?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Background: Previously, we found that patients with borderline personality disorder (BPD) but not healthy controls (HC) showed improved memory retrieval after hydrocortisone administration.
Objective: In this study, we examined whether increases in endogenous cortisol after psychosocial stress are associated with memory function in patients with BPD and in healthy individuals.
Methods: We recruited 49 female patients with BPD and 49 female HC. All participants were exposed to a psychosocial stressor, the Trier Social Stress Test (TSST) and a control condition (Placebo (P-)TSST) in randomized order. Salivary cortisol, alpha amylase (sAA) and blood pressure were measured in response to stress. Subsequently, we examined free recall of a previously learned word list, autobiographical memory, and working memory.
Results: We found a stress*time*group interaction effect for the cortisol response and for sAA to stress, which is mainly triggered by a slightly different increase in cortisol between groups from pre to post TSST. Furthermore, BPD patients showed a less pronounced increase in diastolic blood pressure compared to HC after stress. There was no effect of stress on memory performance in any tests, either in healthy controls or in patients with BPD.
Conclusion: Our results suggest a slightly blunted response of the HPA axis and the sympathetic nervous system to stress in BPD compared to healthy women. In contrast to hydrocortisone administration, psychosocial stress did not improve memory retrieval in BPD patients. This might be explained by lower cortisol concentrations and parallel increases in norepinephrine and negative affect after stress.
HIGHLIGHTS
• Previously, we found that patients with BPD showed improved memory retrieval after hydrocortisone. • Here, we examined the effects of psychosocial stress on memory in patients with BPD. • Patients with BPD had a blunted response of the sympathetic nervous system to stress. • There were only small differences in cortisol release between BPD patients and healthy controls. • Psychosocial stress did not improve memory retrieval in BPD patients.
Antecedentes: Previamente, encontramos que los pacientes con trastorno de personalidad límite (TPL), contrariamente a los controles sanos (CS), mostraron una mejor recuperación de memoria después de la administración de hidrocortisona.
Objetivo: En este estudio, examinamos si los aumentos en el cortisol endógeno, después del estrés psicosocial están asociados con el funcionamiento de la memoria en pacientes con TPL y en individuos sanos.
Métodos: Se reclutaron 49 pacientes de sexo femenino con TPL y 49 mujeres CS. Todas las participantes fueron expuestas a un estresante psicosocial, la Prueba de estrés social de Trier (TSST) y una condición de control (Placebo (P-) TSST) en orden aleatorio. El cortisol salival, la alfa amilasa (sAA) y la presión arterial se midieron en respuesta al estrés. Posteriormente, examinamos la recuperación libre de una lista de palabras previamente aprendida, la memoria autobiográfica y memoria de trabajo.
Resultados: Encontramos un efecto de interacción grupal estrés*tiempo* para la respuesta de cortisol y para sAA al estrés, gatillada principalmente por un aumento ligeramente diferente en el cortisol entre los grupos desde el pre al post TSST. Además, las pacientes con TPL mostraron un aumento menos pronunciado en la presión arterial diastólica en comparación con las CS después del estrés. No hubo efecto del estrés en el rendimiento de la memoria en ninguna prueba, ni en controles sanos ni en pacientes con TLP.
Conclusión: Nuestros resultados sugieren una respuesta ligeramente atenuada del eje Hipotálamo-Hipófisis-Adrenal y del sistema nervioso simpático al estrés en TPL en comparación con mujeres sanas. En contraste con la administración de hidrocortisona, el estrés psicosocial no mejoró la recuperación de la memoria en pacientes con TPL. Esto podría explicarse por menores concentraciones de cortisol y aumentos paralelos de norepinefrina y afecto negativo después del estrés.
前言: 之前我们发现患有边缘人格障碍(BPD)的患者在给予氢化可的松后记忆提取有所改善,但这在健康对照组(HC)中没有出现。在本研究中,我们考查了经历心理社会应激后内源性皮质醇的增加是否与BPD患者和健康个体的记忆功能相关。
方法: 我们招募了49名患有BPD的女性患者和49名女性HC。所有参与者均以随机顺序接触心理社会压力源,Trier社会压力测试(TSST)和对照条件(安慰剂(P-)TSST)。测量应激之后的唾液皮质醇,α淀粉酶(sAA)和血压。随后,我们考查了对学习过的单词列表的自由回忆,自传记忆和工作记忆。
结果: 我们发现皮质醇和sAA的应激*时间*组别相互作用效应,这主要是由于TSST前后的皮质醇增加略有不同。此外,与应激后的HC相比,BPD患者的舒张压增加不太明显。在健康对照组和BPD患者中,任何测试都没有应激对记忆表现的影响。
结论: 我们的研究结果表明,与健康女性相比,BPD患者的HPA轴和交感神经系统对压力的反应略显钝化。与氢化可的松给药相对比,心理社会应激并未改善BPD患者的记忆提取。这可能是由于较低的皮质醇浓度和去甲肾上腺素的平行增加以及压力后的负面影响。
1. Introduction
Patients with borderline personality disorder (BPD) are characterized by severe self-harming and impulsive behaviour, fear of abandonment, unstable relationships and rapidly changing mood states. Acute stress often leads to further worsening of symptoms with stress-related dissociative and paranoid symptoms, anger, aggression or even suicidal behaviour (American Psychiatric Association, Citation2013).
Due to the strong link between BPD symptoms and stress, several studies have investigated whether hypothalamic-pituitary-adrenal (HPA) axis functioning is altered in patients with BPD. In sum, there is evidence for enhanced basal cortisol release, as a marker for HPA axis activation, in concert with reduced feedback sensitivity (Zimmerman & Choi-Kain, Citation2009, Wingenfeld, Spitzer, Rullkotter, et al., Citation2010). In addition, several studies used a well-established psychosocial stressor, the Trier Social Stress Test (TSST), to investigate the psychobiological response to psychosocial stress in BPD patients. Most of these studies reported a blunted or unaltered cortisol response to stress (Aleknaviciute et al., Citation2016; Deckers et al., Citation2015; Ehrenthal, Levy, Scott, & Granger, Citation2018; Nater et al., Citation2010; Scott, Levy, & Granger, Citation2013).
Compared to the HPA axis, the sympathetic nervous system (SNS) reflects a fast-acting neuronal cascade as a response to acute stress. Salivary alpha-amylase (sAA) has found to be a useful marker for stress-induced SNS activation (Nater & Rohleder, Citation2009). Besides alpha amylase, blood pressure and heart rate are important markers for the SNS. However, studies of SNS activation in BPD are equivocal and used different outcome variables. A blunted sAA and heart-rate response after the TSST (Aleknaviciute et al., Citation2016; Nater et al., Citation2010; Scott et al., Citation2013) was found in BPD, but there are also studies which did not confirm a blunted SNS response to stress (Inoue et al., Citation2015; Simeon, Knutelska, Smith, Baker, & Hollander, Citation2007). One study even reported higher stress-induced sAA levels in BPD (Ehrenthal et al., Citation2018).
