ABSTRACT
Contrary to specific phobias, for which Virtual Reality Exposure Therapy (VRET) constitutes an effective treatment, uncertainty still exists regarding the usefulness of VRET for posttraumatic stress disorder (PTSD). Therefore, this meta-analysis investigated the efficacy of VRET for PTSD as compared to waitlist and active comparators. A literature search yielded nine controlled studies encompassing 296 participants (124 VRET, 172 controls). The differences between conditions regarding the primary outcome of PTSD symptom severity and the secondary outcome of depressive and anxiety symptoms post-treatment were calculated using Hedges’ g. Compared to waitlist controls, VRET showed a significantly better outcome for PTSD symptoms (g = 0.62, p = .017) and depressive symptoms (g = 0.50, p = .008). There was no significant difference between VRET and active comparators regarding PTSD symptoms (g = 0.25, p = .356) and depressive symptoms (g = 0.24, p = .340) post-treatment. No significant effects emerged for anxiety symptoms. These findings suggest that VRET may be as effective as active comparators for PTSD patients. However, the results must be interpreted with caution due to the limited number of trials and the substantial number of – predominantly male – military service members studied. Additional controlled trials, considering a wider range of trauma types and balanced gender, are required to strengthen the evidence.
HIGHLIGHTS
• There was evidence of a medium sized effect for VRET over WL for PTSD and depression.• No significant difference between VRET and active controls in reducing PTSD symptoms.• Results indicate no significant changes in anxiety after VRET.
Al contrario de las fobias específicas, para las cuales la Terapia de Exposición de Realidad Virtual (VRET en sus siglas en inglés) constituye un tratamiento efectivo, existe todavía incertidumbre con respecto a la utilidad de la VRET para el trastorno de estrés postraumático (TEPT). Por lo tanto, este meta-análisis investigó la efectividad de la VRET para el TEPT en comparación con la lista de espera y los comparadores activos. Una búsqueda de literatura arrojó nueve estudios controlados involucrando a 296 participantes (124 VRET, 172 controles). Las diferencias entre las condiciones con respecto al resultado principal de la severidad de los síntomas del TEPT y el resultado secundario de los síntomas depresivos y ansiosos luego del tratamiento, fueron calculados usando la g de Hedges. En comparación a los controles de lista de espera, la VRET mostró un resultado significativamente mejor para los síntomas del TEPT (g=0.62, p=.017) y los síntomas depresivos (g=0.50, p=.008). No hubo diferencias significativas entre la VRET y los comparadores activos con respecto a los síntomas del TEPT (g=0.25, p=.356) y los síntomas depresivos (g=0.24, p=.340) luego del tratamiento. No surgieron efectos significativos para los síntomas ansiosos. Estos hallazgos sugieren que la VRET podría ser tan efectiva como los comparadores activos para los pacientes con TEPT. Sin embargo, los resultados deben ser interpretados con cautela debido al número limitado de ensayos y el sustancial número de miembros del servicio militar –predominantemente hombres- estudiados. Ensayos controlados adicionales, que consideren un rango más amplio de tipos de trauma y balanceados en género, son requeridos para fortalecer la evidencia.
