ABSTRACT
Background: According to clinical guidelines, trauma-focused psychotherapies (TF-PT) such as trauma-focused cognitive behavioural therapy (TF-CBT) and eye movement desensitization and reprocessing (EMDR) are recommended as first-line treatments for posttraumatic stress disorder (PTSD). TF-CBT and EMDR are equally effective and have large effect sizes. However, many patients fail to respond or have comorbid symptoms or disorders that only partially decline with TF-PT. Thus, there is growing interest in augmenting TF-PT through adjuvant interventions.
Objective: The current systematic review aims to assess whether adjuvant interventions improve outcome among adult PTSD patients receiving TF-PT.
Methods: We searched the databases PubMed, PILOTS, Web of Science and the Cochrane Library for controlled clinical trials examining whether adjuvant interventions lead to more symptom reduction in adult PTSD patients receiving TF-PT. Thirteen randomized controlled trials fitted the inclusion criteria. These were evaluated for internal risk of bias using the Cochrane Handbook for Systematic Review of Interventions.
Results: Most studies have a substantial risk for internal bias, mainly due to small sample sizes. Thus, no strong conclusion can be drawn from the current empirical evidence. Preliminary evidence suggests that exercise and cortisol administration may have an adjuvant effect on PTSD symptom reduction. Breathing biofeedback showed a trend for an adjuvant effect and an effect for accelerated symptom reduction.
Conclusions: Currently, it is not possible to formulate evidence-based clinical recommendations regarding adjuvants interventions. While several adjuvant interventions hold the potential to boost the effectiveness of TF-PT, the realization of sufficiently powered studies is crucial to separate plausible ideas from interventions proven to work in practice.
HIGHLIGHTS
• Assessment whether adjuvant interventions improve outcome among adult PTSD patients receiving trauma-focused psychotherapy.• No evidence-based recommendations can currently be given.• Several adjuvant interventions are promising.• Further research is needed.
Antecedentes: De acuerdo a las guías clínicas, las psicoterapias con foco en el trauma (TF-PT, por su sigla en inglés), así como la terapia cognitivo-conductual con foco en el trauma (TF-CBT, por su sigla en inglés) y la terapia de reprocesamiento y desensibilización por movimientos oculares (EMDR) son recomendadas como tratamientos de primera línea para el Trastorno de Estrés Postraumático (TEPT). TF-CBT y EMDR son igualmente efectivas y tienen grandes tamaños de efecto. Sin embargo, muchos pacientes no responden, tienen síntomas comórbidos u otros trastornos que sólo disminuyen parcialmente con TF-PT. Por lo tanto, hay un creciente interés en aumentar las TF-PT a través de intervenciones auxiliares.
Objetivo: La presente revisión sistemática busca evaluar si las intervenciones auxiliares mejoran los resultados entre adultos con TEPT que reciben TF-PT.
Métodos: Buscamos en las bases de datos Pubmed, PILOTS, Web of Science y en la Biblioteca Cochrane, estudios clínicos controlados que examinaran si las intervenciones auxiliares llevan a mayor reducción de síntomas en pacientes adultos con TEPT que reciben TF-PT. Trece estudios randomizados controlados cumplieron los criterios de inclusión. En estos se evaluó el riesgo interno de sesgo usando el Manual Cochrane para la Revisión Sistemática de Intervenciones.
Resultados: La mayoría de los estudios tuvo un riesgo sustancial de sesgo interno, principalmente debido al pequeño tamaño muestral. Por lo tanrto, no se puede extraer conclusiones fuertes de la evidencia empírica actual. La evidencia preliminar sugiere que el ejercicio y la administración de cortisol puede tener un efecto auxiliar en la reducción de síntomas de TEPT. El biofeedback de la respiración mostró una tendencia hacia un efecto auxiliar y un efecto en la reducción acelerada de los síntomas.
Conclusiones: Actualmente, no es posible formular recomendaciones clínicas basadas en la evidencia en relación a intervenciones auxiliares. Mientras varias intervenciones auxiliares mantienen potencial para aumentar la efectividad de las TF-PT, la realización de estudios con suficiente poder es crucial para separar las ideas plausibles de las intervenciones con efectividad probada en la práctica.
背景:根据临床指南,创伤型心理治疗(TF-PT)如创伤中心的认知行为疗法(TF-CBT)和眼球运动脱敏和再加工(EMDR)被推荐为创伤后应激障碍( PTSD)的一线治疗方法。 TF-CBT和EMDR同样有效并且具有大的效果量。然而,许多患者对治疗没有反应或患有TF-PT只能部分减轻的共病症状。因此,人们越来越关注通过辅助干预增强TF-PT。
目的:本系统综述旨在评估辅助干预措施是否能改善接受TF-PT的成人PTSD患者的预后。
方法:我们搜索了PubMed,PILOTS,Web of Science和Cochrane的数据库中的对照临床试验,考察辅助干预措施是否会导致接受TF-PT的成人PTSD患者症状减轻。有13项随机对照试验符合纳入标准。使用Cochrane干预系统综述操作手册评估内部偏差风险。
结果:大多数研究存在较大的内部偏差风险,主要是由于样本量较小。因此,目前的经验证据无法得出有力的结论。初步证据表明,运动和皮质醇给药可能对PTSD症状减轻有辅助作用。呼吸生物反馈显示出可能有辅助效应的趋势和加速症状减轻的效果。
结论:目前,还不能制定关于辅助干预的循证临床建议。虽然有几种辅助干预措施具有提高TF-PT有效性的潜力,至关重要的是还需要进行充分的研究将好想法与经证实在实践中起作用的干预措施区分开来。
Posttraumatic stress disorder (PTSD) is a psychological disorder that may develop upon exposure to an extremely threatening event or a series of such events. Symptoms include re-experiencing the traumatic event(s); avoidance of thoughts and memories of the trauma and of activities, people, or situations reminiscent of the event(s); persistent perceptions of heightened current threat; hyperarousal; and negative alterations in cognitions and mood (American Psychological Association, Citation2013). PTSD is associated with a particularly high societal and economic burden (Habetha, Bleich, Weidenhammer, & Fegert, Citation2012) as well as with serious negative physical health consequences (Pacella, Hruska, & Delahanty, Citation2013). In Europe, estimates of the 12-month prevalence of PTSD range between 1.1% and 2.9% (Wittchen et al., Citation2011).
Table 1. Evidence table for included studies.
Trauma-focused psychotherapies (TF-PT), such as trauma-focused cognitive behavioural therapy (TF-CBT), e.g. cognitive therapy (Ehlers, Clark, Hackmann, McManus, & Fennell, Citation2005), prolonged exposure (PE (Foa, Hembree, & Rothbaum, Citation2007)), cognitive processing therapy (CPT (Resick & Schnicke, Citation1993)), and Eye movement desensitization and reprocessing (EMDR (Shapiro, Citation1995)), are recommended as first-line treatments for PTSD (American Psychological Association, Citation2017; Flatten et al., Citation2013; Forbes et al., Citation2007; National Collaborating Centre for Mental Health, Citation2005). Compared to waitlist conditions and treatment as usual (TAU), meta-analyses show large effect sizes for both TF-CBT (SMD = −1.36, 95% CI = −1.77 to −0.94) and EMDR (SMD = −1.08, 95% CI = −1.83 to −0.33) (Cusack et al., Citation2016). Thus, it can be safely concluded that TF-PT works for many patients.
