
- Chapman, D. P., Whitfield, C. L., Felitti, V. J., Dube, S. R., Edwards, V. J., & Anda, R. F. (2004). Adverse childhood experiences and the risk of depressive disorders in adulthood. Journal of Affective Disorders, 82(2), 217–225. https://doi.org/10.1016/j.jad.2003.12.013
- Chen, P. S., & Tan, A. Y. (2007). Autonomic nerve activity and atrial fibrillation. Heart Rhythm, 4(3 Suppl), S61–S64. https://doi.org/10.1016/j.hrthm.2006.12.006
- Chung, M. K., Eckhardt, L. L., Chen, L. Y., Ahmed, H. M., Gopinathannair, R., Joglar, J. A., Noseworthy, P. A., Pack, Q. R., Sanders, P., & Trulock, K. M. (2020). Lifestyle and risk factor modification for reduction of atrial fibrillation: A scientific statement from the American Heart Association. Circulation, 141(16), e750–e772. https://doi.org/10.1161/CIR.0000000000000748
- Corban, M. T., Toya, T., Ahmad, A., Lerman, L. O., Lee, H. C., & Lerman, A. (2021). Atrial fibrillation and endothelial dysfunction: A potential link? Mayo Clinic Proceedings, 96(6), 1609–1621. https://doi.org/10.1016/j.mayocp.2020.11.005
- D'Alessandro, A., Boeckelmann, I., Hammwhöner, M., & Goette, A. (2012). Nicotine, cigarette smoking and cardiac arrhythmia: An overview. European Journal of Preventive Cardiology, 19(3), 297–305. https://doi.org/10.1177/1741826711411738
- Dong, M., Giles, W. H., Felitti, V. J., Dube, S. R., Williams, J. E., Chapman, D. P., & Anda, R. F. (2004). Insights into causal pathways for ischemic heart disease: Adverse childhood experiences study. Circulation, 110(13), 1761–1766. https://doi.org/10.1161/01.CIR.0000143074.54995.7F
- Du, X., Dong, J., & Ma, C. (2017). Is atrial fibrillation a preventable disease? Journal of the American College of Cardiology, 69(15), 1968–1982. https://doi.org/10.1016/j.jacc.2017.02.020
- Elliott, A. D., Middeldorp, M. E., Van Gelder, I. C., Albert, C. M., & Sanders, P. (2023). Epidemiology and modifiable risk factors for atrial fibrillation. Nature Reviews Cardiology, 20(6), 404–417. https://doi.org/10.1038/s41569-022-00820-8
- Fan, M., Sun, D., Zhou, T., Heianza, Y., Lv, J., Li, L., & Qi, L. (2020). Sleep patterns, genetic susceptibility, and incident cardiovascular disease: A prospective study of 385 292 UK Biobank participants. European Heart Journal, 41(11), 1182–1189. https://doi.org/10.1093/eurheartj/ehz849
- Fry, A., Littlejohns, T. J., Sudlow, C., Doherty, N., Adamska, L., Sprosen, T., Collins, R., & Allen, N. E. (2017). Comparison of sociodemographic and health-related characteristics of UK Biobank participants with those of the general population. American Journal of Epidemiology, 186(9), 1026–1034. https://doi.org/10.1093/aje/kwx246
- Gilbert, R., Widom, C. S., Browne, K., Fergusson, D., Webb, E., & Janson, S. (2009). Burden and consequences of child maltreatment in high-income countries. Lancet, 373(9657), 68–81. https://doi.org/10.1016/S0140-6736(08)61706-7
- Giudicessi, J. R., Ackerman, M. J., Fatkin, D., & Kovacic, J. C. (2021). Precision medicine approaches to cardiac arrhythmias: JACC focus seminar 4/5. Journal of the American College of Cardiology, 77(20), 2573–2591. https://doi.org/10.1016/j.jacc.2021.03.325
- Glaesmer, H., Schulz, A., Häuser, W., Freyberger, H. J., Brähler, E., & Grabe, H.-J. (2013). The childhood trauma screener (CTS) - development and validation of cut-off-scores for classificatory diagnostics. Psychiatrische Praxis, 40(4), 220–226. https://doi.org/10.1055/s-0033-1343116
- Godoy, L. C., Frankfurter, C., Cooper, M., Lay, C., Maunder, R., & Farkouh, M. E. (2021). Association of adverse childhood experiences with cardiovascular disease later in life: A review. JAMA Cardiology, 6(2), 228–235. https://doi.org/10.1001/jamacardio.2020.6050
- Ho, F. K., Celis-Morales, C., Gray, S. R., Petermann-Rocha, F., Lyall, D., Mackay, D., Sattar, N., Minnis, H., & Pell, J. P. (2020). Child maltreatment and cardiovascular disease: Quantifying mediation pathways using UK Biobank. BMC Medicine, 18(1), 143. https://doi.org/10.1186/s12916-020-01603-z
