 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Background: Breast cancer (BC) is one of the most common invasive types of cancer among women, with important consequences on both physical and psychological functioning. Patients with BC have a great risk of developing posttraumatic stress disorder (PTSD), but few studies have evaluated the efficacy of psychological interventions to treat it. Furthermore, no neuroimaging studies have evaluated the neurobiological effects of psychotherapeutic treatment for BC-related PTSD.
Objective: The study aimed to evaluate the efficacy of Eye Movement Desensitization and Reprocessing therapy (EMDR) as compared to Treatment as Usual (TAU) in BC patients with PTSD, identifying by electroencephalography (EEG) the neurophysiological changes underlying treatments effect and their correlation with clinical symptoms.
Method: Thirty patients with BC and PTSD diagnosis were included, receiving either EMDR (n = 15) or TAU (n = 15). Patients were assessed before and after treatments with clinical questionnaires and EEG. The proportion of patients who no longer meet criteria for PTSD after the intervention and changes in clinical scores, both between and within groups, were evaluated. Two-sample permutation t-tests among EEG channels were performed to investigate differences in power spectral density between groups. Pearson correlation analysis was carried out between power bands and clinical scores.
Results: At post-treatment, all patients treated with EMDR no longer met criteria for PTSD, while all patients treated with TAU maintained the diagnosis. A significant decrease in depressive symptoms was found only in the EMDR group, while anxiety remained stable in all patients. EEG results corroborated these findings, showing significant differences in delta and theta bands in left angular and right fusiform gyri only in the EMDR group.
Conclusions: It is essential to detect PTSD symptoms in patients with BC, in order to offer proper interventions. The efficacy of EMDR therapy in reducing cancer-related PTSD is supported by both clinical and neurobiological findings.
HIGHLIGHTS
• In accordance with DSM-5 criteria, the prevalence of cancer-related PTSD diagnosis in our sample of women with breast cancer was 8.1%.• EMDR was significantly superior to TAU in reducing PTSD and associated depressive symptoms.• Clinical improvement following EMDR was associated with an increase in neuronal synchronization in left angular and in right fusiform gyri.
Antecedentes: El cáncer de mama (CM) es uno de los tipos de cáncer invasivo más comunes entre las mujeres, con importantes consecuencias tanto en el funcionamiento físico como psicológico. Los pacientes con CM tienen un gran riesgo de desarrollar trastorno de estrés postraumático (TEPT), pero solo unos pocos estudios han evaluado la eficacia de las intervenciones psicológicas para tratarlo. Adicionalmente, ningún estudio de neuroimagen ha evaluado los efectos neurobiológicos del tratamiento psicoterapéutico para el TEPT relacionado con CM.
Objetivo: el estudio buscó evaluar la eficacia de la terapia de desensibilización y reprocesamiento con movimientos oculares (EMDR) en comparación con el tratamiento habitual (TAU) en pacientes con TEPT en CM, identificando también mediante EEG los cambios neurofisiológicos que subyacen al efecto de los tratamientos y su correlación con los síntomas clínicos.
Método: se incluyeron treinta pacientes con diagnóstico de CM y TEPT, recibiendo EMDR (n = 15) o TAU (n = 15). Los pacientes fueron evaluados antes y después de los tratamientos con cuestionarios clínicos y electroencefalografía (EEG). Se evaluó la proporción de pacientes que dejaron de cumplir con los criterios para trastorno de estrés postraumático después de la intervención y los cambios en las puntuaciones clínicas, tanto al interior como entre los grupos. Se realizaron pruebas t de permutación de dos muestras entre canales EEG para investigar las diferencias en la Densidad del Espectro de Potencia entre los grupos. Se realizó un análisis de correlación de Pearson entre bandas de potencia y puntuaciones clínicas.
Resultados: En el postratamiento, todos los pacientes tratados con EMDR ya no cumplían con los criterios para TEPT, mientras que todos los pacientes tratados con TAU mantuvieron el diagnóstico. Se encontró una disminución significativa de síntomas depresivos solo en el grupo EMDR, mientras que la ansiedad se mantuvo estable en todos los pacientes. Los resultados del EEG corroboraron estos hallazgos, mostrando diferencias significativas en las bandas delta y theta en los giros angular izquierdo y fusiforme derecho solo en el grupo EMDR.
Conclusiones: es esencial detectar los síntomas de TEPT también en pacientes con CM para poder ofrecer intervenciones adecuadas. La eficacia de la terapia EMDR en reducir el trastorno de estrés postraumático relacionado con el cáncer es apoyado tanto por los hallazgos clínicos como neurobiológicos.
背景:乳腺癌(BC)是女性中最常见的侵袭性癌症之一,对身心功能有重要影响。
BC患者发生创伤后应激障碍(PTSD)的风险很大,但只有少数研究评估了心理干预对其治疗效果。此外,也没有神经影像学研究评估心理治疗对BC相关PTSD的神经生物学效应。
目的:本研究旨在评估眼动脱敏和再加工疗法(EMDR)与普通治疗组(TAU)相比对BC患者PTSD的疗效,同时通过EEG确定治疗效果的神经生理学变化及其与临床症状的相关性。
方法:纳入30例同时患BC和PTSD的患者,接受EMDR(n = 15)或TAU(n = 15)。通过临床问卷和脑电图(EEG)对患者进行治疗前和治疗后的评估。评估干预后不再符合PTSD标准的患者比例以及组间和组内临床评分的变化。进行EEG通道之间的双样本置换t检验以研究组间功率谱密度的差异。在功率带和临床评分之间进行Pearson相关分析。
结果:在治疗后,所有接受EMDR治疗的患者均不再符合PTSD标准,而所有接受TAU治疗的患者均维持诊断。仅在EMDR组中发现抑郁症状显著减少,而在所有患者中焦虑保持稳定。 EEG结果证实了这些发现,仅显示了EMDR组中左角和右侧梭形回旋区的δ和θ带的显著差异。
结论:为了提供适当的干预措施,在BC患者中检测PTSD症状至关重要。临床和神经生物学研究结果支持EMDR治疗在减少癌症相关PTSD方面的功效。
1. Introduction
Affecting over 1.5 million women each year, breast cancer (BC) is one of the most invasive types of cancer, also causing the greatest number of cancer-related deaths (WHO, Citation2018). BC diagnosis and subsequent treatment is an highly stressful condition, with devastating effects on patient’s psychological functioning and quality of life in addition to physical suffering. For some patients this experience is decisively traumatic, with psychological consequences that might result in posttraumatic stress disorder (PTSD; Parikh et al., Citation2015).