Stress and cortisol are known to affect cognitive processes. Circulating cortisol can pass the blood brain barrier and acts on the brain by binding to two specific receptors: the glucocorticoid and the mineralocorticoid receptor (GR and MR). Two brain regions with a high density of these receptors are the hippocampus and the prefrontal cortex (PFC), which are strongly involved in cognition, such as memory (de Kloet, Citation2014). In healthy individuals, studies show unanimously that cortisol administration or psychosocial stress impairs memory retrieval (de Quervain, Aerni, Schelling, & Roozendaal, Citation2009; Shields, Sazma, McCullough, & Yonelinas, Citation2017; Wolf, Citation2017) and executive function (Schlosser et al., Citation2010; Shields, Sazma, & Yonelinas, Citation2016). Importantly, reduced memory performance after stress has been shown predominantly in exclusively male samples (Kuhlmann, Piel, & Wolf, Citation2005; Schoofs, Preuss, & Wolf, Citation2008).
In BPD, studies investigating the influence of psychosocial stress on memory performance are rare but found comparable effects in patients and healthy individuals. Kaess and colleagues investigated differences between BPD and healthy controls on executive function in a single and dual task paradigm while participants were stressed through noise compared to a control condition (no noise) (Kaess, Parzer, Koenig, Resch, & Brunner, Citation2016). Stress led to impaired task performance in the dual task, but this was independent of group. This is in line with a study which showed that psychosocial stress enhanced facial emotion recognition. Again, this effect was comparable in BPD patients and healthy individuals (Deckers et al., Citation2015).
In one of our own studies, we used a pharmacological approach in which we investigated the impact of exogenous hydrocortisone administration on memory performance in BPD. Patients and healthy controls first had to perform two long-term declarative memory tasks, one semantic (delayed recall of a word list) and one episodic memory (autobiographical memory). Furthermore, a short-term memory task (working memory) was conducted. All memory tasks took place after having received either 10 mg of hydrocortisone or a placebo. Interestingly, BPD patients showed enhanced memory retrieval after hydrocortisone treatment compared to placebo, whereas healthy controls displayed the expected pattern of impaired memory retrieval (Wingenfeld et al., Citation2013). The finding of improved memory function after cortisol in BPD is somewhat contra-intuitive because from a clinical point of view stress rather worsens BPD symptoms. However, pharmacological hydrocortisone administration is not comparable to psychosocial stress. First, the activation of the SNS as well as the psychological stress response is lacking. Second, the cortisol increase is higher in a pharmacological approach compared to the ‘physiological’ stress response. Third, interaction effects between perceived stress, copings strategies, resilience factors, symptomatology and the individuals physiological stress response might be more complex in a psychosocial stress paradigm in contrast to drug administration.
Therefore, the aim of our study was twofold: First, we aimed to investigate the physiological stress response to the well-validated TSST (Kirschbaum, Pirke, & Hellhammer, Citation1993) in comparison to a non-stressful control condition, the so-called Placebo-TSST (Het, Rohleder, Schoofs, Kirschbaum, & Wolf, Citation2009). This is important as most studies in the field abstain from using a control situation. Our hypothesis was that BPD patients show a blunted cortisol and sAA response to the TSST compared to healthy controls. Based on our finding of improved memory performance after hydrocortisone administration in BPD, the second aim was to investigate, whether BPD patients show improved memory retrieval also after a psychosocial stress-induced endogenous cortisol increase. We hypothesized impaired semantic, episodic and working memory performance after the TSST compared to the control condition in healthy controls but improved memory performance in BPD patients after TSST compared to the control condition.
2. Methods and material
2.1. Participants
We recruited 49 female patients with BPD and 49 healthy control (HC) women. In the BPD group, a current episode of major depressive disorder (MDD) as comorbid disorder led to exclusion from the study, because MDD influences HPA axis function and cortisol effects on cognition differ between BPD patients with and without MDD (Wingenfeld et al., Citation2013).
Further exclusion criteria were schizophrenia, schizoaffective disorder, bipolar disorder, anorexia, alcohol dependence or drug dependence (all assessed by DSM-IV SCID axis I Interview). CNS diseases or severe somatic diseases, metabolic or endocrine disorders, autoimmune diseases, current infections, pregnancy, and a body mass index below 17.5 or above 30 also led to exclusion. In addition, healthy controls were excluded if they met diagnostic criteria for any DSM-IV axis I or axis II disorder or reported a history of psychiatric or psychotherapeutic treatment. Intake of any medication also led to exclusion in the control group.
Recruitment of inpatients and outpatients was conducted at the Clinic for Psychiatry and Psychotherapy, Charité Berlin, Campus Benjamin Franklin, Germany. Outpatients and healthy controls were additionally recruited via online advertisement and received financial remuneration (100 euro). Written informed consent was obtained prior to participation. The study was approved by the Local Ethics Committee.
2.2. Procedure
We used the Structured Clinical Interview for DSM-IV (SCID) Axis I and II for assessment of mental disorders (Wittchen, Zaudig, & Fydrich, Citation1997). In addition, the short version of the Borderline Symptom List (BSL-23) was conducted (Bohus et al., Citation2009). Participants are asked to rate their current symptoms on a 5-step Likert scale (0 = not at all, 4 = very strong). In a second part of the questionnaire, dysfunctional behaviour is assessed with 11 items, again on a 5-step Likert scale. To assess childhood trauma we used the Childhood Trauma Questionnaire (CTQ) (Bernstein, Ahluvalia, Pogge, & Handelsman, Citation1997, Wingenfeld, Spitzer, Mensebach, et al., Citation2010).
A crossover study was performed, in which all participants underwent a psychosocial stressor, the TSST and a control condition in randomized order (within-subject design). The TSST consists of a 10 minutes preparation phase, a job interview (5 minutes) and an arithmetic task (5 minutes) (Kirschbaum et al., Citation1993).
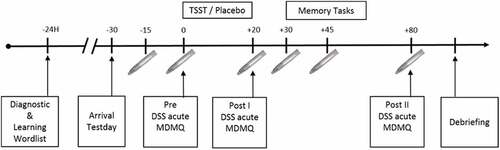
The control condition comprised the Placebo-TSST (P-TSST), which is similar to the TSST except for stressful fragments (Het et al., Citation2009). In addition, participants were asked to report their current mood state, stress appraisal and dissociative symptoms prior, directly after and 80 minutes after treatment onset (see Supplemental data for detailed description of methods and results).
Saliva for cortisol and sAA measurement was collected six times during each investigation: two baseline measurements −15 minutes and directly before (P-)TSST, as well as +20, +30, +45 and +80 minutes after (P-)TSST onset. Participants were not allowed to drink immediately before collecting salivary samples. One hour before taking part in the study, participants were deprived of food, caffeine and sugar consisting drinks, but were allowed to drink water during investigation.
The afternoon before the TSST and P-TSST, respectively, the participants learned a word list. On the next day, all tests were conducted between 16:00 and 20:00. TSST and P-TSST were followed by a free recall of the word list (learned the day before), a working memory task (word suppression test, WST) and an autobiographical memory test (AMT) starting 30 minutes after (P-)TSST onset. The TSST and P-TSST were conducted at least one week apart. Due to the repeated measurement design of the study, parallel versions of the memory test were used, except for the WST. Of note, we have shown that the WST is appropriate for repeated measures (Kuffel et al., Citation2014). For a detailed schedule of the test-days see .