虚拟现实暴露疗法(VRET)可有效治疗特定恐惧症,与之相比,VRET对创伤后应激障碍(PTSD)的可用性仍不确定。因此,这项元分析研究了VRET对创伤后应激障碍的疗效,及其与与等候名单和有效对照疗法的比较。文献检索产生了9项对照研究,包括296名参与者(124名VRET,172名对照组)。使用Hedges’g计算关于接受不同治疗后PTSD症状严重程度的差异,以及抑郁和焦虑症状的差异。与等候名单对照组相比,VRET显示出PTSD症状(g = 0.62,p = .017)和抑郁症状(g = 0.50,p = .008)的显著更好的结果。 VRET和有效对照疗法之间在治疗后PTSD症状(g = 0.25,p = .356)和抑郁症状(g = 0.24,p = .340)上没有显著差异。对焦虑症状没有显著效果。这些发现表明,VRET可能与PTSD患者的有效对照疗法一样有效。但是,因为纳入试验数量有限,并且研究了大量以男性为主的军事服务人员,必须谨慎解释研究结果。需要进一步的对照试验以加强证据,未来的研究需要考虑更广泛的创伤类型和对参与者性别进行平衡。
1. Introduction
According to the Diagnostic and Statistical Manual of Mental Disorders (DSM-5, American Psychiatric Association [APA], Citation2013), posttraumatic stress disorder (PTSD) develops as a result of directly experiencing, witnessing, or being repeatedly exposed to aversive details of, a potentially traumatic event such as death, combat, sexual assault or serious injury. While a substantial number of people show considerable resilience post-exposure, up to a third of those confronted with a traumatic event subsequently develop clinically relevant PTSD symptoms such as re-experiencing, avoidance, hyperarousal and alterations in mood and cognitions (e.g. persistent, negative and distorted cognitions; Cusack et al., Citation2016).
A recent study (Koenen et al., Citation2017) reported a cross-national lifetime prevalence of PTSD of 3.9% in the general population and 5.6% among persons who had previously been exposed to a traumatic event. Furthermore, up to half of those diagnosed with PTSD tend to show persistent symptoms and an unremitting chronic course (Atwoli, Stein, Koenen, & McLaughlin, Citation2015; Cusack et al., Citation2016; Koenen et al., Citation2017). The disorder is associated with particularly high social and health care costs. In a sample of US veterans, the two-year social costs were estimated at $923 million (Kilmer, Eibner, Ringel, & Pacula, Citation2011), and in a representative sample of US women, the median annual health care costs lay at $1283 million (Walker et al., Citation2003). In light of these high costs, as well as the debilitating nature of the disorder, timely and effective intervention is paramount.
A broad range of psychological treatments exist for PTSD. Current reviews (e.g. Cusack et al., Citation2016) suggest that trauma-focused cognitive-behavioural therapy (CBT), cognitive processing therapy (CPT), cognitive therapy (CT), Eye Movement Desensitization and Reprocessing (EMDR), and narrative exposure therapy are efficacious. Moreover, exposure therapy has been found to be highly effective in reducing PTSD symptoms and is regarded as a first-line treatment for PTSD according to numerous guidelines (e.g. APA, Citation2017; International Society for Traumatic Stress Studies [ISTSS], Citation2018). The mechanisms underlying exposure therapy may be explained by the Emotional Processing Theory (Foa & Kozak, Citation1986), which states that a phobic fear structure is activated upon confrontation with trauma-relevant information. Accordingly, mechanisms for symptom reduction involve activation of the fear structure by means of (repeated) confrontation with a feared stimulus (imaginal or in vivo) to achieve habituation and extinction of the anxious reaction. Although emotional engagement is key to treatment outcome (Foa, Huppert, & Cahill, Citation2006), this is particularly difficult to achieve in the context of imaginal exposure, as many patients show problems with visualizing the traumatic event or related details (Rizzo & Shilling, Citation2018). In vivo exposure, in turn, poses the challenge of providing real-life stimuli that are suitable for a systematic, graded exposure (Bohil, Alicea, & Biocca, Citation2011).
A viable approach to overcoming these intricate problems is provided by virtual reality (VR) technology. VR offers multi-sensory cue representation in a highly interactive, ecologically valid and emotionally engaging virtual environment. It carries the advantages of increased control over stimuli, the possibility to repeat exposure infinitely, and the unique option to simulate environments that challenge the boundaries of everyday surroundings (Difede et al., Citation2007; Gamito et al., Citation2010; Miloff et al., Citation2016). Moreover, VR has been shown to be effective in inducing stress and anxiety reactions which are comparable to those observed in analogous real-life situations (e.g. Dibbets, Citation2019; Kothgassner et al., Citation2016). These characteristics have all contributed to the implementation of VR as a method for exposure therapy. In the past, virtual reality exposure therapy (VRET) has shown to be effective for a wide range of specific phobias (e.g. Morina, Ijntema, Meyerbröker, & Emmelkamp, Citation2015; Opriş et al., Citation2012; Parsons & Rizzo, Citation2008; Powers & Emmelkamp, Citation2008).