PTSD is a mental disorder occurring after exposure to life threatening episodes (criterion A) and is characterized by: intense reliving of the traumatic event through intrusive memories and nightmares (criterion B); avoidance of reminders of the event (criterion C); negative alterations in cognitions and mood (criterion D); hypervigilance toward potential threats in the environment (criterion E); and in some cases persistent or recurrent depersonalization symptoms (American Psychiatric Association, Citation2013).
The publication of DSM-IV-TR (American Psychiatric Association, Citation2000), explicitly including life-threatening illness as a stressor that could elicit PTSD, has stimulated investigations of posttraumatic sequelae in cancer, myocardial infarction, intensive care treatment, HIV and other medical conditions (Swartzman, Booth, Munro, & Sani, Citation2017; Tedstone & Tarrier, Citation2003).
Several studies showed that 5–35% of patients with cancer might suffer from posttraumatic symptoms (Kangas, Henry, & Bryant, Citation2002) and that prevalence of cancer-related PTSD is about 6% (Abbey, Thompson, Hickish, & Heathcote, Citation2015). A recent meta-analysis (Swartzman et al., Citation2017) showed that patients with cancer are at increased risk of PTSD compared to controls with no history of cancer while prevalence of BC-related PTSD is 3–19% (Andrykowski, Cordova, McGrath, Sloan, & Kenady, Citation2000).
In cancer patients, posttraumatic symptoms are displayed as constant worries, fears of recurrence, nightmares about the illness or treatments, and a sense of shortened future. Intrusive symptoms are the preeminent ones, with a prevalence of 11–45% (Matsuoka, Nagamine, & Uchitomi, Citation2006; Whitaker, Brewin, & Watson, Citation2008).
DSM-5 PTSD diagnostic criteria (American Psychiatric Association, Citation2013) advanced a debate about the appropriateness of applying the concept of PTSD to medical conditions such as cancer (Cordova, Riba, & Spiegel, Citation2017; Kangas, Citation2013). To date, no studies have used such criteria for assessing PTSD among patients with cancer, calling for investigations exploring the features of PTSD in patients with cancer and their response to psychotherapies.
In recent years, neuroimaging has disclosed the neurobiological correlates of psychological and psychiatric disorders. As regards PTSD, several studies have shown the involvement of amygdala, medial prefrontal Cortex (mPFC), anterior cingulate cortex (ACC) and hippocampus (Patel, Spreng, Shin, & Girard, Citation2012; Sherin & Nemeroff, Citation2011), being a relative decreased mPFC activity and a parallel increased amygdala activation the neurobiological core of the disorder. These brain regions are also implicated in cancer-related PTSD (Carletto & Pagani, Citation2016), suggesting that effective treatments for ‘typical’ PTSD can be effective also in cancer-related PTSD.
Neuroimaging has also disclosed neurobiological changes associated with the outcome of evidence-based psychotherapeutic PTSD treatments, such as Trauma Focused-Cognitive Behavioural Therapy (TF-CBT) and Eye Movement Desensitization and Reprocessing therapy (EMDR) (Malejko, Abler, Plener, & Straub, Citation2017; Pagani, Högberg, Fernandez, & Siracusano, Citation2013). The latter was found effective in promoting a post-treatment reversal of prefrontal and limbic alterations associated with long-lasting clinical improvements (Högberg et al., Citation2008; Pagani et al., Citation2012).
Few studies have shown that TF-CBT and EMDR are effective in reducing PTSD symptoms in patients with cancer (DuHamel et al., Citation2010; Jarero et al., Citation2015; Kangas, Milross, Taylor, & Bryant, Citation2013). In a direct comparison, EMDR was found to be significantly more effective than TF-CBT in reducing PTSD symptoms (Capezzani et al., Citation2013).
EMDR is a trauma-focused psychotherapy using alternate bilateral stimulation (eye movements, tactile or audio) that seems to promote the reprocessing of dysfunctionally-stored information related to the traumatic event (Jeffries & Davis, Citation2013; Pagani, Amann, Landin-Romero, & Carletto, Citation2017). It has also been suggested to contribute to a better psychological adjustment to the disease in patients with cancer (Faretta & Civilotti, Citation2016; Lantheaume, Citation2018; Murray, Citation2016). Moreover, a specific EMDR therapy protocol for patients with cancer has been recently published (Faretta & Borsato, Citation2016), calling to compare its effectiveness with a supportive psychological intervention usually delivered in psycho-oncological settings, aiming at informing clinicians about best therapeutic options.
In the recent past, electroencephalography (EEG), thanks to its optimal temporal resolution and directly measuring neuronal activity, has emerged as a complementary technique compared to the neuroimaging techniques to deepen the comprehension of the mechanisms underlying psychotherapies efficacy (Pagani et al., Citation2012). Moreover, EEG features correlating with psychological questionnaires might be used as biomarkers to objectively test non-invasively the efficacy of treatment.
The aims of the present study are: (1) to evaluate the efficacy of EMDR as compared to Treatment as Usual (TAU) in a cohort of BC patients with PTSD, diagnosed according to DSM-5 criteria; (2) to identify by EEG the functional changes related to EMDR outcome; (3) to correlate such changes to those in clinical scores.