2.3. Memory tasks
Since we aimed to investigate whether our previous findings of enhanced memory performance after administration of 10 mg hydrocortisone in patients with BPD are also seen after psychosocial stress, we conducted the same memory tests as in this previous study (Wingenfeld et al., Citation2013).
2.3.1. Delayed free recall of wordlist
The verbal memory test is a word list, containing of 21 words (Terfehr et al., Citation2011a). Twenty-four hours before the free recall, participants were presented with the wordlist in a five-trial setting. Words were read out by the investigator with an inter stimulus interval (ISI) of 1–2 seconds. After each trial, participants were asked to recall as many words as possible and were allowed to recall a word multiple times. No time limit was set. On the next day, they were asked to recall as many words as possible. As a score, we use the performance (number of correctly recalled words) of the delayed recall in relation to the fifth trial from the day before as baseline and calculated percentage of correct remembered words.
2.3.2. Autobiographical memory test
We used an adapted version of the AMT (Buss, Wolf, Witt, & Hellhammer, Citation2004; Schlosser et al., Citation2010; Williams & Broadbent, Citation1986). In the AMT, participants were instructed to remember a specific event from their biography by recalling the specific place they were in, the time when it happened and the people which were involved in the event. Therefore, the participants were consecutively presented with six adjectives, which were presented on cards. Participants had to write down a specific memory from their life related to the presented adjectives. An event was stated as specific, when participants were able to recall at least three dimensions (what, where, when, with whom). An event was labelled as ‘non-memory’ when participants were not able to find a memory within one minute. The results were evaluated by two independent raters with respect to specificity. Occurring disagreements were solved by a third independent rater. As a score, we used the sum of all specific memories for each participant. Word stimuli were rated concerning valence in a former study (Kuffel et al., Citation2013).
2.3.3. Working memory (word suppression test)
The working memory task (word suppression test, WST) (Terfehr et al., Citation2011b) comprised of two parts (neutral and negative). Both parts consist of 14 audio recordings with sequences of alternating digits and interference words and were presented via loudspeakers. Therefore, the ISI and intonation were constant over all participants. In the first part, neutral interference words were used, whereas negative words were applied in the second part. The participants consecutively listened to every recorded trial separately and were asked to recall the digits in the presented order but to ignore the interference words. Number of digits and words increased every second trial by one. Rating of the used word stimuli took part in the study by Kuffel et al. (Citation2013). As a score, we used all successful recalled trials for each participant and for neutral and negative words, separately.
2.4. Laboratory parameters
Salivette collection devices (Sarstedt, Germany) were used to sample saliva for assessment of cortisol and sAA. Samples were immediately frozen and stored at −80°C until biochemical analysis, which were performed in the Neurobiology Laboratory of the Department of Psychiatry, Charité Berlin, Germany. Salivary cortisol was analysed using an adapted homogenous time-resolved fluorescence resonance energy transfer (HTR-FRET)-based competitive immunoassay (for detailed description see Duesenberg et al., Citation2016). Intra-assay coefficients of variation were below 8%, inter-assay coefficients of variation were below 10%. The limit of detection of free cortisol was 0.2 nM. sAA activity was determined using a modified protocol of a previously published direct alpha-amylase assay (Rombold et al., Citation2016). Inter- and intra-assay coefficients of variation were both lower than 10% for sAA activity. All samples and standards were measured in duplicates.
2.5. Subjective stress responses
Besides physiological stress responses, we conducted two self-rating scales to measure subjective stress: the Multidimensional Mood State Questionnaire (MDMQ; Steyer, Schwenkmezger, Notz, & Eid, Citation1997), which consists of 16 items related to three dimensions, namely ‘good vs. bad’, ‘calm vs. nervous’ and ‘awake vs. tired’; and the Dissociation-Tension Scale acute (DSS-4; Stiglmayr, Braakmann, Haaf, Stieglitz, & Bohus, Citation2003), to assess dissociative symptoms in the course of the investigation. Participants rated their arousal and dissociation before, directly after and 80 minutes after stress or placebo, respectively. Please see Supplemental data for further information and results.
2.6. Statistical analysis
We used SPSS Version 24.0 to perform statistical analysis. Demographical data were analysed using Student’s t-test for continuous data and Pearson’s Chi2-test for categorical variables.
We controlled all analyses for intake of oral contraceptives by introducing this variable as an additional factor in our analyses. However, because there was no effect of intake of OC in any analyses, this variable was not considered as covariate in our analyses.
Due to violation of normal distribution, we performed a log-transformation on salivary cortisol and sAA as well as blood pressure data for statistical analyses. However, figures present the raw data. Salivary cortisol, sAA and blood pressure were analysed using mixed model ANOVAs with repeated measurements with the within-subject factors time (six measurement points) and stress (TSST vs. P-TSST) and the between-subject factor group (BPD vs. HC). Additionally, Pearson’s correlation between cortisol and sAA increase and CTQ as well as DSS scores were calculated. To do so we calculated baseline to peak scores of cortisol and sAA levels in response to stress (BtP, maximum value of t3 to t6 minus mean of t1 and t2). Effects of stress on memory were also analysed with mixed model ANOVAs with repeated measurements with the factors stress and group.
For all ANOVAs, we report partial η2 as effect size. Greenhouse-Geisser corrected p-values are calculated when appropriate. In case of a significant interaction effect, post hoc t-tests and contrasts were performed for pairwise comparisons and to shed more light on a potential blunted reaction in BPD compared to placebo. For all post-hoc tests, we used the Bonferroni-Holm procedure to correct for multiple testing.
3. Results
3.1. Demographical data and diagnostics
According to the SCID I Interview, BPD patients reported the following current DSM-IV axis I disorders: panic disorder n = 4, agoraphobia n = 3, agoraphobia with panic disorder n = 4, social phobia n = 8, obsessive compulsive disorder n = 7, PTSD n = 22, bulimia nervosa n = 5, generalized anxiety disorder n = 2, substance abuse n = 2, alcohol abuse n = 1.
Overall, 33 BPD patients received psychotropic medication: selective serotonin reuptake inhibitor (SSRI) n = 20, serotonin and noradrenaline reuptake inhibitor (SNRI) n = 7, tricyclic antidepressant n = 2, dopamine and noradrenergic reuptake inhibitor (NDRI) n = 2, antipsychotics n = 9, anticonvulsants n = 8, alpha/beta adrenergic blocker n = 4. Five patients took three and 15 took two different drugs. A total of 16 BPD patients and all healthy controls received no psychotropic medication.
BPD patients and healthy women did not differ with regard to age, years of education and Body Mass Index (BMI). Intake of oral contraceptives (OC) and number of smokers differed significantly between groups.
Overall, 14 patients with BPD took oral contraceptives, 23 were tested in their luteal phase and four in their follicular phase, two were tested while switching menstrual phases from follicular to luteal. In the control group, 22 women took OCs, 14 were tested in their luteal phase, seven in their follicular phase and one participant switched phases during investigation. Sample characteristics are presented in .
Table 1. Sample characteristics.