However, a recent meta-analysis of randomized placebo-controlled trials reported only a small effect size for TF-PT (Carpenter et al., Citation2018). In contrast to the waitlist- or TAU-conditions, placebo conditions were standardized (e.g. present-centred therapy (Schnurr et al., Citation2007)) and therefore controlled for unspecific treatment factors, e.g. time spent with the therapist. Due to this fact, psychological placebo-controlled trials are more conservative as waitlist- and TAU-conditions resulting in smaller effect sizes. Furthermore, up to 30–50% of the patients still fulfil diagnostic criteria of PTSD subsequent to treatment (Bisson et al., Citation2007; Schottenbauer, Glass, Arnkoff, Tendick, & Gray, Citation2008). Many PTSD patients also suffer from comorbid symptoms (e.g. depression) that are not properly addressed by first-line PTSD treatments (Bisson et al., Citation2007; Bradley, Greene, Russ, Dutra, & Westen, Citation2005; Hofmann & Smits, Citation2008).
Given that the World Health Organization (World Health Organization, Citation2013) and the National Institute for Health and Care Excellence (National Collaborating Centre for Mental Health, Citation2005) recommend pharmacotherapy second to TF-PT, it has been assumed that the combination of these two strategies promotes greater effectiveness. However, there is no evidence to date that the combination of TF-PT with pharmacotherapy enhances treatment success (Tolin, Citation2017). Therefore, testing further treatment approaches is crucial to enhance the effectiveness of TF-PT on PTSD core symptoms as well as comorbid symptoms (Hoge & Chard, Citation2018).
Against this background, a broad range of interventions have been examined over the last decade as adjuvants to TF-PT (Marin, Lonak, & Milad, Citation2015; Wynn, Citation2015). However, so far no systematic review exists that evaluates and summarizes the findings of this substantial body of research on adjunct interventions to TF-PT. In order to close this gap, we carried out this systematic review. For clearness, we divided adjuvant interventions into the categories of behaviour-based interventions and cognitive enhancers.
Behaviour-based interventions wish to augment TF-PT by either supporting its mode of action (e.g. enhancing therapeutic learning processes (Asmundson et al., Citation2013)) or by focussing modes of actions not addressed by TF-PT (e.g. non-verbal access to trauma-related memories (Collie, Backos, Malchiodi, & Spiegel, Citation2006)). Cognitive enhancers are pharmacological agents (e.g. cortisol, D-cycloserine) that exert their effects by facilitating cognitive processes underlying therapeutic change (e.g. fear extinction processes and/or the consolidation of helpful non-fear memories (Bentz, Michael, de Quervain, & Wilhelm, Citation2010; Marin et al., Citation2015)).
This systematic review summarizes and discusses the evidence of whether adjunct behaviour-based interventions and cognitive enhancers improve the outcome of TF-PT. If possible, it is also reported whether these interventions improve comorbid symptoms. The review makes the available evidence more accessible for clinicians and researchers and provides a base for future research on adjuvant interventions in PTSD.
1. Methods
1.1. Eligibility criteria
Studies were included if they were controlled trials examining the adjuvant effect of alternative therapies in addition to PTSD first-line treatment for adults (≥18 years) with PTSD. TAU conditions not clearly describing that all patients were treated with TF-PT were excluded. Studies needed to be published in peer-reviewed English-language journals. Further, studies were excluded if they did not report data of self-ratings or clinician-administered ratings on pre- and post-treatment PTSD symptom severity. Studies examining the combination of two established monotherapies in the treatment of PTSD (e.g. TF-PT + sertraline) instead of examining the augmentation of a monotherapy by an alternative treatment approach (e.g. TF-PT + cortisol) were excluded. Moreover, results of meta-analyses were included if they reported analyses for (sub-) samples of studies meeting the mentioned inclusion criteria.
1.2. Data sources and search strategies
PubMed, the Cochrane Library, PILOTS and Web of Science were searched for articles published in English until 1 August 2018. We searched keywords, titles and abstracts using the following set of search terms:
randomized controlled trial OR meta-analysis OR controlled clinical trial;
acupoint* OR acupuncture* OR D-cycloserine OR hypnotherapy OR hypnosis OR neurofeedback OR biofeedback OR imagery rescripting OR exercise OR art* OR music* OR animal assisted* OR body psychotherapy OR ergotherapy OR conjoint* OR family* OR meditation OR yoga OR augmentation OR adjunctive OR adjuvant OR add-on OR enhancement;
PTSD OR post-traumatic stress disorder OR posttraumatic stress disorder;
#1 AND #2 AND #3.
Additionally, reference lists of all included studies, previous literature reviews and meta-analyses were searched for relevant studies.
1.3. Study selection
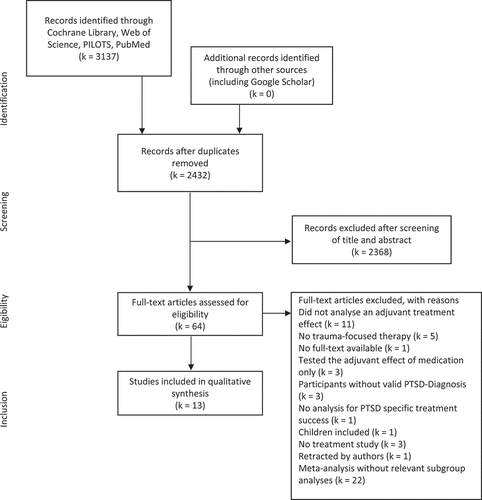
The literature search resulted in 3137 hits across databases of which 705 were removed as duplicates. The remaining 2432 records were screened based on title and abstract. Following the screening, 2368 studies were excluded since they did not meet inclusion criteria (see for a detailed overview of reasons for exclusion). Thus, 64 full texts were assessed for eligibility of which 13 met the inclusion criteria. All included studies are randomized controlled trials (RCTs). Adjuvant treatment with art therapy, behavioural family therapy (BFT), breathing biofeedback, exercise, hypnosis, cortisol, oxytocin, yohimbine, methylene blue were each examined in one of the eligible studies. Four eligible studies examined the adjuvant effect of D-cycloserine (DCS).
Figure 1. Adapted PRISMA flow diagram (Moher, Liberati, Tetzlaff, & Altman, Citation2009).
1.4. Adjuvant interventions
As mentioned above, eligible adjuvant interventions are grouped into two categories. Art therapy, BFT, biofeedback, exercise and hypnosis are summarized in the category behaviour-based interventions. The category cognitive enhancers comprises cortisol, DCS, methylene blue, oxytocin, and yohimbine. In the following, these approaches are introduced by category and alphabetical order, not by relevance.
1.4.1. Behaviour-based interventions
1.4.1.1. Arts therapies
Creative arts therapies (e.g. art therapy, music therapy, dance/movement therapy) wish to provide nonverbal access to trauma-related experiences through sensory processes. It is assumed that the nonverbal character of these therapies helps the patients to process their traumatic experiences by expressing trauma-related emotions (Malchiodi, Citation2015).
1.4.1.2. Behavioural family therapy
For a large number of veterans, PTSD symptoms are a source of family stress (Batten et al., Citation2009). The main goals of family therapy interventions in the treatment of adult PTSD are to improve communication within the family and to reduce parenting problems (Sherman & Larsen, Citation2018; Sherman, Larsen, Straits-Troster, Erbes, & Tassey, Citation2015; Whealin et al., Citation2017). Primary target points of BFT are psychoeducation of family members, improving problem-solving abilities and reducing aggression (Glynn et al., Citation1995).