2. Methods
2.1. Design
This was a quasi-experimental study comparing the neurobiological correlates of two different therapeutic interventions (EMDR and TAU) for treating cancer-related PTSD in women with BC.
2.2. Setting
The participants were recruited at the Breast Unit service of Città della Salute e della Scienza of Turin between September 2016 and November 2017. The study was approved by the local Research Ethics Committee. Informed written consent was obtained from all participants.
2.3. Participants
The subjects were patients with BC and a diagnosis of cancer-related PTSD. Inclusion criteria were: (1) diagnosis of advanced breast cancer (stage III or IV); (2) age between 18 and 70 years; (3) diagnosis of PTSD according to DSM-5 criteria; (4) fluent Italian speaker; (5) legal capacity to consent to the treatment; (6) willingness to suspend all concomitant psychological treatment; (7) suspension of all psychotropic medications at least one month before the treatment or maintenance at baseline level throughout the study.
Exclusion criteria were: (1) presence of major psychiatric disorders, including severe major depression disorder, schizophrenia, bipolar disorder, dissociative disorders, current abuse of alcohol or other substances; (2) inability to complete psychological assessments due to visual impairment or cognitive difficulties; (3) presence of uncontrolled symptoms due to the medical illness or therapies.
2.4. Recruitment and measures
Proposal to participate was made by two psychologists during a clinical visit. Participants were recruited with a three-step screening.
Impact of Event Scale-Revised (IES-R) was administered to all eligible patients fitting the inclusion criteria and who considered only the illness as the traumatic event. The IES-R (Weiss & Marmar, Citation1997) is a 22-item self-report questionnaire consisting of three subscales: intrusion, avoidance and hyperarousal. The scale assesses subjective distress caused by traumatic events. A cut-off equal to or above 33 is considered indicative of posttraumatic stress symptoms (Creamer, Bell, & Failla, Citation2003).
Patients with scores equal to or above 33 were assessed with the PTSD module of Structured Clinical Interview for DSM-5 (SCID-5; First, Williams, Karg, & Spitzer, Citation2015) in order to confirm diagnosis of PTSD.
Patients were assessed with the Clinician-Administered PTSD Scale (CAPS-5; Weathers et al., Citation2018), a semi-structured interview based on the DSM-5, considered the gold standard for diagnosing PTSD. Severity rating is calculated by combining frequency and intensity scores. During the CAPS-5 administration, the narrative of the worst traumatic event related to BC history (target event) was audio-recorded and, if patients accepted their participation in the study, was used as script during the EEG recordings.
The research protocol with an explanation of the aims of the study was proposed to all consecutive patients with confirmed PTSD diagnosis, asking whether they were willing to receive a more intensive psychotherapeutic intervention (EMDR) other than TAU. On reaching the maximum number of patients in the EMDR group, the remaining patients were assigned to the TAU group. Upon agreement, they signed the written informed consent and were asked to complete the following psychological questionnaires.
Trauma Antecedent Questionnaire (TAQ; Luxenberg, Spinazzola, & Van Der Kolk, Citation2001) a self-administered instrument that gathers past information about adverse childhood experiences and other traumatic life experiences, assessed at four different age periods: early childhood (birth to six years), latency (seven to 12 years), adolescence (13 to 18 years) and adulthood. This questionnaire was administered only at baseline.
State-Trait Anxiety Inventory (STAI-Y; Spielberger, Citation1983) used to measure the presence and severity of current symptoms of anxiety (state anxiety; STAI-1) and a generalized propensity to be anxious (trait anxiety; STAI-2). Range of scores for each subtest is 20–80, with the higher score indicating greater anxiety.
Beck Depression Inventory-II (BDI-II; Beck & Steer, Citation1993), a 21-item self-report instrument that assesses the presence and severity of depression symptoms. A score above 13 indicates presence of depression symptoms.
Psychological assessment was performed by psychologists independent of the research protocol and blinded to treatment group, using the same timing and tools in both groups, i.e. at baseline before the first session of treatment (T0) and after treatment’s end (T1).
2.5. Treatments
2.5.1. Treatment as usual
The TAU group received four sessions of supportive therapy, one every other week over a period of two months. They consisted of standard treatment to support patients to better deal with the psychological symptoms related to BC. TAU was performed by two psychotherapists at MSc level or higher, with a minimum of three years of experience in treating patients with BC. They received supervision by an experienced colleague for the duration of the study.
2.5.2. Eye movement desensitization and reprocessing
Participants received 10 EMDR sessions over a period that varied between two and three months. EMDR was administered in accordance with Shapiro’s protocol for traumatic events (Shapiro, Citation2001) and particularly it followed the EMDR specific protocol for oncological patients (Faretta & Borsato, Citation2016). In the first two sessions patients were trained to stabilization techniques, such as the Safe Place or mindfulness-based practices. The remaining sessions focused on trauma reprocessing, starting from the target event (identified in the CAPS-5 interview and used as a script during the EEG recording) and then moving to other related traumatic material and events. EMDR was provided by three practitioners with a minimum of three years of experience in the liaison setting. They received supervision from a certified senior EMDR supervisor for the entire duration of the study.
2.6. EEG data recordings
EEG was recorded by a Galileo system (EBNeuro, Florence, Italy) with patients seated on a comfortable chair in a quiet room. Thirty-seven active electrodes were applied to the scalp using a pre-cabled electrode cap. Data were collected and digitalized at a sampling rate of 1024 Hz.
Participants were assessed using EEG at two separate time points: at baseline (T0), all participants received two resting state EEG measurements, with eyes open and eyes closed and one EEG measurement during script-driven imagery, and then two other resting state EEG measurements, again with eyes open and eyes closed. After the last session of EMDR or TAU treatment (T1), the EEG resting state (eyes open and eyes closed) and script-driven imagery sessions were repeated.