3.2. Physiological data
3.2.1. Cortisol
Three patients with BPD refused to collect saliva, because they felt awkward to chew on salivettes. Main finding of the mixed model ANOVA was a stress*time*group interaction (F2,169 = 3.88, p = .019, = .05), indicating an increase of cortisol over time depending on treatment and group affiliation. ) shows the increase of cortisol after TSST but not after P-TSST in both groups. Furthermore, we revealed a main effect of stress (F1,77 = 22.3, p < .001,
=.23), time (F2,179 = 37.56, p < .001,
= .33) a stress*time interaction (F2,169 = 21.47, p < .000,
= .22) and a time*group interaction (F2,179 = 3.28, p = .033,
= .04). There was no significant main effect of group. Post-hoc paired sampled t-tests showed that each groups’ cortisol (BPD and HC) release was higher at +30 min, +45 and +80 minutes after onset of TSST compared to the P-TSST (all p < .01, corrected for multiple testing). The healthy controls additionally showed a significant increase at +20 minutes (p < .01, corrected for multiple testing). Post-hoc t-tests for independent samples revealed no differences between groups in their reaction to the TSST.
Figure 2. (a). Salivary cortisol concentration 15 minutes, immediately prior to (0) and 20 minutes, 30 minutes, 45 minutes and 80 minutes after TSST and P-TSST, respectively. BPD = Borderline Personality Disorder, HC = Healthy Controls, TSST = Trier Social Stress Test, P-TSST = Placebo TSST. (b). Salivary α-Amylase concentration 15 minutes, immediately prior to (0) and 20 minutes, 30 minutes, 45 minutes and 80 minutes after TSST and P-TSST, respectively. BPD = Borderline Personality Disorder, HC = Healthy Controls, TSST = Trier Social Stress Test, P-TSST = Placebo TSST.
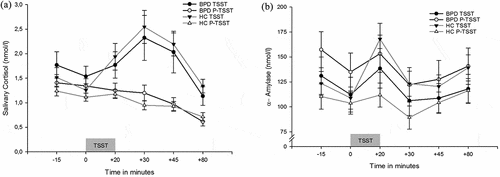
However, to shed more light on the question of a potential blunted reaction in BPD compared to HC, we additionally conducted a mixed model ANOVA, only with the measurement points pre and post TSST and ran post-hoc contrasts to compare the increase of cortisol from pre to post TSST in matters of group affiliation. The contrasts showed a significant change in cortisol for HC (Pillais’ Trace = .185, F1, 88 = 19.93, p < .001), but only on trend level in BPD (Pillais’ Trace = .032, F1, 88 = 2.95, p = .089). There was no significant correlation between cortisol release (BtP) and CTQ sum score and dissociation before and after the stressor, respectively.
3.2.2. Alpha amylase
In matters of alpha amylase, the main finding was a stress*time*group interaction effect (F3,272 = 2.63, p = .04, =.03) (see also )). Furthermore, we revealed a main effect of time (F3,312 = 12.17, p < .001,
=.13) and a stress*group interaction (F1,81 = 20.82, p < .001,
= .20). There were no main effects of group and stress.
Post hoc t-tests showed that, in healthy controls, sAA was higher after the TSST at +20 minutes and +35 minutes compared to the P-TSST (all p < .01, corrected for multiple testing). BPD patients showed no significant differences between TSST and P-TSST in matters of alpha amylase, except for the two baseline measurement points prior to treatment (all p < .01, corrected for multiple testing), where the alpha amylase was higher in the placebo condition compared to TSST. Furthermore, post hoc t-tests for independent samples did not reveal any differences between groups in matters of stress.
Additionally, we again conducted a mixed model ANOVA only with the two measurement points pre and post TSST and ran post-hoc contrasts to gain information about a possible blunted reaction in BPD. However, the contrasts showed a significant change in cortisol for HC (Pillais’ Trace = .178, F1, 89 = 19.21, p < .001) and on trend level in BPD (Pillais’ Trace = .037, F1, 89 = 3.39, p = .069). Results reflect an increase in sAA in response to the TSST in both groups, which was more levelled in the BPD group.
Additionally, we found a significant negative correlation between sAA release (BtP) and CTQ in the TSST (r = .-25, p = .02). There were no significant associations between dissociation and sAA.
3.2.3. Blood pressure
For systolic blood pressure, we found a significant stress*time interaction (F4,371 = 7.38, p < .001,= .08), which reflects an increase in blood pressure in response to stress but not after the P-TSST in (see )). Furthermore, a main effect of stress (F1,85 = 10.13, p = .002,
= .11) and time (F4,353 = 17.66, p < .001,
= .17) were revealed.
Figure 3. (a). Systolic blood pressure 15 minutes, immediately prior to (0) and 20 minutes, 30 minutes, 45 minutes and 80 minutes after TSST and P-TSST, respectively. BPD = Borderline Personality Disorder, HC = Healthy Controls, TSST = Trier Social Stress, P-TSST = Placebo TSST. (b). Diastolic blood pressure 15 minutes, immediately prior to (0) and 20 minutes, 30 minutes, 45 minutes and 80 minutes after TSST and P-TSST, respectively. BPD = Borderline Personality Disorder, HC = Healthy Controls, TSST = Trier Social Stress Test, P-TSST = Placebo TSST.
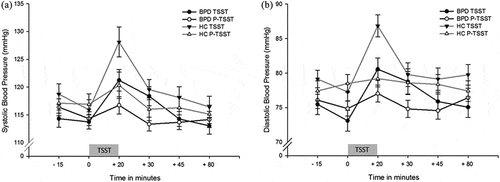
Across groups, post-hoc t-tests indicate higher systolic blood pressure at measurement point +20 min and at + 30 min in the TSST compared to the P-TSST (all p < .01, corrected for multiple testing). Post-hoc contrasts revealed that BPD patients and healthy participants did not differ in matters of their stress response.
Main finding for diastolic blood pressure was a stress*time*group interaction (F4,370 = 2.44, p = .04,= .03). Furthermore, we revealed a main effect of group (F1,86 = 4.34, p = .04,
= .05), stress (F1,86 = 8.16, p = .005,
= .09) and time (F4, 369 = 13.66, p < .001,
= .14), as well as a stress*time interaction effect (F4,370 = 5.69, p < .001,
= .06).
Post-hoc tests showed that directly after the TSST diastolic blood pressure was significantly increased in healthy controls (p < .001) and on trend level in BPD patients (p = .078, corrected for multiple testing). Furthermore, independent t-tests showed that BPD patients had lower diastolic blood pressure compared to healthy controls at +20 min in the TSST condition on trend level (p = .06, corrected for multiple testing). However, post-hoc contrasts indicate no differences in the increase of diastolic blood pressure between HC and BPD from pre to post TSST (see )).
3.3. Effects of stress on memory
3.3.1. Verbal memory retrieval
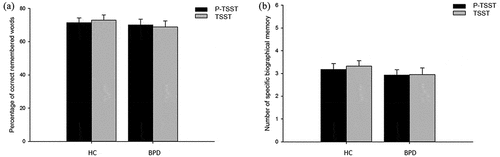
We analysed the percentage of remembered words relative to the words recalled after the fifth learning trial on the day before. Patients and controls did not differ in remembered words after the fifth learning trial (p = .49). Mixed model ANOVAs revealed no main effect of group, stress, or any interaction effect (all p > .49, all < .01) (see )).