1.4.1.3. Biofeedback
Breathing biofeedback is an intervention that indirectly enhances heart-rate variability (HRV) by breathing slowly at a frequency of six breaths per minute (Lehrer & Gevirtz, Citation2014). Several studies show that HRV is a biomarker of autonomous nervous system functioning that is linked to physical and mental health (Thayer, Åhs, Fredrikson, Sollers, & Wager, Citation2012). Increasing HRV is assumed to support TF-PT by reducing physiological arousal both in exposure sessions and in the long term (Tan, Dao, Farmer, Sutherland, & Gevirtz, Citation2011).
1.4.1.4. Exercise
Exercise is assumed to augment first-line PTSD treatments by various mechanisms such as an increase of brain-derived neurotrophic factor (BDNF), improvement of sleep, and increase of self-efficacy (Asmundson et al., Citation2013). Therefore, exercise is assumed to support TF-PT by both its short-term (e.g. facilitating exposure learning by increasing cortisol (Peake et al., Citation2014)) and its long-term effects (e.g. normalizing the reactivity of the hypothalamic–pituitary–adrenal (HPA) axis (Hackney, Citation2006)).
1.4.1.5. Hypnosis
The use of hypnosis has a long tradition in both psychoanalytic treatments and CBT, albeit its presumed mode of action (e.g. reduction of hyperarousal or facilitating integration of traumatic memories) differs depending on its specific use. Hypnosis targets both the key symptoms of PTSD and comorbid symptoms (e.g. dissociation, pain or insomnia) (Cardeña, Maldonado, van der Hart, & Spiegel, Citation2000).
1.4.2. Cognitive enhancers
1.4.2.1. Cortisol
Cortisol is a stress hormone and potent modulator of learning, memory processes, and neuroplasticity. There is extensive evidence from animal and human studies showing that HPA axis activation due to stress enhances memory consolidation when there is concomitant activation of the sympathetic nervous system while it inhibits the retrieval of previously acquired information (de Quervain, Schwabe, & Roozendaal, Citation2017). Thus, it has been argued that cortisol could be used to augment psychotherapy for fear-related disorders by enhancing the consolidation of the therapeutically acquired no-fear memory (Lass-Hennemann & Michael, Citation2014).
1.4.2.2. D-cycloserine
DCS is an N-methyl-d-aspartate (NMDA) receptor agonist. Both animal and human studies suggest that DCS could improve fear extinction, facilitate generalizability of extinction learning and reduce fear reinstatement (Davis, Ressler, Rothbaum, & Richardson, Citation2006). Therefore, DCS is assumed to enhance exposure therapy by modulating amygdala-based fear extinction processes.
1.4.2.3. Methylene blue
Methylene blue is a metabolic enhancer that increases mitochondrial respiration, thereby facilitating memory processes (Riha, Rojas, & Gonzalez-Lima, Citation2011). Methylene blue has been shown to facilitate fear extinction memory in rats (Gonzalez-Lima & Bruchey, Citation2004) and to improve retention of extinction in humans (Telch et al., Citation2014).
1.4.2.4. Oxytocin
Oxytocin is a neuropeptide that is involved in attachment and bonding behaviour. It is assumed to enhance trauma-focused psychotherapy by promoting fear extinction processes, reducing distress and anxiety as well as by facilitating social functioning and hence augmenting therapeutic alliance (Acheson et al., Citation2013).
1.4.2.5. Yohimbine
The alpha-2 adrenergic receptor antagonist yohimbine is assumed to facilitate fear extinction by elevating noradrenergic activity (Singewald, Schmuckermair, Whittle, Holmes, & Ressler, Citation2015). It is assumed that the increased noradrenergic activity heightens exposure-related arousal, thereby improving treatment effects through elevated inhibitory learning.
1.5. Risk of bias evaluation
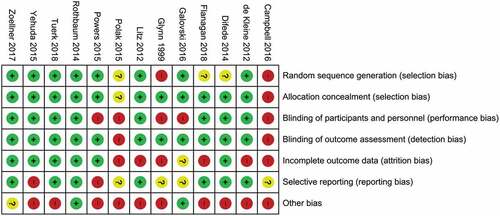
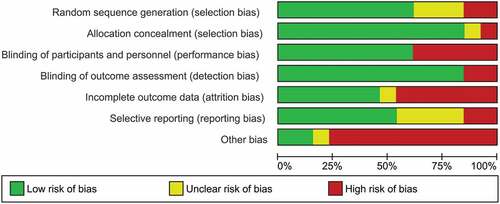
For all included studies, a risk of internal bias evaluation based on the Cochrane Handbook for Systematic Review of Interventions (Higgins & Green, Citation2011) was conducted. Risk of bias assessment included seven domains: random sequence generation (low risk of bias if all participants had the same chances to be concealed to each group), allocation concealment (low risk of bias if group allocation was unpredictable for staff members examining fulfilment of eligibility criteria), blinding of participants and personnel (low risk of bias if both participants and personnel were blind for group allocation), blinding for outcome assessment (low risk of bias if outcome assessors were blind for group allocation), incomplete outcome data (low risk of bias if either data were complete or missing data were appropriately handled, e.g. with intent-to-treat analysis), selective reporting (low risk of bias if an available preregistration of the trial indicated that all acquired measurements were reported), other source of bias (low risk of bias if no other reasons for risk of bias were identified, e.g. small sample size). If criteria for a low risk of bias judgment were not fulfilled, the risk of bias was judged as high. If the information required for the risk of bias evaluation were not available, the risk of bias was judged as unclear. Two independent raters (CS and HM) assessed the internal risk of bias per study. Inter-rater reliability was analysed using kappa statistic with kappa values between 0.40 and 0.59 reflecting fair agreement, between 0.60 and 0.74 reflecting good agreement and above 0.75 reflecting excellent agreement (Orwin, Cooper, & Hedges, Citation1994). The kappa statistics for the domains random sequence generation (K = .876), blinding of participants and personnel (K = 1) and selective outcome reporting (K = .865) revealed excellent inter-rater reliability. For allocation concealment (K = .639), other sources for bias (K = .662), blinding for outcome assessment (K = .639) inter-rater reliability was good. For the domain incomplete outcome data (K = .536) inter-rater reliability was fair. Overall kappa statistics revealed good interrater reliability (K = .718). In case of inter-rater disagreement, a third author (ME) assessed studies. Thereby, a consensus was reached in all cases.
2. Results
2.1. Risk of bias analysis
Methodological quality varied considerably across included studies. The main sources of internal bias were small samples (less than 30 participants (Campbell, Decker, Kruk, & Deaver, Citation2016; Difede et al., Citation2014; Flanagan, Sippel, Wahlquist, Moran-Santa Maria, & Back, Citation2018; Glynn et al., Citation1999; Litz et al., Citation2012; Polak, Witteveen, Denys, & Olff, Citation2015; Powers et al., Citation2015; Tuerk et al., Citation2018; Yehuda et al., Citation2015; Zoellner et al., Citation2017)). In addition to small sample size, the study of Powers et al. (Powers et al., Citation2015) was at risk for bias due to a lack of inferential statistical analyses. Only 5 of the remaining 12 trials used complete intent to treat analyses (Difede et al., Citation2014; Rothbaum et al., Citation2014; Tuerk et al., Citation2018; Yehuda et al., Citation2015; Zoellner et al., Citation2017). Due to their double-blind designs, studies investigating cognitive enhancers had a smaller risk of internal bias than the other studies. A graphical representation of the overall risk of internal bias for each study and each domain is available in and , respectively (for a detailed evaluation see Supplementary Material 1). Given this substantial risk of internal bias, the findings of the current review should be interpreted with caution.