2.7. EEG data analysis
Only the data during the listening of the script were analysed in the EEGLAB environment (http://www.sccn.ucsd.edu/eeglab/index.html) and a collection of scripts running under Matlab R2014b (Mathworks Inc., Natick, MA). All EEG signals were bandpass filtered (1–40 Hz) since muscular artefacts were present in the data due to the clinical condition of the participants and average referenced was applied before further analysis. Artefactual non-cerebral source activities (eye blinks and movements, microsaccadic, cardiac and muscle/electromyographic activity) were identified and rejected using a semiautomatic procedure based on Independent Component Analysis (Barbati, Porcaro, Zappasodi, Rossini, & Tecchio, Citation2004; Porcaro, Medaglia, & Krott, Citation2015). Power spectral density (PSD) was estimated for each channel, using the Welch procedure (1024 ms duration, Hanning window and 60% overlap). We investigated the spectral properties in the classical frequency bands, such as delta band (1–3 Hz), theta band (4–7 Hz), alpha band (8–13 Hz) and beta band (14–32 Hz) (IFSECN 1974).
We used an equivalent current dipole (ECD) with four concentric conductive spheres model (see routine DIPFIT2 of EEGLAB v11.0, available at http://www.sccn.ucsd.edu/eeglab) to obtain ECD positions in Talairach space for delta plus theta power difference between T1 minus T0 for the EMDR group. Talairach coordinates were transformed in Montreal Neurological Institute (MNI) space and plotted on MNI template using MRICro (www.mricro.com).
2.8. Statistical analyses
Data were processed and analysed using the Statistical Package for Social Sciences (SPSS version 22.0; Chicago, IL, USA). Both parametric and nonparametric tests were used, in accordance with Shapiro–Wilk as a test for normality. Baseline group differences were assessed using Student’s t-test or Mann–Whitney U test to compare the two groups for continuous measures and Fisher’s Exact Test for categorical measures.
Fisher’s Exact Test was used to evaluate the association between the treatment group (EMDR vs. TAU) and the PTSD diagnosis at T1. Cramer’s V was used to calculate the effect size for the proportion of participants that no longer meet PTSD diagnosis as measured with CAPS at T1.
A GLM repeated measures multivariate ANOVA (RM-MANOVA) was used to analyse the main pre- and post-intervention effects and interactions both between and within the EMDR and TAU groups. Pairwise comparison between groups were made by simple contrast and are reported as means difference with the Sidak correction 95% confidence interval (95% CI) for multiple comparisons.
A multivariable linear regression analysis was performed to determine the association between group (EMDR vs. TAU) and each clinical outcome. Two models for each outcome were performed: in the first model the association between the clinical score at T1 and treatment was adjusted only with the clinical score at T0; in the second model an additional adjustment for possible confounding variables (the ones that resulted significant at T0, i.e. age and CAPS-C) was carried out. To verify the goodness-of-fitness of the models, the adjusted R2 value was computed.
Moreover, two-sample permutation t-tests (10,000 permutations, avoiding multiple comparisons problem) among channels were performed to investigate differences in PSD at T0 and T1 between the TAU and EMDR groups. Pearson correlation analysis was also performed between power bands (delta, theta, alpha and beta) and the clinical scores. A p < .05 was considered statistically significant throughout all of the analyses.
3. Results
A total of 567 patients with BC were screened with IES-R; 224 presented a IES-R score above the cut-off (39.5%). Of those, 86 were excluded on the basis of the inclusion/exclusion criteria (38.4%). Of the remaining 138, 73 were not available or refused to continue the evaluation (52.9%). Only 46 met the criteria of PTSD diagnosis (8.1% of the total) according to CAPS interview. Later, 16 patients declined to participate to the study (reasons given for refusal were mainly the distance of patients’ place of residence from the hospital and the inability to attend the intervention sessions; refusal rate: 34.8%).
Therefore, a total of 30 patients were enrolled in the study: 15 were assigned to EMDR and 15 to TAU. Only one drop-out was registered in the EMDR group.
presents at baseline (T0) the socio-demographic characteristics of these patients. There were no significant differences in demographics between the two groups, except for patients in the EMDR group being slightly older than those in the TAU group ().
Table 1. Demographic data of participants at baseline.
At T0 there were no significant differences in clinical characteristics between the two groups, except for patients in the EMDR group showing higher avoidance symptoms (CAPS-C) than the TAU group (). Patients in the EMDR group (14 out of 14, 100%) did not meet PTSD diagnostic criteria after treatment, while all patients in the TAU group maintained their baseline clinical diagnosis, with a statistically significant difference (p ≤ .001; Cramer’s V = 1.000).
Table 2. Comparison of clinical variables for the two groups (EMDR and TAU).
A repeated-measures MANOVA was performed on the pre- and post-intervention clinical scores comparing group and time effects and interactions between group and time yielding a significant pre–post main effect (F(9, 19) = 21.664, p < .001; η2p = .911), and a significant interaction between the pre–post measures and the treatment condition (F(9, 19) = 13.504, p < .001; η2p = .865).
Significant time effects were found across both groups for all variables except for STAI-1 and STAI-2, indicating that the mean participant scores improved from T0 to T1 on all variables, except for anxiety symptoms ().
Group-by-time interaction effects were found for IES-R, CAPS-TOT, CAPS-B, CAPS-C, CAPS-D, CAPS-E and BDI-II scores, indicating that clinical improvements regarding the related symptoms were different in the two treatment groups. No group-by-time interaction was found for STAI-1 and STAI-2, confirming that no change was observed on these measures ().
Planned post hoc analyses of simple pre–post effects were conducted for all variables with a significant group-by-time effect by GLM pairwise comparisons using the Sidak adjustment for multiple comparisons.