- Huxley, R. R., Lopez, F. L., Folsom, A. R., Agarwal, S. K., Loehr, L. R., Soliman, E. Z., Maclehose, R., Konety, S., & Alonso, A. (2011). Absolute and attributable risks of atrial fibrillation in relation to optimal and borderline risk factors: The Atherosclerosis Risk in Communities (ARIC) study. Circulation, 123(14), 1501–1508. https://doi.org/10.1161/CIRCULATIONAHA.110.009035
- Khurshid, S., Choi, S. H., Weng, L. C., Wang, E. Y., Trinquart, L., Benjamin, E. J., Ellinor, P. T., & Lubitz, S. A. (2018). Frequency of cardiac rhythm abnormalities in a half million adults. Circulation: Arrhythmia and Electrophysiology, 11(7), e006273. https://doi.org/10.1161/CIRCEP.118.006273
- Kivimaki, M., Nyberg, S. T., Batty, G. D., Kawachi, I., Jokela, M., Alfredsson, L., Bjorner, J. B., Borritz, M., Burr, H., Dragano, N., Fransson, E. I., Heikkila, K., Knutsson, A., Koskenvuo, M., Kumari, M., Madsen, I. E. H., Nielsen, M. L., Nordin, M., Oksanen, T., … consortium, I. P.-W. (2017). Long working hours as a risk factor for atrial fibrillation: A multi-cohort study. European Heart Journal, 38(34), 2621–2628. https://doi.org/10.1093/eurheartj/ehx324
- Kivimäki, M., & Steptoe, A. (2018). Effects of stress on the development and progression of cardiovascular disease. Nature Reviews Cardiology, 15(4), 215–229. https://doi.org/10.1038/nrcardio.2017.189
- La Gerche, A., & Heidbuchel, H. (2014). Can intensive exercise harm the heart? You can get too much of a good thing. Circulation, 130(12), 992–1002. https://doi.org/10.1161/CIRCULATIONAHA.114.008141
- Li, X., Zhou, T., Ma, H., Huang, T., Gao, X., Manson, J. E., & Qi, L. (2021). Healthy sleep patterns and risk of incident arrhythmias. Journal of the American College of Cardiology, 78(12), 1197–1207. https://doi.org/10.1016/j.jacc.2021.07.023
- Liang, Y., Ai, S., Weng, F., Feng, H., Yang, L., He, Z., Xue, H., Zhou, M., Shu, X., Chen, Y., Ma, H., Guo, L., Geng, Q., & Zhang, J. (2022). Associations of childhood maltreatment and genetic risks with incident heart failure in later life. Journal of the American Heart Association, 11, e026536. https://doi.org/10.1161/jaha.122.026536
- Liu, M. (2023). Potential risk factors of cardiovascular diseases and mental disorders. Heart and Mind, 7(2), 55–56. https://doi.org/10.4103/hm.HM-D-23-00018
- Lourida, I., Hannon, E., Littlejohns, T. J., Langa, K. M., Hyppönen, E., Kuzma, E., & Llewellyn, D. J. (2019). Association of lifestyle and genetic risk with incidence of dementia. JAMA, 322(5), 430–437. https://doi.org/10.1001/jama.2019.9879
- Martin, A. R., Kanai, M., Kamatani, Y., Okada, Y., Neale, B. M., & Daly, M. J. (2019). Clinical use of current polygenic risk scores may exacerbate health disparities. Nature Genetics, 51(4), 584–591. https://doi.org/10.1038/s41588-019-0379-x
- McLaughlin, K. A., Sheridan, M. A., Alves, S., & Mendes, W. B. (2014). Child maltreatment and autonomic nervous system reactivity: identifying dysregulated stress reactivity patterns by using the biopsychosocial model of challenge and threat. Psychosomatic Medicine, 76(7), 538–546. https://doi.org/10.1097/PSY.0000000000000098
- Patel, K. H. K., Reddy, R. K., Sau, A., Sivanandarajah, P., Ardissino, M., & Ng, F. S. (2022). Obesity as a risk factor for cardiac arrhythmias. BMJ Medicine, 1(1), e000308. https://doi.org/10.1136/bmjmed-2022-000308
- Qi, Q., Chu, A. Y., Kang, J. H., Huang, J., Rose, L. M., Jensen, M. K., Liang, L., Curhan, G. C., Pasquale, L. R., Wiggs, J. L., De Vivo, I., Chan, A. T., Choi, H. K., Tamimi, R. M., Ridker, P. M., Hunter, D. J., Willett, W. C., Rimm, E. B., Chasman, D. I., … Qi, L. (2014). Fried food consumption, genetic risk, and body mass index: gene-diet interaction analysis in three US cohort studies. BMJ, 348(mar19 1), g1610. https://doi.org/10.1136/bmj.g1610
- Reuben, A., Moffitt, T. E., Caspi, A., Belsky, D. W., Harrington, H., Schroeder, F., Hogan, S., Ramrakha, S., Poulton, R., & Danese, A. (2016). Lest we forget: Comparing retrospective and prospective assessments of adverse childhood experiences in the prediction of adult health. Journal of Child Psychology and Psychiatry, 57(10), 1103–1112. https://doi.org/10.1111/jcpp.12621