2.2. Effectiveness of adjuvant interventions
In the following, results are presented by category and alphabetical order, not by relevance (see for summary of results).
2.3. Behaviour-based interventions
2.3.1. Art therapy
An RCT with traumatized veterans (Campbell et al., Citation2016) examined the effects of CPT (eight sessions) compared to CPT (eight sessions) plus art therapy (eight sessions). Art therapy included the visualisation of the trauma narrative (e.g. imaging traumatic lost), non-verbal expression and regulation of emotions (mask making, mind mapping) and the construction of a new self-concept and worldview. PTSD and depressive symptoms improved in both treatment groups. There was no significant group-time-interaction, but a non-significant trend towards a greater reduction of depressive symptoms in the CPT plus art therapy condition.
2.3.2. Behavioural family therapy
In an RCT, combat veterans with PTSD were assigned to either a waiting list condition, exposure therapy (twice a week; 18 sessions) or exposure therapy followed by BFT (16 sessions; veterans and a family member) (Glynn et al., Citation1999). There was no adjuvant effect of BFT in addition to exposure therapy regarding PTSD symptom reduction.
2.3.3. Biofeedback
One RCT (Polak et al., Citation2015) analysed the effectiveness of breathing biofeedback as an adjunct to TF-CBT. Eight participants were randomized to either TF-CBT only or TF-CBT plus breathing biofeedback. The number of total TF-CBT sessions ranged from 5 to 18 between participants. The TF-CBT plus breathing biofeedback group practised the breathing biofeedback every day. From TF-CBT session 3 onwards, patients were additionally instructed to use breathing biofeedback during imaginal exposure. There was no significant adjuvant effect of biofeedback regarding PTSD symptom reduction; however, there was a faster symptom reduction in the TF-CBT plus breathing biofeedback group and a trend towards an enhanced treatment effect.
2.3.4. Exercise
One study reports preliminary findings regarding an adjuvant effect of exercise in addition to PE for PTSD (Powers et al., Citation2015). Nine patients with PTSD were randomized to either PE plus exercise or PE only. PE consisted of 12 manualized treatment sessions with 90-min duration on a weekly basis. In the PE plus exercise condition, exercise took place immediately before each PE session. Exercise sessions lasted 30 min and were performed with moderate intensity (70% of maximal heart rate) on a treadmill. In addition to the effect of exercise on symptom-reduction, its effect on BDNF serum concentration was examined. Results revealed a large short-term effect (d = 2.65) of exercise in addition to PE for the improvement of PTSD symptoms. Adjuvant exercise also leads to an increased BDNF serum concentration (d = 1.08).
2.3.5. Hypnosis
Galovski et al. (Citation2016) examined the augmentative effect of sleep-directed hypnosis (3x/week over three weeks) preceding CPT (once per week over 12 weeks) compared to daily monitoring of sleep and PTSD symptoms (over three weeks) preceding CPT (control group) in female adults with PTSD. In addition to its effect on PTSD key symptoms and depression, the effect of adjuvant hypnosis on sleep difficulties was assessed. After completion of the hypnosis-session-block, patients showed greater symptom reduction in various sleep parameters. However, after the CPT-session-block, this superiority disappeared except for sleep latency. PTSD symptom reduction was not larger in the hypnosis plus CPT group. However, there was a significant effect of the CPT plus hypnosis group regarding the reduction of depressive symptom at post-treatment, but not at 3-month follow-up.
2.4. Cognitive enhancers
2.4.1. Cortisol
One double-blind RCT investigated the adjuvant effect of cortisol in addition to PE (Yehuda et al., Citation2015). Both groups received 10 sessions of manualized PE. A quantity of 30 mg of placebo (control group) or hydrocortisone (experimental group) was administered 20 min before PE session 3 to 10. Short- and long-term effects were not analysed separately. Instead, data for the analyses varied across patients and represented the assessment with the lowest CAPS total score (being post-assessment or follow-up). Regarding PTSD symptoms, these analyses revealed a significantly enhanced treatment effect for cortisol compared with placebo.
2.4.2. D-cycloserine
Four double-blind RCTs examined the augmentative effect of DCS in samples of PTSD patients (de Kleine, Hendriks, Kusters, Broekman, & van Minnen, Citation2012; Difede et al., Citation2014; Litz et al., Citation2012; Rothbaum et al., Citation2014). In all studies, DCS and placebo were repeatedly administered prior to TF-PT sessions [prolonged exposure (PE (de Kleine et al., Citation2012; Rothbaum et al., Citation2014))] imaginal exposure (IE (Litz et al., Citation2012)) or virtual reality exposure (VRE (Difede et al., Citation2014)). Between studies, treatment duration varied from 6 (Litz et al., Citation2012) to 12 (Difede et al., Citation2014) weeks and the weekly DCS-dose was either 50 mg (de Kleine et al., Citation2012; Litz et al., Citation2012; Rothbaum et al., Citation2014) or 100 mg (Difede et al., Citation2014). All studies used intent-to-treat (ITT) analyses; however, de Kleine et al. (Citation2012) excluded eight patients for dropping out before the first exposure session. Rothbaum et al. (Citation2014) did not report an overall enhancement effect of DCS administration adjunct to VRE. De Kleine et al. (Citation2012) reported that DCS yielded higher symptom reduction in patients with more severe pre-treatment PTSD who needed longer treatment. Difede et al. (Citation2014) provide preliminary evidence for an enhancement of VRE by DCS (Hedges g = 1.13) in the long term. They found a similar pattern for depression, anger expression, and sleep. PTSD remission rates were greater for the VRE + DCS group. Contrary to these findings, Litz et al. (Citation2012) reported a lower performance of IE + DCS compared to exposure and placebo conditions in the short term.
2.4.3. Methylene blue
In a double-blind RCT (Zoellner et al., Citation2017), participants were randomized to either IE plus methylene blue, IE alone or a waitlist control group. TF-PT consisted of six daily sessions of 50-min IE based on a PE protocol. A quantity of 260 mg methylene blue or placebo was administered after five of six IE treatment sessions. Both treatment groups were superior to waiting list controls, but there was no adjuvant effect of methylene blue in addition to IE regarding PTSD symptoms and depression.
2.4.4. Oxytocin
Flanagan et al. (Citation2018) investigated the augmentative effect of oxytocin to PE in a double-blind RCT. Intranasal oxytocin (40 IU) and placebo were self-administered 45 min prior to each of 10 manualized PE sessions. Completer analysis regarding PTSD and depressive symptoms revealed no significant differences in symptom reduction. Nevertheless, the symptom scores at PE session number three were significantly lower in the oxytocin group, which may indicate preliminary evidence for accelerated symptom reduction.
2.4.5. Yohimbine
The adjuvant effect of the alpha-2 adrenergic receptor antagonist yohimbine was investigated in a double-blind RCT (Tuerk et al., Citation2018). For both groups, TF-PT consisted of manualized PE sessions delivered on a weekly basis. A quantity of 21.6 mg yohimbine or placebo was administered once before the first exposure session. The primary endpoint was trauma cued heart-rate reactivity. The secondary endpoints included change of PTSD symptom severity. The yohimbine group showed a significantly stronger reduction of the trauma cued heart-rate reactivity. However, ITT analysis detected no adjuvant effect of yohimbine on PTSD and depressive symptoms.