As for posttraumatic symptoms, both groups had an improvement in CAPS-TOT score, but the EMDR group scored significantly lower compared to the TAU group at post-treatment (). These results were partially confirmed by IES-R, which showed an improvement only in the EMDR group ().
Table 3. Comparison between T0 and T1 of clinical variables for the two groups (EMDR and TAU).
A more in-depth evaluation of different clusters of posttraumatic symptoms assessed with CAPS subscales indicated that only the EMDR group had an improvement in intrusive (CAPS-B), avoidance (CAPS-C) and hyper-arousal (CAPS-E) symptoms () scoring significantly lower than the TAU group at T1 (). Both groups improved in negative alterations in mood and cognitions cluster (CAPS-D), but the EMDR group scored significantly better compared to the TAU group at post-treatment ().
As for BDI-II, there was a significant improvement in the EMDR group while no difference was found in the TAU group () and the EMDR group scored significantly lower than the TAU group at post-treatment ().
In order to check whether the variables that were significantly different between groups at T0 (i.e. age and CAPS-C) could have influenced the observed clinical outcomes at T1, a multivariable linear regression analysis was performed. As reported in , no evidence of confounding due to age and CAPS-C was found. All models, except for STAI-1 and STAI-2 showed a good fit, thus confirming that being treated with EMDR resulted in an improvement from T0 to T1 on all variables, except for anxiety ().
Table 4. Multivariable linear regression models for each clinical score at T1. β values regard the effect of receiving EMDR (vs. TAU).
3.1. Power spectral density results
3.1.1. Channels selection
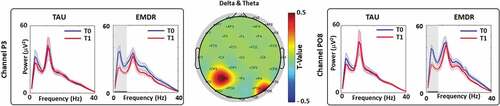
We found that the permutation t-test on the PSD among channels showed statistically significant differences (T0 vs. T1) in delta (1–3 Hz) and theta (4–7 Hz) bands for P3 and PO8 channels, respectively, only for patients treated with EMDR (see ).
Figure 1. T0 vs. T1 power spectral density analysis (PSD).
3.1.2. Localization analysis
Localization analysis on the EMDR power difference between T0 vs. T1 showed activations on left Angular Gyri (left BA39 – MNI coordinates: −32; −76; 44) and right Fusiform Gyri (right BA37 – MNI coordinates: 31; −78; 2) see .
3.1.3. Correlation analysis power bands vs. clinical scores
To understand the neural correlates of symptom improvement following EMDR treatment, Pearson correlation analysis was performed between delta values of the power bands and the delta values of the clinical score. The delta was calculated as follows:
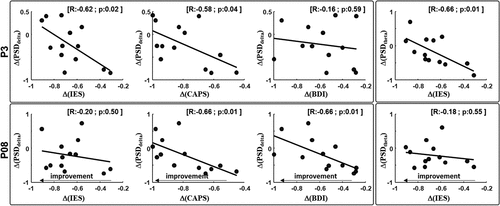
where value indicates PSD in delta, theta, alpha and beta bands or the clinical score. We found that Δ(IES-R) and Δ(CAPS-TOT) negatively correlated with Δ(PSDdelta) for channel P3 ().
Furthermore, for the same channel, Δ(IES-R) negatively correlated with Δ(PSDtheta). Δ(CAPS-TOT) and Δ(BDI-II) negatively correlated with Δ(PSDdelta) for channel PO8 ( and ). These results indicate that symptoms reduction was associated with an increased PSD delta between T0 and T1. No correlation was observed in the TAU group in any of the PSD bands. Results from correlations indicate that the higher the Δ(clinical score) (higher negative value) the higher the power increase in delta and theta bands after EMDR.
Figure 2. T0 vs. T1 EMDR PSD difference localization analysis.
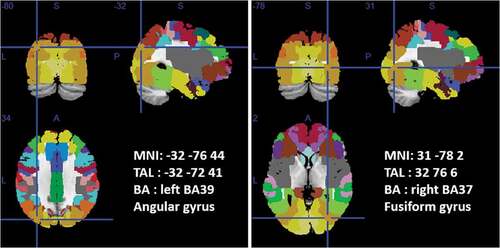
Figure 3. Correlation analysis.
4. Discussion
To our knowledge, this is the first study to apply DSM-5 diagnostic criteria for assessing PTSD in patients with cancer, and to evaluate therapy outcomes by EEG neuroimaging. Our results show that the prevalence of BC-related PTSD is in line with that reported by previous studies which used DSM-IV criteria (Abbey et al., Citation2015; Andrykowski et al., Citation2000; Swartzman et al., Citation2017). Since DSM-5 stated that medical illness per se could no longer be considered as a potential traumatic event, it became a matter of controversy whether the diagnostic category of Adjustment Disorder (AD), rather than PTSD, better describes cancer patients’ experiences in dealing with their disease (Cordova et al., Citation2017; Kangas, Citation2013). Our results show that a fifth of BC patients who exhibit posttraumatic stress symptoms (i.e. IES score ≥ 33) met full criteria for PTSD, thereby suggesting the appropriateness of applying the concept of PTSD to this medical condition and limiting the AD diagnosis to patients exhibiting posttraumatic stress symptoms without meeting full PTSD diagnostic criteria (Kangas, Citation2013).
One of the factors contributing to PTSD symptoms in response to cancer is the presence of pre-cancer diagnosis adverse/traumatic experiences (Cordova et al., Citation2017; Green et al., Citation2000). Accordingly, in our study a great majority of patients have experienced previous adverse childhood experiences or traumatic events (), suggesting that the assessment of patients’ trauma history should be a standard part of clinical routine, as already stated by the National Comprehensive Cancer Network’s clinical practice guidelines (Citation2018).