- Roselli, C., Chaffin, M. D., Weng, L. C., Aeschbacher, S., Ahlberg, G., Albert, C. M., Almgren, P., Alonso, A., Anderson, C. D., Aragam, K. G., Arking, D. E., Barnard, J., Bartz, T. M., Benjamin, E. J., Bihlmeyer, N. A., Bis, J. C., Bloom, H. L., Boerwinkle, E., Bottinger, E. B., … Ellinor, P. T. (2018). Multi-ethnic genome-wide association study for atrial fibrillation. Nature Genetics, 50(9), 1225–1233. https://doi.org/10.1038/s41588-018-0133-9
- Soares, A. L. G., Hammerton, G., Howe, L. D., Rich-Edwards, J., Halligan, S., & Fraser, A. (2020). Sex differences in the association between childhood maltreatment and cardiovascular disease in the UK Biobank. Heart, 106(17), 1310–1316. https://doi.org/10.1136/heartjnl-2019-316320
- Staerk, L., Sherer, J. A., Ko, D., Benjamin, E. J., & Helm, R. H. (2017). Atrial fibrillation: epidemiology, pathophysiology, and clinical outcomes. Circulation Research, 120(9), 1501–1517. https://doi.org/10.1161/CIRCRESAHA.117.309732
- Suglia, S. F., Koenen, K. C., Boynton-Jarrett, R., Chan, P. S., Clark, C. J., Danese, A., Faith, M. S., Goldstein, B. I., Hayman, L. L., Isasi, C. R., Pratt, C. A., Slopen, N., Sumner, J. A., Turer, A., Turer, C. B., & Zachariah, J. P. (2018). Childhood and adolescent adversity and cardiometabolic outcomes: A scientific statement from the American Heart Association. Circulation, 137(5), e15–e28. https://doi.org/10.1161/CIR.0000000000000536
- Tarullo, A. R., & Gunnar, M. R. (2006). Child maltreatment and the developing HPA axis. Hormones and Behavior, 50(4), 632–639. https://doi.org/10.1016/j.yhbeh.2006.06.010
- Tyrka, A., Price, L., Gelernter, J., Schepker, C., Anderson, G., & Carpenter, L. (2009). Interaction of childhood maltreatment with the corticotropin-releasing hormone receptor gene: Effects on hypothalamic-pituitary-adrenal axis reactivity. Biological Psychiatry, 66(7), 681–685. https://doi.org/10.1016/j.biopsych.2009.05.012
- Uradu, A., Wan, J., Doytchinova, A., Wright, K. C., Lin, A. Y. T., Chen, L. S., Shen, C., Lin, S. F., Everett, T. H. t., & Chen, P. S. (2017). Skin sympathetic nerve activity precedes the onset and termination of paroxysmal atrial tachycardia and fibrillation. Heart Rhythm, 14(7), 964–971. https://doi.org/10.1016/j.hrthm.2017.03.030
- Urquijo, H., Soares, A. G., Fraser, A., Howe, L. D., & Carter, A. R. (2023). Investigating effect modification between childhood maltreatment and genetic risk for cardiovascular disease in the UK Biobank. PLoS One, 18(5), e0285258. https://doi.org/10.1371/journal.pone.0285258
- Wang, T. J., Parise, H., Levy, D., D’Agostino, R. B., Wolf, P. A., Vasan, R. S., & Benjamin, E. J. (2004). Obesity and the risk of new-onset atrial fibrillation. JAMA, 292(20), 2471–2477. https://doi.org/10.1001/jama.292.20.2471
- Wei, D., Olofsson, T., Chen, H., Janszky, I., Fang, F., Ljung, R., Yu, Y., Li, J., & Laszlo, K. D. (2021). Death of a child and the risk of atrial fibrillation: A nationwide cohort study in Sweden. European Heart Journal, 42(15), 1489–1495. https://doi.org/10.1093/eurheartj/ehaa1084
- Weng, L. C., Preis, S. R., Hulme, O. L., Larson, M. G., Choi, S. H., Wang, B., Trinquart, L., McManus, D. D., Staerk, L., Lin, H., Lunetta, K. L., Ellinor, P. T., Benjamin, E. J., & Lubitz, S. A. (2018). Genetic predisposition, clinical risk factor burden, and lifetime risk of atrial fibrillation. Circulation, 137(10), 1027–1038. https://doi.org/10.1161/CIRCULATIONAHA.117.031431
- Zeppenfeld, K., Tfelt-Hansen, J., de Riva, M., Winkel, B. G., Behr, E. R., Blom, N. A., Charron, P., Corrado, D., Dagres, N., de Chillou, C., Eckardt, L., Friede, T., Haugaa, K. H., Hocini, M., Lambiase, P. D., Marijon, E., Merino, J. L., Peichl, P., Priori, S. G., … Slade, A. (2022). 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. European Heart Journal, 43(40), 3997–4126. https://doi.org/10.1093/eurheartj/ehac262