2.5. Discussion
To our knowledge, this is the first systematic review assessing the effectiveness of adjuvant interventions in adult PTSD patients treated with TF-PT. Thirteen RCTs were included in this review. The methodological quality of the included studies varied considerably but tended to be low. All but one study had a high risk of internal bias. Thus, only tentative conclusions can be drawn from the available empirical evidence and clinical recommendations are made with reservations. Nevertheless, there is promising preliminary evidence for some adjuvant interventions.
2.6. Evaluation of the current body of evidence
2.6.1. Behaviour-based interventions
Within the category behaviour-based interventions, breathing biofeedback and exercise seem to be the most promising adjuncts regarding PTSD symptom outcome measures.
The reported results of breathing biofeedback are encouraging (Polak et al., Citation2015). Biofeedback has not only been examined as an adjuvant intervention to TF-PT but also as an adjuvant to TAU and as a stand-alone therapy for PTSD. Studies examining its effect on TAU conditions show quite heterogeneous results (Lande, Williams, Francis, Gragnani, & Morin, Citation2010; Tan et al., Citation2011; Zucker, Samuelson, Muench, Greenberg, & Gevirtz, Citation2009). One study investigating HRV-biofeedback as an adjuvant intervention to TAU (Lande et al., Citation2010) did not find that it resulted in increased PTSD and depressive symptom reduction. However, one study (Tan et al., Citation2011) found that HRV-biofeedback augmented TAU with respect to PTSD symptoms, and one study showed a positive effect on comorbid depressive symptoms (Zucker et al., Citation2009). Furthermore, the results of breathing biofeedback as a stand-alone therapy are quite promising. Several studies show that biofeedback employed as a stand-alone treatment is successful in reducing PTSD symptoms (Askovic, Watters, Aroche, & Harris, Citation2017; Gapen et al., Citation2016; Reiter, Andersen, & Carlsson, Citation2016). Taken together, the evidence suggests that HRV-biofeedback is feasible and acceptable to PTSD patients (Foa et al., Citation2007; Morina et al., Citation2012; Polak et al., Citation2015; Tan et al., Citation2011) and that its use as an adjuvant intervention warrants further research.
Exercise showed a substantial adjuvant effect on PE (Powers et al., Citation2015). However, due to the small sample size and the lack of inferential statistical analysis, the results of Powers et al. (Powers et al., Citation2015) have to be interpreted with caution. A study assessing the adjuvant effect of exercise on TAU (Rosenbaum, Sherrington, & Tiedemann, Citation2015) also showed that it resulted in enhanced PTSD and depressive symptom reduction. Exercise also seems to be an effective stand-alone intervention for PTSD (Fetzner & Asmundson, Citation2015; Goldstein et al., Citation2018). Furthermore, exercise is time- and cost-effective, has beneficial side effects on physical health and is popular among patients (Chapman et al., Citation2017). Therefore, exercise seems a particularly promising behaviour-based adjuvant intervention that should be investigated further.
2.6.2. Cognitive enhancers
Among the cognitive enhancers, cortisol seems to be the most promising substance augmenting TF-PT. In the reviewed study, cortisol administration as an adjunct to PE was associated with a greater reduction in PTSD symptoms compared to PE plus placebo. The study results are in line with studies on specific phobias (de Quervain et al., Citation2011; Soravia et al., Citation2014); therefore, further research on enhancing TF-PT by augmenting cortisol levels seems warranted. The largest body of research on cognitive enhancers focuses on DCS. Given that three out of four reviewed studies did not report an overall effect for DCS, the current evidence does not support the assumption that DCS augments TF-PT. This conclusion is in line with a recent meta-analysis, including both samples of adults and children with PTSD (Bürkner, Bittner, Holling, Buhlmann, & Hashimoto, Citation2017). The reviewed trials on methylene blue, oxytocin and yohimbine also did not detect significant adjuvant treatment effects.
2.6.3. Main limitations of the reviewed studies
The main methodological limitation of the reviewed studies is their lack of statistical power. Of all studies, only two trials examining the effectiveness of DCS and hypnosis had a sufficient power to detect at least moderate adjuvant treatment effect sizes. Another limitation is that the heterogeneous study designs result in a restricted comparability of the results. Especially in the category of the cognitive enhancers, dosage level (e.g. 50 mg (de Kleine et al., Citation2012; Litz et al., Citation2012; Rothbaum et al., Citation2014) versus 100 mg (Difede et al., Citation2014) for DCS trials), total number of doses (single dose (Tuerk et al., Citation2018) versus multiple doses (e.g. Zoellner et al., Citation2017)), treatment duration (5 days (Zoellner et al., Citation2017) to 12 weeks, e.g. Difede et al., Citation2014), and time point of drug administration (30 min (e.g. Rothbaum et al., Citation2014 to 90 min (Difede et al., Citation2014)) before TF-PT or immediately after TF-PT (Zoellner et al., Citation2017)) differ between the studies. Among behaviour-based interventions, heterogeneity in study designs is also present; for example, trials examining adjuvant effects of exercise on mental disorders differ regarding treatment duration, intensity, and frequency (e.g. Powers et al., Citation2015; Rosenbaum et al., Citation2015).
2.7. Future directions
Adaptive designs (ADs) could be a possible way to approach these methodological problems efficiently. Mostly used in trials evaluating drugs, ADs can also be applied to evaluate behaviour-based interventions. Based on planned interim analyses that have been defined before the first patient is enrolled in the study, ADs allow for modifications to key aspects of the study design (Pallmann et al., Citation2018). Typical pre-planned changes in ADs are sample size re-estimation to ensure the desired statistical power and adaptive dose ranging to identify the most promising dose. In the research area of adjuvant interventions in the treatment of PTSD, an AD could be conducted for instance to identify the most efficient application of exercise augmenting TF-PT starting with two intensities (moderate versus high), two frequencies (once a week versus three times a week), and two time points (before or after TF-PT) of exercise. Based on a priori planned adaptions, the conditions can be reduced to the most promising ones during the trial resulting in a more time and cost-effective identification of promising treatment approaches and application forms. Multi-centre-studies could be another way to overcome the main limitations of the current body of evidence. Through the combination of various recruitment locations, large sample sizes could be acquired in a more time- and cost-effective manner (Bellomo, Warrillow, & Reade, Citation2009). Furthermore, internal bias (e.g. regarding group allocation concealment or blinding of staff members) could be reduced through task division between research-centres.
2.7.1. Further promising adjuvant treatments
Promising interventions, not yet examined in studies eligible for this review, are inter alias acupuncture, meditation-based interventions and the cognitive enhancer 3,4-Methylene-dioxy methamphetamine (MDMA). Meta-analyses revealed preliminary significant effects on PTSD-symptoms for meditation-based interventions and acupuncture as stand-alone treatments (Grant et al., Citation2018; Hilton et al., Citation2017). Furthermore, acupuncture and MDMA successfully augment active control conditions in adult PTSD samples (e.g. Engel et al., Citation2014; Mithoefer, Wagner, Mithoefer, Jerome, & Doblin, Citation2011). However, up to now, no studies exist examining the adjuvant effects of these interventions when combined with TF-PT, but future research on these interventions using TF-PT control conditions seems eligible.