Figure 4. (a). Percentage of correct remembered words in a 24 hours delayed recall. Displayed for BPD patients and healthy controls (HC). Error bars indicate standard error of the mean (SEM). BPD = Borderline Personality Disorder, HC = Healthy Controls, TSST = Trier Social Stress Test, P-TSST = Placebo TSST. (b). Number of remembered specific autobiographical memories in the AMT. Displayed for BPD patients and healthy controls (HC). Error bars indicate standard error of the mean (SEM). BPD = Borderline Personality Disorder, HC = Healthy Controls, TSST = Trier Social Stress Test, P-TSST = Placebo TSST.
3.3.2. Autobiographical memory retrieval
To indicate autobiographic memory retrieval, number of specific events served as dependent variable. Again, no main effects of group, stress or interaction effects (all p > .35, all < .01) were revealed (see )).
3.3.3. Word suppression test
Number of correctly remembered digits served as dependent variable in the Word Suppression Test. Two independent ANOVAs were performed for each test part, i.e. neutral and negative interference words, as we did in our previous study (Wingenfeld et al., Citation2013).
ANOVAs revealed a main effect of group related to both subtests of the working memory task (neutral: F1,90 = 5.59, p = .02, = .06; negative: F1,90 = 4.21, p = .04,
= .05), with more remembered digits in the control group across conditions (see ).
Figure 5. Number of correct recalled trials in the WST. Displayed for BPD patients, healthy controls (HC) and for negative and neutral trials. Error bars indicate standard error of the mean (SEM). BPD = Borderline Personality Disorder, HC = Healthy Controls, TSST = Trier Social Stress Test, P-TSST = Placebo TSST.
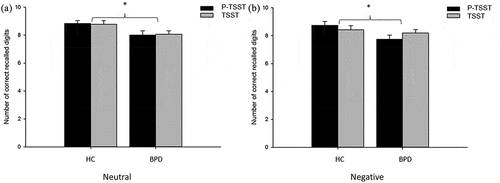
Controlling these analyses for dissociation scores (see Supplemental data) did not change the results with respect to verbal memory retrieval, autobiographical memory retrieval and working memory (negative test part). In the neutral part of the WST the main effect of group was not found to be significant after controlling for dissociation.
3.3.4. Comparison between patients with BPD with and without psychotropic medication and with and without comorbid PTSD
We used mixed model ANOVAs to compare patients with BPD with (n = 33) and without (n = 16) medication to check for potential influences of medication on physiological responses to the TSST. No main effect of medication or interaction with medication intake occurred, except for diastolic blood pressure, where we found a stress*time*medication interaction effect (F5,195 = 2.42, p = .04, = .06). Post hoc t-tests indicated that only medicated BPD patients revealed the expected increase of diastolic blood pressure after TSST. Concerning memory performance, no impact of medication intake was found.
We also analysed whether BPD patients with a comorbid PTSD differed in their physiological reaction to the TSST and P-TSST compared to BPD patients without PTSD. We only revealed a main effect of group for systolic blood pressure (F1,38 = 5.97, p = .02, = .14), displaying a higher systolic blood pressure in BPD patients with PTSD compared to the BPD patients without PTSD. Groups did not differ with regard to memory performance or the influence of stress on memory.
4. Discussion
In this study, we investigated the effects of psychosocial stress on (1) biological variables such as salivary cortisol, sAA and blood pressure and (2) memory performance in patients with BPD and healthy women. To examine these questions, we conducted an intra-individual cross-over study so that every participant was tested during and after psychosocial stress and a non-stressful control condition.
4.1. Biological response to psychosocial stress
Overall, patients with BPD and healthy women responded with an almost similar increase in cortisol when exposed to psychosocial stress (TSST). However, when only including the pre and post TSST measurement points, the slope of the cortisol increase differs slightly between BPD patients and HC, suggesting a blunted cortisol increase in BPD. Furthermore, there were no group differences in cortisol during the control condition. In contrast to healthy women, there was no increase in sAA and a less pronounced increase in diastolic blood pressure in response to stress in the BPD group directly after TSST. Again, post-hoc contrasts indicate an alleviated alpha amylase increase in BPD compared to healthy controls, when only comparing the pre and post TSST measurement points. Thus, our data suggest a slightly blunted cortisol and SNS increase from pre to post TSST during acute stress in BPD patients.
Our results contrast findings of a clearly blunted cortisol reactivity in response to psychosocial stress in BPD patients compared to healthy controls in earlier studies since we only found small differences between groups from pre to post TSST (Aleknaviciute et al., Citation2016; Ehrenthal et al., Citation2018; Nater et al., Citation2010; Scott et al., Citation2013). However, literature on HPA axis function in BPD is heterogeneous and several studies did not find a blunted cortisol response to stress (Deckers et al., Citation2015; Simeon et al., Citation2007). There is evidence that comorbid mental disorders, such as PTSD and MDD (Rinne et al., Citation2002; Wingenfeld & Wolf, Citation2015) contribute to HPA axis functioning in BPD patients. Therefore, it is important to take comorbidity with other mental disorders into account. Of note, in our study we excluded patients with comorbid major depressive disorder and found no effect of PTSD on cortisol release. Other authors suggested that childhood trauma (Ehrenthal et al., Citation2018) and dissociation (Simeon et al., Citation2007) affect cortisol release to stress in BPD. However, we did not find any significant correlations between CTQ and dissociation scores and cortisol release. Future studies should further examine which factors affect HPA axis regulation in BPD and under which circumstances alterations occur.
Furthermore, we found a slightly blunted SNS response to stress as indicated by (a) a reduced sAA level from pre to post TSST in BPD and (b) a lower peak reaction in diastolic blood pressure in BPD compared to HC directly after TSST. These results again are in line with existing literature since some studies found a reduced activity of sAA (Nater et al., Citation2010; Scott et al., Citation2013) and a blunted heart rate response (Aleknaviciute et al., Citation2016) after TSST in this patient group. Other studies did not reveal differences between BPD and HC (Ehrenthal et al., Citation2018; Kaess et al., Citation2012; Simeon et al., Citation2007). A blunted stress reaction could be potentially explained by adverse events experienced during childhood (Lovallo, Farag, Sorocco, Cohoon, & Vincent, Citation2012; Voellmin et al., Citation2015). Childhood trauma is frequent in BPD and we found a significant association between CTQ scores and reduced sAA. Longitudinal studies are needed to further evaluate this hypothesis. Again, comorbid mental disorders might affect the physiological stress response. In our BPD sample, we found higher blood pressure in those with comorbid PTSD as well as in medicated patients. Of note, a hyperactive noradrenergic system has been frequently reported for patients with PTSD (Delahanty, Nugent, Christopher, & Walsh, Citation2005; Wingenfeld, Whooley, Neylan, Otte, & Cohen, Citation2015; Young, Tolman, Witkowski, & Kaplan, Citation2004). Thus, differences in study samples might in part explain diverging results in the literature.
In sum, our results in a relatively large sample suggest only slightly blunted cortisol and SNS reactivity to stress in female BPD patients. Of note, we excluded MDD as comorbid mental disorder. Furthermore, we controlled our analyses for comorbid PTSD and found no difference between BPD patients with and without PTSD. Future studies are needed to disentangle the effect of BPD symptomatology and childhood trauma. Furthermore, additional physiological stress markers, such as ACTH, CRH or skin conductance, might be useful to characterize potential alterations in the stress response in patients with BPD.