Development of appropriate treatment pathways in the oncology setting that can optimize detection and management of cancer-related PTSD is claimed by both clinicians and researchers (Cordova et al., Citation2017). One aim of the present study was to evaluate if EMDR was more effective than TAU in reducing posttraumatic symptoms in women with BC-related PTSD. As expected, EMDR was superior to TAU in reducing the proportion of patients with a PTSD clinical diagnosis. Only EMDR resulted in a clinically significant improvement, reaching scores below the clinical threshold in intrusive, avoidant and hyper-arousal symptoms. These results are in accordance with guidelines that indicate that trauma-focused therapies, such as EMDR and TF-CBT, are more effective than non-trauma focused intervention in treating PTSD (Bisson & Andrew, Citation2007; National Collaborating Centre for Mental Health, Citation2005).
Furthermore, in the present study, only EMDR was found to be effective in reducing depressive symptoms, as previously demonstrated in other patient groups (Carletto et al., Citation2017; Chen et al., Citation2014; Ostacoli et al., Citation2018).
Neither EMDR nor TAU were effective in reducing anxiety symptoms, in contrast with previous research on PTSD (Chen et al., Citation2014). This might be due to the peculiar medical condition of patients with cancer, in whom sustained anxiety could be related to realistic concerns about the recurrence and the progression of the disease. Moreover, future oriented fears differ from intrusive memories or re-experiencing of cancer-related stressors that have already occurred (Cordova et al., Citation2017).
The superiority of EMDR in treating cancer-related PTSD in women with BC was also supported by EEG results in which the power spectral density extracted from single channels allows us to quantitatively characterize neuronal changes occurring in different conditions. The clinical improvement in patients following EMDR, as expressed by the scores of psychological tests, was associated with an increased difference (increased Δ) of the EEG power in delta and theta bands. Such change is due either to a higher local neuronal synchronization or to a higher recruitment of neurons, implying better communication between different brain regions. Consequently, neuronal desynchronization or inhibition of neuronal networking might be one of the neurobiological mechanisms underlying PTSD symptomatology.
Locations in which such increases took place are P3 and PO8, approximately corresponding to the left angular (BA 39) and right fusiform gyri (BA 37), respectively. The angular gyrus lies in correspondence to the temporo-parieto-occipital junction (Brodmann area 37), which has been previously described as the brain region whose cortical electric activity was most associated with symptom improvements following EMDR (Pagani et al., Citation2012). Upon symptom disappearance the posterior multisensory associative cortex was found to fire during the reliving of the traumatic experience. This result supports the hypothesis that preferential brain activation shifted, after EMDR therapy, from limbic regions with an emotional valence to cortical regions with cognitive and semantic valence.
As for changes occurring in the right fusiform gyrus, a dysfunction of this region was found in patients with PTSD and could be related to an altered visual perception and sensory integration (Kennis, van Rooij, van Den Heuvel, Kahn, & Geuze, Citation2016; Mueller-Pfeiffer et al., Citation2013) that might explain the difficulties that patients with PTSD have in spatial and temporal contextualization. The restored activity of this region after successful EMDR could facilitate an elaboration at higher cognitive level of the images related to the traumatic event, allowing better processing and contextualization. This finding is in accordance with previous results showing an increased right fusiform gyrus electrical activation upon traumatic script during the last session of successful EMDR (Pagani et al., Citation2012).
Therefore, EMDR might increase the cortical connections between different brain regions resulting in a better capability to adapt the traumatic experience into associative cortices. These results are also in accordance with a recently proposed two-stage cortical coherence model (Keller, Stevens, Lui, Murray, & Yaggie, Citation2014; Yaggie et al., Citation2015), which hypothesizes that EMDR could facilitate the reprocessing of traumatic memories by increasing neural interconnectivity. This aspect might be a target point for future interventions to re-establish correct neuronal functioning.
Limitations of the study are the relatively small number of patients and the lack of random allocation to intervention groups. This was mainly due to the exploratory nature and the complexity of the research design, and to the high costs of neuroimaging procedure and analyses. Moreover, other limitations of the study are related to the different treatment dose received by patients (i.e. 10 sessions for EMDR and four sessions for TAU) and the possible expectancy effect (i.e. EMDR was presented as a more intensive psychotherapeutic intervention in respect to TAU). These difference should be taken into account when interpreting the results, and future studies should be designed with more balanced interventions.
Finally, another limitation of the study relates to the nature of the EEG techniques, which tend to be more sensitive to cortical activity rather than to activity in subcortical areas, which have also been widely implicated in PTSD. Further studies are needed to extend and replicate these findings.
In conclusion, the present study emphasizes the importance of detecting PTSD symptoms in patients with BC in order to offer them appropriate and effective treatments. EMDR could be an ideal candidate to treat cancer-related PTSD and associated psychological symptoms in current clinical practice as its functional effects are supported from both a clinical and a neurobiological point of view.
Acknowledgments
The authors would like to thank the participants in the study for their time and effort. The authors are also very grateful to Cristina Barile, Stefano Cerrato, Massimo Castaldo, Manuela Negro, Antonella Sammartino, Erica Sguazzotti and Ilaria Soncin for their contributions to the study.
Disclosure statement
IF is the president of EMDR Europe Association and EMDR Italy Association. LO, SC, and MP have been invited speakers at EMDR conferences. The other authors declare no potential conflicts of interest.
Additional information
Funding
References
- Abbey, G., Thompson, S. B. N., Hickish, T., & Heathcote, D. (2015). A meta-analysis of prevalence rates and moderating factors for cancer-related post-traumatic stress disorder: Cancer-related PTSD meta-analysis. Psycho-Oncology, 24(4), 371–12.
- American Psychiatric Association. (2000). Diagnostic and statistical manual of mental disorders, fourth edition, revised: DSM-IV-TR®. Washington DC: American Psychiatric Pub.
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders: Dsm-5. Washington DC: American Psychiatric Pub Incorporated.