3. Conclusion
Overall, research investigating the effect of TF-PT in combination with adjuvant interventions is rare and lacks high-quality trials. To date, only a small number of studies have assessed the augmenting effects of adjuvant interventions in adult PTSD patients receiving TF-PT. Actually, not a single study included in this review tested the utility of adjuvant interventions in EMDR, with the majority of studies drawing heavily on exposure. Risk of internal bias was high for all but one of the reviewed studies, particularly due to small sample sizes. Given that first-line treatments for PTSD are fortunately highly effective and that most adjuvant intervention probably only shows small to medium effect sizes, future research should be conducted with appropriate sample sizes. While the preliminary results of some adjuvant interventions are encouraging with respect to both PTSD-specific and comorbid symptoms, clinical recommendations can only be made with reservations. The available data indicate that cortisol administration, breathing biofeedback and exercise are currently the interventions with the strongest empirical support. In summary, adjuvant interventions might hold high potential for boosting the effectiveness of TF-PT, but high-quality research is needed before this claim can be confirmed or refuted.
Supplemental Material
Download MS Word (19 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental Material
Supplementary data for this article can be accessed here
References
- Acheson, D., Feifel, D., Wilde, S., McKinney, R., Lohr, J., & Risbrough, V. (2013). The effect of intranasal oxytocin treatment on conditioned fear extinction and recall in a healthy human sample. Psychopharmacology, 229(1), 199–13. PubMed PMID: 89657567.
- American Psychological Association. (2013). Diagnostic and statistical manual of mental disorders. Washington, DC: American Psychiatric Pub.
- American Psychological Association. (2017). Clinical practice guideline for the treatment of Posttraumatic Stress Disorder (PTSD) in adults. American Psychological Association guideline development panel for the treatment of PTSD in adults. Retrieved from https://www.apa.org/ptsd-guideline/ptsd.pdf
- Askovic, M., Watters, A. J., Aroche, J., & Harris, A. W. F. (2017). Neurofeedback as an adjunct therapy for treatment of chronic posttraumatic stress disorder related to refugee trauma and torture experiences: Two case studies. Australasian Psychiatry, 25(4), 358–363.
- Asmundson, G. J., Fetzner, M. G., DeBoer, L. B., Powers, M. B., Otto, M. W., & Smits, J. A. (2013). Let’s get physical: A contemporary review of the anxiolytic effects of exercise for anxiety and its disorders. Depression and Anxiety, 30(4), 362–373.
- Batten, S. V., Drapalski, A. L., Decker, M. L., DeViva, J. C., Morris, L. J., Mann, M. A., & Dixon, L. B. (2009). Veteran interest in family involvement in PTSD treatment. Psychological Services, 6(3), 184.
- Bellomo, R., Warrillow, S. J., & Reade, M. C. (2009). Why we should be wary of single-center trials. Critical Care Medicine, 37(12), 3114–3119.
- Bentz, D., Michael, T., de Quervain, D. J. F., & Wilhelm, F. H. (2010). Enhancing exposure therapy for anxiety disorders with glucocorticoids: From basic mechanisms of emotional learning to clinical applications. Journal of Anxiety Disorders, 24, 223–230.
- Bisson, J. I., Ehlers, A., Matthews, R., Pilling, S., Richards, D., & Turner, S. (2007). Psychological treatments for chronic post-traumatic stress disorder - Systematic review and meta-analysis. British Journal of Psychiatry, 190, 97–104.
- Bradley, R., Greene, J., Russ, E., Dutra, L., & Westen, D. (2005). A multidimensional meta-analysis of psychotherapy for PTSD. American Journal of Psychiatry, 162(2), 214–227.
- Bürkner, P.-C., Bittner, N., Holling, H., Buhlmann, U., & Hashimoto, K. (2017). D-cycloserine augmentation of behavior therapy for anxiety and obsessive-compulsive disorders: A meta-analysis. PloS One, 12(3), 1–19.
- Campbell, M., Decker, K. P., Kruk, K., & Deaver, S. P. (2016). Art therapy and cognitive processing therapy for combat-related PTSD: A randomized controlled trial. Art Therapy, 33(4), 169–177.
- Cardeña, E., Maldonado, J., van der Hart, O., & Spiegel, D. (2000). Hypnosis. In E. B. Foa, T. M. Keane, & M. J. Friedman (Eds.), Effective treatments for PTSD (pp. 247–279). NewYork: Guilford.
- Carpenter, J. K., Andrews, L. A., Witcraft, S. M., Powers, M. B., Smits, J. A. J., & Hofmann, S. G. (2018). Cognitive behavioral therapy for anxiety and related disorders: A meta‐analysis of randomized placebo‐controlled trials. Depression and Anxiety, 35(6), 502–514.
- Chapman, J. J., Coombes, J. S., Brown, W. J., Khan, A., Chamoli, S., Pachana, N. A., & Burton, N. W. (2017). The feasibility and acceptability of high-intensity interval training for adults with mental illness: A pilot study. Mental Health and Physical Activity, 13, 40–48.
- Collie, K., Backos, A., Malchiodi, C., & Spiegel, D. (2006). Art therapy for combat-related PTSD: Recommendations for research and practice. Art Therapy, 23(4), 157–164.
- Cusack, K., Jonas, D. E., Forneris, C. A., Wines, C., Sonis, J., Middleton, J. C., … Gaynes, B. N. (2016). Psychological treatments for adults with posttraumatic stress disorder: A systematic review and meta-analysis. Clinical Psychology Review, 43, 128–141.
- Davis, M., Ressler, K., Rothbaum, B. O., & Richardson, R. (2006). Review: Effects of D-cycloserine on extinction: Translation from preclinical to clinical work. Biological Psychiatry, 60, 369–375.
- de Kleine, R. A., Hendriks, G.-J., Kusters, W. J., Broekman, T. G., & van Minnen, A. (2012). A randomized placebo-controlled trial of D-cycloserine to enhance exposure therapy for posttraumatic stress disorder. Biological Psychiatry, 71(11), 962–968.
- de Quervain, D., Schwabe, L., & Roozendaal, B. (2017). Stress, glucocorticoids and memory: Implications for treating fear-related disorders. Nature Reviews Neuroscience, 18(1), 7.
- de Quervain, D. J. F., Bentz, D., Michael, T., Bolt, O. C., Wiederhold, B. K., Margraf, J., & Wilhelm, F. H. (2011). Glucocorticoids enhance extinction-based psychotherapy. Proceedings of the National Academy of Sciences, 108(16), 6621–6625. PubMed PMID: edsbas.6C0FDA32
- Difede, J., Cukor, J., Wyka, K., Olden, M., Hoffman, H., Lee, F. S., & Altemus, M. (2014). D-cycloserine augmentation of exposure therapy for post-traumatic stress disorder: A pilot randomized clinical trial. Neuropsychopharmacology, 39(5), 1052–1058.
- Ehlers, A., Clark, D. M., Hackmann, A., McManus, F., & Fennell, M. (2005). Cognitive therapy for post-traumatic stress disorder: Development and evaluation. Behaviour Research and Therapy, 43(4), 413–431.
- Engel, C. C., Cordova, E. H., Benedek, D. M., Liu, X., Gore, K. L., Goertz, C., … Ursano, R. J. (2014). Randomized effectiveness trial of a brief course of acupuncture for posttraumatic stress disorder. Medical Care, 52(12), S57–S64.
- Fetzner, M. G., & Asmundson, G. J. G. (2015). Aerobic exercise reduces symptoms of posttraumatic stress disorder: A randomized controlled trial. Cognitive Behaviour Therapy, 44(4), 301–313.