4.2. Impact of stress on memory systems
Second aim of our study was to shed more light on cognitive alterations in BPD during acute stress. In a previous study, we found improved memory performance in female patients with BPD after the intake of 10 mg hydrocortisone compared to placebo (Wingenfeld et al., Citation2013). We tested whether these effects were also seen after psychosocial stress and used the same tasks, namely a word list learning test, a test for working memory and an autobiographical memory test, as in our previous study. In contrast to our hypothesis, we did not find an effect of stress on episodic memory retrieval and working memory, either in healthy control women or in BPD patients.
Against our hypothesis, the TSST did not affect memory in healthy controls. While the impairing effects of hydrocortisone administration on memory retrieval appears to be a relatively robust effect (Het, Ramlow, & Wolf, Citation2005), the effects of psychosocial stress induction are not that clear (Hidalgo, Almela, Villada, & Salvador, Citation2014; Luettgau, Schlagenhauf, & Sjoerds, Citation2018; Schoofs & Wolf, Citation2009; Wolf, Schommer, Hellhammer, Reischies, & Kirschbaum, Citation2002). However, a recent meta-analysis suggested stress to impair memory retrieval (Shields et al., Citation2017). Interestingly, reduced memory performance after stress has been shown predominantly in male samples (Kuhlmann et al., Citation2005; Schoofs et al., Citation2008). Another study found an association between cortisol release to stress and reduced memory retrieval only in male but not female participants (Wolf, Schommer, Hellhammer, McEwen, & Kirschbaum, Citation2001), which is in line with other observations (Espin et al., Citation2013). Thus, the lacking stress effects on memory might be – in part – due to the fact that we investigated only females (Merz & Wolf, Citation2017). Intake of oral contraceptives and menstrual cycle might also contribute to the lacking stress effects on memory retrieval in our study (Kuhlmann & Wolf, Citation2005). Most but not all free cycling women were tested in the luteal phase of the menstrual cycle. Interestingly, especially in the luteal phase, stress related memory effects seem to be less pronounced or even absent (Schoofs & Wolf, Citation2009). However, OC intake did not influence the results in our sample. Additionally, the used memory tests might not be sensitive enough for the relatively subtle effects of stress compared to pharmacological stimulation. As we recently reported effects of psychosocial stress on emotional empathy (Wingenfeld et al., Citation2018), one might suggest that more arousing stimuli, such as emotional pictures, might have been more appropriate.
In BPD patients, memory performance was not affected by psychosocial stress induction. This contrasts our earlier findings of improved memory retrieval after hydrocortisone administration in BPD patients (Wingenfeld et al., Citation2013). Of note, hydrocortisone treatment, at least at the dose of 10 mg, leads to much higher cortisol levels than a laboratory stressor. Possibly, cortisol induced memory improvement in BPD only occurs under very high cortisol levels.
Interestingly, stimulation of the MR with fludrocortisone led to impaired memory performance in a visuo-spatial memory task and a word list learning test in BPD patients (Wingenfeld et al., Citation2015). Endogenous cortisol release through stress activates both receptor types. Thus, in this study memory retrieval was measured during MR and GR activation. In sum, it is possible that in BPD pronounced GR activation through hydrocortisone administration improves memory performance (Wingenfeld et al., Citation2013), while MR activation alone reduces memory performance (Wingenfeld et al., Citation2015). Endogenous cortisol release through psychosocial stress, however, will stimulate both receptor types in a ‘physiological’ manner, which seems not to affect memory retrieval in BPD. This might be due to a more balanced GR/MR activation in the context of moderately enhanced corticoid levels.
Furthermore, psychosocial stress induction differs in other important aspects from a pharmacological approach, such as hydrocortisone or fludrocortisone administration. The TSST also activates the SNS and comprises an interactional/affective component. It has been shown that glucocorticoid effects on memory rely on (co-)activation of the SNS, namely noradrenergic activation (Roozendaal, Okuda, de Quervain, & McGaugh, Citation2006). Thus, the observed blunted SNS response in our BPD sample might also account for the lacking stress effects on memory. Additionally, during stress additional HPA axis hormones such as ACTH and CRH are released. Of note, CRH has been shown to influence learning and memory (Roozendaal, Schelling, & McGaugh, Citation2008). In sum it seems that enhancing effects on memory in BPD are only found during high cortisol concentration without a full activation of the HPA axis and without noradrenergic activation.
4.3. Strengths and limitations
A strength of our study is the intra-individual crossover design using a well-established control condition in addition to the TSST. Thus, possible inter-individual differences have been reduced. In addition, the sample size of our within-subject design led to sufficient statistical power. Moreover, a growing body of evidence shows that especially PTSD and MDE as co-morbidities are strongly related to HPA axis alterations (Wingenfeld, Spitzer, Rullkotter, et al., Citation2010, Wingenfeld et al., Citation2013). Thus, all patients with MDE were excluded from our study. In terms of PTSD, we recruited half of our BPD sample with a current PTSD diagnosis to be able to compare both subgroups.
As mentioned above, most free cycling women were tested in the luteal phase of the menstrual cycle, which might have contributed to the lacking stress effects on memory (Schoofs & Wolf, Citation2009). However, the number of women in the follicular and with different phases at the two testing days was too small to perform subgroup analyses. Importantly, we confirmed that OC intake did not influence the results.
Comorbid mental disorders except MDE and PTSD have not been considered in our study, because our relatively large sample was still too small to conduct subgroup analyses with sufficient power. Anxiety disorders like social phobia might be of interest (Lieb, Zanarini, Schmahl, Linehan, & Bohus, Citation2004; Zimmerman & Mattia, Citation1999). Furthermore, many of our patients took psychotropic medication but BPD patients with and without medication did not differ with regard to the outcome variable. As only women were included in this study, no conclusions can be drawn with regard to men.
4.4. Conclusion
In sum, our results suggest a slightly blunted cortisol and SNS response to psychosocial stress in BPD. Memory performance in BPD was unchanged in comparison to healthy controls and after stress. This is remarkable as many BPD symptoms worsen under stressful circumstances. Emotional empathy for instance is reduced after stress in these patients (Wingenfeld et al., Citation2018). Thus, future studies should examine which kind of stressor leads to worsening of cognitive functioning and which factors mediate this process.
Supplemental Material
Download MS Word (32.6 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental data
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Aleknaviciute, J., Tulen, J. H., Kamperman, A. M., de Rijke, Y. B., Kooiman, C. G., & Kushner, S. A. (2016). Borderline and cluster C personality disorders manifest distinct physiological responses to psychosocial stress. Psychoneuroendocrinology, 72, 131–13.
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Washington, DC: American Psychiatric Association.
- Bernstein, D. P., Ahluvalia, T., Pogge, D., & Handelsman, L. (1997). Validity of the childhood trauma questionnaire in an adolescent psychiatric population. Journal of the American Academy of Child and Adolescent Psychiatry, 36(3), 340–348.
- Bohus, M., Kleindienst, N., Limberger, M. F., Stieglitz, R.-D., Domsalla, M., Chapman, A. L., … Wolf, M. (2009). The short version of the borderline symptom list (BSL-23): Development and initial data on psychometric properties. Psychopathology, 42(1), 32–39.