- Andrykowski, M. A., Cordova, M. J., McGrath, P. C., Sloan, D. A., & Kenady, D. E. (2000). Stability and change in posttraumatic stress disorder symptoms following breast cancer treatment: A 1-year follow-up. Psycho-Oncology, 9(1), 69–78.
- Barbati, G., Porcaro, C., Zappasodi, F., Rossini, P. M., & Tecchio, F. (2004). Optimization of an independent component analysis approach for artifact identification and removal in magnetoencephalographic signals. Clinical Neurophysiology, 115(5), 1220–1232.
- Beck, A., & Steer, R. (1993). Manual for the Beck depression inventory. San Antonio, TX: Psychological Corporation.
- Bisson, J., & Andrew, M. (2007). Psychological treatment of post-traumatic stress disorder (PTSD). The Cochrane Database of Systematic Reviews, (3), CD003388.
- Capezzani, L., Ostacoli, L., Cavallo, M., Carletto, S., Fernandez, I., Solomon, R., … Cantelmi, T. (2013). EMDR and CBT for cancer patients: Comparative study of effects on PTSD, anxiety, and depression. Journal of EMDR Practice and Research, 7(3), 134–143.
- Carletto, S., Ostacoli, L., Colombi, N., Calorio, L., Oliva, F., Fernandez, I., & Hofmann, A. (2017). EMDR for depression: A systematic review of controlled studies. Clinical Neuropsychiatry: Journal of Treatment Evaluation, 14(5), 306–312.
- Carletto, S., & Pagani, M. (2016). Neurobiological impact of EMDR in cancer. Journal of EMDR Practice and Research, 10(3), 153–161.
- Chen, Y.-R., Hung, K.-W., Tsai, J.-C., Chu, H., Chung, M.-H., Chen, S.-R., … Chou, K.-R. (2014). Efficacy of eye-movement desensitization and reprocessing for patients with posttraumatic-stress disorder: A meta-analysis of randomized controlled trials. PLoS ONE, 9, 8.
- Cordova, M. J., Riba, M. B., & Spiegel, D. (2017). Post-traumatic stress disorder and cancer. The Lancet Psychiatry, 4(4), 330–338.
- Creamer, M., Bell, R., & Failla, S. (2003). Psychometric properties of the impact of event scale—revised. Behaviour Research and Therapy, 41(12), 1489–1496.
- DuHamel, K. N., Mosher, C. E., Winkel, G., Labay, L. E., Rini, C., Meschian, Y. M., … Redd, W. H. (2010). Randomized clinical trial of telephone-administered cognitive-behavioral therapy to reduce post-traumatic stress disorder and distress symptoms after hematopoietic stem-cell transplantation. Journal of Clinical Oncology, 28(23), 3754–3761.
- Faretta, E., & Borsato, T. (2016). EMDR therapy protocol for oncological patients. Journal of EMDR Practice and Research, 10(3), 162–175.
- Faretta, E., & Civilotti, C. (2016). EMDR therapy in psycho-oncology: A bridge between mind and body. Journal of EMDR Practice and Research, 10(3), 138–152.
- First, M. B., Williams, J. B. W., Karg, R. S., & Spitzer, R. L. (2015). Structured clinical interview for DSM-5—Research version (SCID-5 for DSM-5, Research Version; SCID-5-RV). Arlington, VA, US: American Psychiatric Association.
- Green, B. L., Krupnick, J. L., Rowland, J. H., Epstein, S. A., Stockton, P., Spertus, I., & Stern, N. (2000). Trauma history as a predictor of psychologic symptoms in women with breast cancer. Journal of Clinical Oncology, 18(5), 1084.
- Högberg, G., Pagani, M., Sundin, Ö., Soares, J., Åberg-Wistedt, A., Tärnell, B., & Hällström, T. (2008). Treatment of post-traumatic stress disorder with eye movement desensitization and reprocessing: Outcome is stable in 35-month follow-up. Psychiatry Research, 159(1–2), 101–108.
- Jarero, I., Artigas, L., Uribe, S., García, L. E., Cavazos, M. A., & Givaudan, M. (2015). Pilot research study on the provision of the eye movement desensitization and reprocessing integrative group treatment protocol with female cancer patients. Journal of EMDR Practice and Research, 9(2), 98–105.
- Jeffries, F. W., & Davis, P. (2013). What is the role of eye movements in eye movement desensitization and reprocessing (EMDR) for post-traumatic stress disorder (PTSD)? a review. Behavioural and Cognitive Psychotherapy, 41(3), 290–300.
- Kangas, M. (2013). DSM-5 trauma and stress-related disorders: Implications for screening for cancer-related stress. Frontiers in Psychiatry, 4, 122.
- Kangas, M., Henry, J. L., & Bryant, R. A. (2002). Posttraumatic stress disorder following cancer. Clinical Psychology Review, 22(4), 499–524.
- Kangas, M., Milross, C., Taylor, A., & Bryant, R. A. (2013). A pilot randomized controlled trial of a brief early intervention for reducing posttraumatic stress disorder, anxiety and depressive symptoms in newly diagnosed head and neck cancer patients. Psycho-Oncology, 22(7), 1665–1673.
- Keller, B., Stevens, L., Lui, C., Murray, J., & Yaggie, M. (2014). The effects of bilateral eye movements on EEG coherence when recalling a pleasant memory. Journal of EMDR Practice and Research, 8(3), 113–128.
- Kennis, M., van Rooij, S. J. H., van Den Heuvel, M. P., Kahn, R. S., & Geuze, E. (2016). Functional network topology associated with posttraumatic stress disorder in veterans. NeuroImage: Clinical, 10, 302–309.
- Lantheaume, S. (2018). Utilisation de la thérapie EMDR dans le traitement d’un ESPT après cancer du sein. Journal de Thérapie Comportementale et Cognitive, 28(1), 3–16.