- Flanagan, J. C., Sippel, L. M., Wahlquist, A., Moran-Santa Maria, M. M., & Back, S. E. (2018). Augmenting prolonged exposure therapy for PTSD with intranasal oxytocin: A randomized, placebo-controlled pilot trial. Journal of Psychiatric Research, 98, 64–69.
- Flatten, G., Gast, U., Hofmann, A., Knaevelsrud, C., Lampe, A., Liebermann, P., ... Wöller, W. (2013). Posttraumatische Belastungsstörung: S3-Leitlinie und Quellentexte (pp. 147). Stuttgart: Schattauer.
- Foa, E. B., Hembree, E. A., & Rothbaum, B. O. (2007). Prolonged exposure therapy for PTSD: Emotional processing of traumatic experiences; therapist guide (pp. 2007). Oxford: Oxford University Press.
- Forbes, D., Creamer, M., Phelps, A., Bryant, R., McFarlane, A., Devilly, G. J., … Newton, S. (2007). Australian guidelines for the treatment of adults with acute stress disorder and post-traumatic stress disorder. The Australian and New Zealand Journal of Psychiatry, 41(8), 637–648.
- Galovski, T. E., Harik, J. M., Blain, L. M., Elwood, L., Gloth, C., & Fletcher, T. D. (2016). Augmenting cognitive processing therapy to improve sleep impairment in PTSD: A randomized controlled trial. Journal of Consulting and Clinical Psychology, 84(2), 167.
- Gapen, M., van der Kolk, B. A., Hamlin, E., Hirshberg, L., Suvak, M., & Spinazzola, J. (2016). A pilot study of neurofeedback for chronic PTSD. Applied Psychophysiology and Biofeedback, 41(3), 251–261.
- Glynn, S. M., Eth, S., Randolph, E. T., Foy, D. W., Leong, G. B., Paz, G. G., ... Katzman. (1995). Behavioral family therapy for Vietnam combat veterans with posttraumatic stress disorder. The Journal of Psychotherapy Practice and Research, 4(3), 214.
- Glynn, S. M., Eth, S., Randolph, E. T., Foy, D. W., Urbaitis, M., Boxer, L., … Crothers, J. (1999). A test of behavioral family therapy to augment exposure for combat-related posttraumatic stress disorder. Journal of Consulting and Clinical Psychology, 67(2), 243–251.
- Goldstein, L. A., Mehling, W. E., Metzler, T. J., Cohen, B. E., Barnes, D. E., Choucroun, G. J., … Neylan, T. C. (2018). Veterans group exercise: A randomized pilot trial of an integrative exercise program for veterans with posttraumatic stress. Journal of Affective Disorders, 227, 345–352.
- Gonzalez-Lima, F., & Bruchey, A. K. (2004). Extinction memory by the metabolic enhancer improvement methylene blue. Learning & Memory, 11(5), 633–640.
- Grant, S., Colaiaco, B., Motala, A., Shanman, R., Sorbero, M., & Hempel, S. (2018). Acupuncture for the treatment of adults with posttraumatic stress disorder: A systematic review and meta-analysis. Journal of Trauma & Dissociation, 19(1), 39–58.
- Habetha, S., Bleich, S., Weidenhammer, J., & Fegert, J. M. (2012). A prevalence-based approach to societal costs occurring in consequence of child abuse and neglect. Child and Adolescent Psychiatry and Mental Health, 6(1), 35.
- Hackney, A. C. (2006). Stress and the neuroendocrine system: The role of exercise as a stressor and modifier of stress. Expert Review of Endocrinology & Metabolism, 1(6), 783–792.
- Higgins, J. P., & Green, S. (2011, March). Cochrane handbook for systematic review of interventions version 5.1. The Cochrane Collaboration. Retrieved from https://training.cochrane.org/handbook
- Hilton, L., Maher, A. R., Colaiaco, B., Apaydin, E., Sorbero, M. E., Booth, M., … Hempel, S. (2017). Meditation for posttraumatic stress: Systematic review and meta-analysis. Psychological Trauma: Theory, Research, Practice, and Policy, 9(4), 453–460.
- Hofmann, S. G., & Smits, J. A. J. (2008). Cognitive-behavioral therapy for adult anxiety disorders: A meta-analysis of randomized placebo-controlled trials. Journal of Clinical Psychiatry, 69(4), 621–632.
- Hoge, C. W., & Chard, K. M. (2018). A window into the evolution of trauma-focused psychotherapies for posttraumatic stress disorder. JAMA, 319(4), 343–345.
- Lande, R. G., Williams, L. B., Francis, J. L., Gragnani, C., & Morin, M. L. (2010). Efficacy of biofeedback for post-traumatic stress disorder. Complementary Therapies in Medicine, 18(6), 256–259.
- Lass-Hennemann, J., & Michael, T. (2014). Endogenous cortisol levels influence exposure therapy in spider phobia. Behaviour Research and Therapy, 60, 39–45.
- Lehrer, P. M., & Gevirtz, R. (2014). Heart rate variability biofeedback: How and why does it work? Frontiers in Psychology, 5, 756.
- Litz, B. T., Salters-Pedneault, K., Steenkamp, M. M., Hermos, J. A., Bryant, R. A., Otto, M. W., & Hofmann, S. G. (2012). A randomized placebo-controlled trial of D-cycloserine and exposure therapy for posttraumatic stress disorder. Journal of Psychiatric Research, 46(9), 1184–1190.
- Malchiodi, C. (2015). Neurobiology, creative interventions, and childhood trauma. In C. A. Malchiodi (Ed.), Creative interventions with traumatized children, 2nd ed. (pp. 3–23). New York, NY: Guilford Press.
- Marin, M.-F., Lonak, S. F., & Milad, M. R. (2015). Augmentation of evidence-based psychotherapy for PTSD with cognitive enhancers. Current Psychiatry Reports, 17(6), 39.
- Mithoefer, M. C., Wagner, M. T., Mithoefer, A. T., Jerome, L., & Doblin, R. (2011). The safety and efficacy of (plus minus) 3,4-methylenedioxymethamphetamine-assisted psychotherapy in subjects with chronic, treatment-resistant posttraumatic stress disorder: The first randomized controlled pilot study. Journal of Psychopharmacology, 25(4), 439.
- Moher, D., Liberati, A., Tetzlaff, J., & Altman, D. G. (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Annals of Internal Medicine, 151(4), 264–269.
- Morina, N., Maier, T., Bryant, R., Knaevelsrud, C., Wittmann, L., Rufer, M., … Müller, J. (2012). Combining biofeedback and Narrative Exposure Therapy for persistent pain and PTSD in refugees: A pilot study. European Journal of Psychotraumatology, 3, 17660.
- National Collaborating Centre for Mental Health. (2005). Post-traumatic stress disorder: The management of PTSD in adults and children in primary and secondary care. Leicester (UK): Gaskell.
- Orwin, R., Cooper, H., & Hedges, L. (1994). The handbook of research synthesis (pp. 139–162). New York, NY: Russell Sage Foundation.
- Pacella, M. L., Hruska, B., & Delahanty, D. L. (2013). The physical health consequences of PTSD and PTSD symptoms: A meta-analytic review. Journal of Anxiety Disorders, 27(1), 33–46.
- Pallmann, P., Bedding, A. W., Choodari-Oskooei, B., Dimairo, M., Flight, L., Hampson, L. V., … Jaki, T. (2018). Adaptive designs in clinical trials: Why use them, and how to run and report them. BMC Medicine, 16–29.