- Buss, C., Wolf, O. T., Witt, J., & Hellhammer, D. H. (2004). Autobiographic memory impairment following acute cortisol administration. Psychoneuroendocrinology, 29(8), 1093–1096.
- de Kloet, E. R. (2014). From receptor balance to rational glucocorticoid therapy. Endocrinology, 155(8), 2754–2769.
- de Quervain, D. J., Aerni, A., Schelling, G., & Roozendaal, B. (2009). Glucocorticoids and the regulation of memory in health and disease. Frontiers in Neuroendocrinology, 30(3), 358–370.
- Deckers, J. W., Lobbestael, J., van Wingen, G. A., Kessels, R. P., Arntz, A., & Egger, J. I. (2015). The influence of stress on social cognition in patients with borderline personality disorder. Psychoneuroendocrinology, 52, 119–129.
- Delahanty, D. L., Nugent, N. R., Christopher, N. C., & Walsh, M. (2005). Initial urinary epinephrine and cortisol levels predict acute PTSD symptoms in child trauma victims. Psychoneuroendocrinology, 30(2), 121–128.
- Duesenberg, M., Weber, J., Schulze, L., Schaeuffele, C., Roepke, S., Hellmann-Regen, J., … Wingenfeld, K. (2016). Does cortisol modulate emotion recognition and empathy? Psychoneuroendocrinology, 66, 221–227.
- Ehrenthal, J. C., Levy, K. N., Scott, L. N., & Granger, D. A. (2018). Attachment-related regulatory processes moderate the impact of adverse childhood experiences on stress reaction in borderline personality disorder. Journal of Personality Disorders, 32(Supplement), 93–114.
- Espin, L., Almela, M., Hidalgo, V., Villada, C., Salvador, A., & Gomez-Amor, J. (2013). Acute pre-learning stress and declarative memory: Impact of sex, cortisol response and menstrual cycle phase. Hormones and Behavior, 63(5), 759–765.
- Het, S., Ramlow, G., & Wolf, O. T. (2005). A meta-analytic review of the effects of acute cortisol administration on human memory. Psychoneuroendocrinology, 30(8), 771–784.
- Het, S., Rohleder, N., Schoofs, D., Kirschbaum, C., & Wolf, O. T. (2009). Neuroendocrine and psychometric evaluation of a placebo version of the ‘trier social stress test’. Psychoneuroendocrinology, 34(7), 1075–1086.
- Hidalgo, V., Almela, M., Villada, C., & Salvador, A. (2014). Acute stress impairs recall after interference in older people, but not in young people. Hormones and Behavior, 65(3), 264–272.
- Inoue, A., Oshita, H., Maruyama, Y., Tanaka, Y., Ishitobi, Y., Kawano, A., … Akiyoshi, J. (2015). Gender determines cortisol and alpha-amylase responses to acute physical and psychosocial stress in patients with borderline personality disorder. Psychiatry Research, 228(1), 46–52.
- Kaess, M., Hille, M., Parzer, P., Maser-Gluth, C., Resch, F., & Brunner, R. (2012). Alterations in the neuroendocrinological stress response to acute psychosocial stress in adolescents engaging in nonsuicidal self-injury. Psychoneuroendocrinology, 37(1), 157–161.
- Kaess, M., Parzer, P., Koenig, J., Resch, F., & Brunner, R. (2016). Dual-task performance under acute stress in female adolescents with borderline personality disorder. European Child & Adolescent Psychiatry, 25(9), 1027–1035.
- Kirschbaum, C., Pirke, K. M., & Hellhammer, D. H. (1993). The ‘trier social stress test’–a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology, 28(1–2), 76–81.
- Kuffel, A., Eikelmann, S., Terfehr, K., Mau, G., Kuehl, L. K., Otte, C., … Wingenfeld, K. (2014). Noradrenergic blockade and memory in patients with major depression and healthy participants. Psychoneuroendocrinology, 40, 86–90.
- Kuffel, A., Terfehr, K., Uhlmann, C., Schreiner, J., Lowe, B., Spitzer, C., & Wingenfeld, K. (2013). Messwiederholung von Gedächtnistests unter Berücksichtigung der Valenz des Testmaterials: Verbales Gedächtnis, Arbeitsgedächtnis und autobiografisches Gedächtnis [Repeated measurement of memory with valenced test items: Verbal memory, working memory and autobiographic memory]. Fortschritte der Neurologie-Psychiatrie, 81(7), 390–397.
- Kuhlmann, S., Piel, M., & Wolf, O. T. (2005). Impaired memory retrieval after psychosocial stress in healthy young men. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 25(11), 2977–2982.
- Kuhlmann, S., & Wolf, O. T. (2005). Cortisol and memory retrieval in women: Influence of menstrual cycle and oral contraceptives. Psychopharmacology, 183(1), 65–71.
- Lieb, K., Zanarini, M. C., Schmahl, C., Linehan, M. M., & Bohus, M. (2004). Borderline personality disorder. The Lancet, 364(9432), 453–446. doi:10.1016/S0140-6736(04)16770-6
- Lovallo, W. R., Farag, N. H., Sorocco, K. H., Cohoon, A. J., & Vincent, A. S. (2012). Lifetime adversity leads to blunted stress axis reactivity: Studies from the Oklahoma family health patterns project. Biological Psychiatry, 71(4), 344–349.
- Luettgau, L., Schlagenhauf, F., & Sjoerds, Z. (2018). Acute and past subjective stress influence working memory and related neural substrates. Psychoneuroendocrinology, 96, 25–34.
- Merz, C. J., & Wolf, O. T. (2017). Sex differences in stress effects on emotional learning. Journal of Neuroscience Research, 95(1–2), 93–105.
- Nater, U. M., Bohus, M., Abbruzzese, E., Ditzen, B., Gaab, J., Kleindienst, N., … Ehlert, U. (2010). Increased psychological and attenuated cortisol and alpha-amylase responses to acute psychosocial stress in female patients with borderline personality disorder. Psychoneuroendocrinology, 35, 1565–1572.
- Nater, U. M., & Rohleder, N. (2009). Salivary alpha-amylase as a non-invasive biomarker for the sympathetic nervous system: Current state of research. Psychoneuroendocrinology, 34(4), 486–496.
- Rinne, T., de Kloet, E. R., Wouters, L., Goekoop, J. G., DeRijk, R. H., & van Den Brink, W. (2002). Hyperresponsiveness of hypothalamic-pituitary-adrenal axis to combined dexamethasone/corticotropin-releasing hormone challenge in female borderline personality disorder subjects with a history of sustained childhood abuse. Biological Psychiatry, 52(11), 1102–1112.
- Rombold, F., Wingenfeld, K., Renneberg, B., Hellmann-Regen, J., Otte, C., & Roepke, S. (2016). Influence of the noradrenergic system on the formation of intrusive memories in women: An experimental approach with a trauma film paradigm. Psychological Medicine, 46(12), 2523–2534.
- Roozendaal, B., Okuda, S., de Quervain, D. J., & McGaugh, J. L. (2006). Glucocorticoids interact with emotion-induced noradrenergic activation in influencing different memory functions. Neuroscience, 138(3), 901–910.