- Luxenberg, T., Spinazzola, J., & Van Der Kolk, B. (2001). Complex trauma and the Disorders of Extreme Stress (DESNOS) diagnosis, part one: Assessment. Directions in Psychiatry, 11, 373–393.
- Malejko, K., Abler, B., Plener, P. L., & Straub, J. (2017). Neural correlates of psychotherapeutic treatment of post-traumatic stress disorder: A systematic literature review. Frontiers in Psychiatry, 8, 85.
- Matsuoka, Y., Nagamine, M., & Uchitomi, Y. (2006). Intrusion in women with breast cancer. In N. Kato, M. Kawata, & R. K. Pitman (Eds.), PTSD: Brain mechanism and clinical implications (pp. 169–178). Tokyo, Japan: Springer-Verlag.
- Mueller-Pfeiffer, C., Schick, M., Schulte-Vels, T., O’Gorman, R., Michels, L., Martin-Soelch, C., … Hasler, G. (2013). Atypical visual processing in posttraumatic stress disorder. NeuroImage: Clinical, 3, 531–538.
- Murray, K. (2016). EMDR resource methods for women with breast cancer. Journal of EMDR Practice and Research, 10(3), 176–188.
- National Collaborating Centre for Mental Health. (2005). Post-traumatic stress disorder: The management of PTSD in adults and children in primary and secondary care. Leicester (UK): National Institute for Health and Clinical Excellence Guidance.
- National Comprehensive Cancer Network. (2018). NCCN clinical practice guidelines in oncology (NCCN guidelines): Distress management, version 2.2018. Retrieved from https://www.nccn.org/professionals/physician_gls/default.aspx
- Ostacoli, L., Carletto, S., Cavallo, M., Baldomir-Gago, P., Di Lorenzo, G., Fernandez, I., … Hofmann, A. (2018). Comparison of eye movement desensitization reprocessing and cognitive behavioral therapy as adjunctive treatments for recurrent depression: The European Depression EMDR Network (EDEN) randomized controlled trial. Frontiers in Psychology, 9, 74.
- Pagani, M., Amann, B. L., Landin-Romero, R., & Carletto, S. (2017). Eye movement desensitization and reprocessing and slow wave sleep: A putative mechanism of action. Frontiers in Psychology, 8, 1935.
- Pagani, M., Di Lorenzo, G., Verardo, A. R., Nicolais, G., Monaco, L., Lauretti, G., … Siracusano, A. (2012). Neurobiological correlates of EMDR monitoring - An EEG study. PLoS ONE, 7(9), e45753.
- Pagani, M., Högberg, G., Fernandez, I., & Siracusano, A. (2013). Correlates of EMDR therapy in functional and structural neuroimaging: A critical summary of recent findings. Journal of EMDR Practice and Research, 7(1), 29–38.
- Parikh, D., De Ieso, P., Garvey, G., Thachil, T., Ramamoorthi, R., Penniment, M., & Jayaraj, R. (2015). Post-traumatic stress disorder and post-traumatic growth in breast cancer patients-a systematic review. Asian Pacific Journal of Cancer Prevention: APJCP, 16(2), 641–646.
- Patel, R., Spreng, R. N., Shin, L. M., & Girard, T. A. (2012). Neurocircuitry models of posttraumatic stress disorder and beyond: A meta-analysis of functional neuroimaging studies. Neuroscience and Biobehavioral Reviews, 36(9), 2130–2142.
- Porcaro, C., Medaglia, M. T., & Krott, A. (2015). Removing speech artifacts from electroencephalographic recordings during overt picture naming. NeuroImage, 105, 171–180.
- Shapiro, F. (2001). Eye movement desensitization and reprocessing (EMDR): Basic principles, protocols, and procedures (2nd ed.). New York: Guilford Press.
- Sherin, J. E., & Nemeroff, C. B. (2011). Post-traumatic stress disorder: The neurobiological impact of psychological trauma. Dialogues in Clinical Neuroscience, 13(3), 263–278.
- Spielberger, C. (1983). Manual for the state-trait anxiety inventory (STAI) (Form Y: Self-evaluation questionnaire). Redwood City, CA: Mind Garden.
- Swartzman, S., Booth, J. N., Munro, A., & Sani, F. (2017). Posttraumatic stress disorder after cancer diagnosis in adults: A meta-analysis. Depression and Anxiety, 34(4), 327–339.
- Tedstone, J. E., & Tarrier, N. (2003). Posttraumatic stress disorder following medical illness and treatment. Clinical Psychology Review, 23(3), 409–448.
- Weathers, F. W., Bovin, M. J., Lee, D. J., Sloan, D. M., Schnurr, P. P., Kaloupek, D. G., … Marx, B. P. (2018). The clinician-administered PTSD scale for DSM–5 (CAPS-5): Development and initial psychometric evaluation in military veterans. Psychological Assessment, 30(3), 383–395.
- Weiss, D. S., & Marmar, C. R. (1997). The impact of event scale-revised. In J. P. Wilson & T. M. Keane (Eds.), Assessing psychological trauma and PTSD (pp. 399–411). New York, NY: Guilford Press.
- Whitaker, K. L., Brewin, C. R., & Watson, M. (2008). Intrusive cognitions and anxiety in cancer patients. Journal of Psychosomatic Research, 64(5), 509–517.
- World Health Organization (WHO). (2018). Breast Cancer. Retrieved from http://www.who.int/cancer/prevention/diagnosis-screening/breast-cancer/en/
- Yaggie, M., Stevens, L., Miller, S., Abbott, A., Woodruff, C., Getchis, M., … Daiss, S. (2015). Electroencephalography coherence, memory vividness, and emotional valence effects of bilateral eye movements during unpleasant memory recall and subsequent free association: Implications for eye movement desensitization and reprocessing. Journal of EMDR Practice and Research, 9(2), 78–97.