- Peake, J. M., Tan, S. J., Markworth, J. F., Broadbent, J. A., Skinner, T. L., & Cameron-Smith, D. (2014). Metabolic and hormonal responses to isoenergetic high-intensity interval exercise and continuous moderate-intensity exercise. American Journal of Physiology-Heart and Circulatory Physiology, 307(7), E539–E552.
- Polak, A. R., Witteveen, A. B., Denys, D., & Olff, M. (2015). Breathing biofeedback as an adjunct to exposure in cognitive behavioral therapy hastens the reduction of PTSD symptoms: A pilot study. Applied Psychophysiology and Biofeedback, 40(1), 25–31.
- Powers, M. B., Medina, J. L., Burns, S., Kauffman, B. Y., Monfils, M., Asmundson, G. J. G., … Smits, J. A. J. (2015). Exercise augmentation of exposure therapy for PTSD: Rationale and pilot efficacy data. Cognitive Behaviour Therapy, 44(4), 314–327.
- Reiter, K., Andersen, S. B., & Carlsson, J. (2016). Neurofeedback treatment and posttraumatic stress disorder effectiveness of neurofeedback on posttraumatic stress disorder and the optimal choice of protocol. Journal of Nervous and Mental Disease, 204(2), 69–77.
- Resick, P. A., & Schnicke, M. (1993). Cognitive processing therapy for rape victims: A treatment manual. Newbury Park: Sage Publications.
- Riha, P. D., Rojas, J. C., & Gonzalez-Lima, F. (2011). Beneficial network effects of methylene blue in an amnestic model. Neuroimage, 54(4), 2623–2634.
- Rosenbaum, S., Sherrington, C., & Tiedemann, A. (2015). Exercise augmentation compared with usual care for post‐traumatic stress disorder: A randomized controlled trial. Acta Psychiatrica Scandinavica, 131(5), 350–359.
- Rothbaum, B. O., Price, M., Jovanovic, T., Norrholm, S. D., Gerardi, M., Dunlop, B., … Ressler, K. J. (2014). A randomized, double-blind evaluation of D-cycloserine or alprazolam combined with virtual reality exposure therapy for posttraumatic stress disorder in Iraq and Afghanistan War veterans. The American Journal of Psychiatry, 171(6), 640–648.
- Schnurr, P. P., Friedman, M. J., Engel, C. C., Foa, E. B., Shea, M. T., Chow, B. K., … Bernardy, N. (2007). Cognitive behavioral therapy for posttraumatic stress disorder in women: A randomized controlled trial. JAMA, 297(8), 820–830.
- Schottenbauer, M. A., Glass, C. R., Arnkoff, D. B., Tendick, V., & Gray, S. H. (2008). Nonresponse and dropout rates in outcome studies on PTSD: Review and methodological considerations. Psychiatry: Interpersonal and Biological Processes, 71(2), 134–168.
- Shapiro, F. (1995). Eye movement desensitization and reprocessing: Basic principles, protocols, and procedures (Vol. 3, pp. 1995). New York: Guilford. print.
- Sherman, M. D., Larsen, J., Straits-Troster, K., Erbes, C., & Tassey, J. (2015). Veteran-child communication about parental PTSD: A mixed methods pilot study. Journal of Family Psychology, 29(4), 595–603.
- Sherman, M. D., & Larsen, J. L. (2018). Family-focused interventions and resources for veterans and their families. Psychological Services, 15(2), 146–153.
- Singewald, N., Schmuckermair, C., Whittle, N., Holmes, A., & Ressler, K. (2015). Pharmacology of cognitive enhancers for exposure-based therapy of fear, anxiety and trauma-related disorders. Pharmacology & Therapeutics, 149, 150–190.
- Soravia, L. M., Heinrichs, M., Winzeler, L., Fisler, M., Schmitt, W., Horn, H., … de Quervain, D. J.-F. (2014). Glucocorticoids enhance in vivo exposure‐based therapy of spider phobia. Depression & Anxiety, 31(5), 429–435.
- Tan, G., Dao, T. K., Farmer, L., Sutherland, R. J., & Gevirtz, R. (2011). Heart Rate Variability (HRV) and Posttraumatic Stress Disorder (PTSD): A pilot study. Applied Psychophysiology and Biofeedback, 36(1), 27–35.
- Telch, M. J., Bruchey, A. K., Rosenfield, D., Cobb, A. R., Smits, J., Pahl, S., & Gonzalez-Lima, F. (2014). Effects of post-session administration of methylene blue on fear extinction and contextual memory in adults with claustrophobia. The American Journal of Psychiatry, 171(10), 1091–1098.
- Thayer, J. F., Åhs, F., Fredrikson, M., Sollers, J. J., III, & Wager, T. D. (2012). A meta-analysis of heart rate variability and neuroimaging studies: Implications for heart rate variability as a marker of stress and health. Neuroscience & Biobehavioral Reviews, 36(2), 747–756.
- Tolin, D. F. (2017). Can cognitive behavioral therapy for anxiety and depression be improved with pharmacotherapy? A meta-analysis. Psychiatric Clinics, 40(4), 715–738.
- Tuerk, P. W., Wangelin, B. C., Powers, M. B., Smits, J. A., Acierno, R., Myers, U. S., ... Hamner, M. B. (2018). Augmenting treatment efficiency in exposure therapy for PTSD: A randomized double-blind placebo-controlled trial of yohimbine HCl. Cognitive Behaviour Therapy, 14(5), 1–21.
- Whealin, J. M., Yoneda, A. C., Nelson, D., Hilmes, T. S., Kawasaki, M. M., & Yan, O. H. (2017). A culturally adapted family intervention for rural pacific Island veterans with PTSD. Psychological Services, 14(3), 295–306.
- Wittchen, H. U., Jacobi, F., Rehm, J., Gustavsson, A., Svensson, M., Jonsson, B., … Steinhausen, H.-C. (2011). The size and burden of mental disorders and other disorders of the brain in Europe 2010. European Neuropsychopharmacology, 21(9), 655–679.
- World Health Organization. (2013). Guidelines for the management of conditions specifically related to stress. Geneva, Switzerland: WHO.
- Wynn, G. H. (2015). Complementary and alternative medicine approaches in the treatment of PTSD. Current Psychiatry Reports, 17(8), 62.
- Yehuda, R., Bierer, L. M., Pratchett, L. C., Lehrner, A., Koch, E. C., Van Manen, J. A., … Hildebrandt, T. (2015). Cortisol augmentation of a psychological treatment for warfighters with posttraumatic stress disorder: Randomized trial showing improved treatment retention and outcome. Psychoneuroendocrinology, 51, 589–597.
- Zoellner, L. A., Telch, M., Foa, E. B., Farach, F. J., McLean, C. P., Gallop, R., … Gonzalez-Lima, F. (2017). Enhancing extinction learning in posttraumatic stress disorder with brief daily imaginal exposure and methylene blue: A randomized controlled trial. The Journal of Clinical Psychiatry, 78(7), E782–E789.
- Zucker, T. L., Samuelson, K. W., Muench, F., Greenberg, M. A., & Gevirtz, R. N. (2009). The effects of respiratory sinus arrhythmia biofeedback on heart rate variability and posttraumatic stress disorder symptoms: A pilot study. Applied Psychophysiology and Biofeedback, 34(2), 135–143.