- Roozendaal, B., Schelling, G., & McGaugh, J. L. (2008). Corticotropin-releasing factor in the basolateral amygdala enhances memory consolidation via an interaction with the beta-adrenoceptor-cAMP pathway: Dependence on glucocorticoid receptor activation. The Journal of Neuroscience :The Official Journal of the Society for Neuroscience, 28(26), 6642–6651.
- Schlosser, N., Wolf, O. T., Fernando, S. C., Riedesel, K., Otte, C., Muhtz, C., … Wingenfeld, K. (2010). Effects of acute cortisol administration on autobiographical memory in patients with major depression and healthy controls. Psychoneuroendocrinology, 35(2), 316–320.
- Schoofs, D., Preuss, D., & Wolf, O. T. (2008). Psychosocial stress induces working memory impairments in an n-back paradigm. Psychoneuroendocrinology, 33(5), 643–653.
- Schoofs, D., & Wolf, O. T. (2009). Stress and memory retrieval in women: No strong impairing effect during the luteal phase. Behavioral Neuroscience, 123(3), 547–554.
- Scott, L. N., Levy, K. N., & Granger, D. A. (2013). Biobehavioral reactivity to social evaluative stress in women with borderline personality disorder. Personality Disorders, 4(2), 91–100.
- Shields, G. S., Sazma, M. A., McCullough, A. M., & Yonelinas, A. P. (2017). The effects of acute stress on episodic memory: A meta-analysis and integrative review. Psychological Bulletin, 143(6), 636–675.
- Shields, G. S., Sazma, M. A., & Yonelinas, A. P. (2016). The effects of acute stress on core executive functions: A meta-analysis and comparison with cortisol. Neuroscience and Biobehavioral Reviews, 68, 651–668.
- Simeon, D., Knutelska, M., Smith, L., Baker, B. R., & Hollander, E. (2007). A preliminary study of cortisol and norepinephrine reactivity to psychosocial stress in borderline personality disorder with high and low dissociation. Psychiatry Research, 149(1–3), 177–184.
- Steyer, R., Schwenkmezger, P., Notz, P., & Eid, M. (1997). MDBF–Mehrdimensionaler Befindlichkeitsfragebogen. Göttingen, Germany: Hogrefe.
- Stiglmayr, C. E., Braakmann, D., Haaf, B., Stieglitz, R.-D., & Bohus, M. (2003). Development and characteristics of dissociation-tension-scale acute (DSS-Akute). Psychotherapie, Psychosomatik, medizinische Psychologie, 53(7), 287–294.
- Terfehr, K., Wolf, O. T., Schlosser, N., Fernando, S. C., Otte, C., Muhtz, C., … Wingenfeld, K. (2011a). Effects of acute hydrocortisone administration on declarative memory in patients with major depressive disorder: A placebo-controlled, double-blind crossover study. The Journal of Clinical Psychiatry, 72, 1644–1650.
- Terfehr, K., Wolf, O. T., Schlosser, N., Fernando, S. C., Otte, C., Muhtz, C., … Wingenfeld, K. (2011b). Hydrocortisone impairs working memory in healthy humans, but not in patients with major depressive disorder. Psychopharmacology, 215(1), 71–79.
- Voellmin, A., Winzeler, K., Hug, E., Wilhelm, F. H., Schaefer, V., Gaab, J., … Bader, K. (2015). Blunted endocrine and cardiovascular reactivity in young healthy women reporting a history of childhood adversity. Psychoneuroendocrinology, 51, 58–67.
- Williams, J. M., & Broadbent, K. (1986). Autobiographical memory in suicide attempters. Journal of Abnormal Psychology, 95(2), 144–149.
- Wingenfeld, K., Driessen, M., Terfehr, K., Schlosser, N., Fernando, S. C., Otte, C., … Wolf, O. T. (2013). Effects of cortisol on memory in women with borderline personality disorder: Role of co-morbid post-traumatic stress disorder and major depression. Psychological Medicine, 43(3), 495–505.
- Wingenfeld, K., Duesenberg, M., Fleischer, J., Roepke, S., Dziobek, I., Otte, C., & Wolf, O. T. (2018). Psychosocial stress differentially affects emotional empathy in women with borderline personality disorder and healthy controls. Acta Psychiatrica Scandinavica, 137(3), 206–215.
- Wingenfeld, K., Kuehl, L. K., Janke, K., Hinkelmann, K., Eckert, F. C., Roepke, S., & Otte, C. (2015). Effects of mineralocorticoid receptor stimulation via fludrocortisone on memory in women with borderline personality disorder. Neurobiology of Learning and Memory, 120, 94–100.
- Wingenfeld, K., Spitzer, C., Mensebach, C., Grabe, H. J., Hill, A., Gast, U., … Driessen, M. (2010). The German version of the Childhood Trauma Questionnaire (CTQ): Preliminary psychometric properties. Psychotherapie, Psychosomatik, medizinische Psychologie, 60(11), 442–450.
- Wingenfeld, K., Spitzer, C., Rullkotter, N., & Löwe, B. (2010). Borderline personality disorder: Hypothalamus pituitary adrenal axis and findings from neuroimaging studies. Psychoneuroendocrinology, 35(1), 154–170.
- Wingenfeld, K., Whooley, M. A., Neylan, T. C., Otte, C., & Cohen, B. E. (2015). Effect of current and lifetime posttraumatic stress disorder on 24-h urinary catecholamines and cortisol: Results from the mind your heart study. Psychoneuroendocrinology, 52, 83–91.
- Wingenfeld, K., & Wolf, O. T. (2015). Effects of cortisol on cognition in major depressive disorder, posttraumatic stress disorder and borderline personality disorder - 2014 Curt Richter Award Winner. Psychoneuroendocrinology, 51, 282–295.
- Wittchen, H.-U., Zaudig, M., & Fydrich, T. (1997). Strukturiertes Klinisches Interview für DSM-IV. Göttingen: Hogrefe.
- Wolf, O. T. (2017). Stress and memory retrieval: Mechanisms and consequences. Current Opinion in Behavioral Sciences, 14, 40–46.
- Wolf, O. T., Schommer, N. C., Hellhammer, D. H., McEwen, B. S., & Kirschbaum, C. (2001). The relationship between stress induced cortisol levels and memory differs between men and women. Psychoneuroendocrinology, 26(7), 711–720.
- Wolf, O. T., Schommer, N. C., Hellhammer, D. H., Reischies, F. M., & Kirschbaum, C. (2002). Moderate psychosocial stress appears not to impair recall of words learned 4 weeks prior to stress exposure. Stress, 5(1), 59–64.
- Young, E. A., Tolman, R., Witkowski, K., & Kaplan, G. (2004). Salivary cortisol and posttraumatic stress disorder in a low-income community sample of women. Biological Psychiatry, 55(6), 621–626.
- Zimmerman, D. J., & Choi-Kain, L. W. (2009). The hypothalamic-pituitary-adrenal axis in borderline personality disorder: A review. Harvard Review of Psychiatry, 17(3), 167–183.
- Zimmerman, M., & Mattia, J. I. (1999). Axis I diagnostic comorbidity and borderline personality disorder. Comprehensive Psychiatry, 40(4), 245–252. doi:10.1016/S0010-440X(99)90123-2