In contrast to specific phobias, data regarding the efficacy of VRET in PTSD patients are still inconclusive. Early studies on VRET in veterans with chronic, treatment-resistant PTSD (Rothbaum et al., Citation1999; Rothbaum, Hodges, Ready, Graap, & Alarcon, Citation2001) generally yielded promising outcomes for VR-based exposure, and a more recent qualitative review (Gonçalves, Pedrozo, Coutinho, Figueira, & Ventura, Citation2012) concluded that VRET is potentially useful in the treatment of different types of trauma. Similarly, VRET has been included in the ISTSS Guidelines (Citation2018), for instance as a treatment with emerging evidence for PTSD, although the guidelines also emphasize that while the evidence is promising, it is still insufficient to fully support VRET as a standard treatment. To date, most meta-analytic research on the efficacy of VRET has considered anxiety disorders in general, and the analyses have only included small numbers of PTSD related studies (Carl et al., Citation2019; Fodor et al., Citation2018; Opriş et al., Citation2012; Parsons & Rizzo, Citation2008; Powers & Emmelkamp, Citation2008), rendering it difficult to draw specific conclusions for PTSD. Two recently published meta-analyses on VRET for anxiety disorders found small (Fodor et al., Citation2018) and medium (Carl et al., Citation2019) effect sizes in favour of VRET versus control comparisons in terms of reducing PTSD symptom severity. Nevertheless, due to the small sample sizes of the included studies and the lack of active treatment controls, these results have to be regarded as a first step, and further evidence is needed.
In light of the aforementioned evidence, the objective of the current meta-analysis was to evaluate the efficacy of VRET for PTSD on the basis of existing controlled trials comparing VRET to waitlist control conditions as well as to active control conditions. We hypothesized that VRET would have a significant positive effect on outcome measures (i.e. overall symptom severity, anxiety and depression-related symptoms) relative to waitlist controls, as well as a comparable effect to active controls.
2. Method
2.1. Inclusion criteria and search strategy
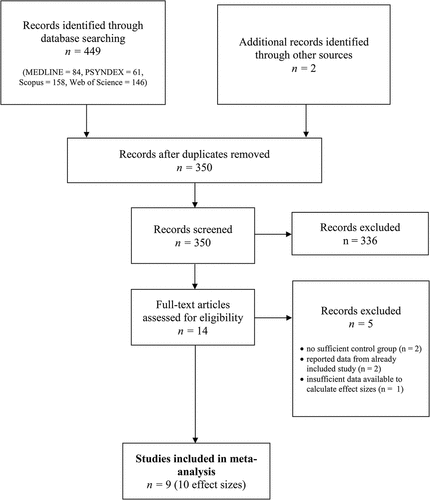
A search of MEDLINE, PSYNDEX, Scopus and Web of Science was conducted using the keywords ‘PTSD OR trauma AND VR’, ‘VR OR Virtual Reality AND PTSD’, ‘VRET OR Virtual Reality Exposure Therapy AND PTSD’ from the beginning of database records until December 2018. Studies were included in the meta-analysis if controlled trials evaluated the efficacy of VR-based treatment in samples with PTSD according to the DSM-IV, DSM-5, or the International Statistical Classification of Diseases and Related Health Problems; ICD-10. The primary outcome was PTSD symptom severity. Secondary outcomes included symptoms of depression and anxiety. All control group interventions were included in the present meta-analysis, and multi-group comparisons were coded separately. No further restrictions on age of participants, type of trauma, comorbidity, medication, and language or publication status were applied. In addition, Google Scholar alerts were enabled to ensure the inclusion of accepted articles and articles in preprint, and authors with a research focus on this specific field were contacted to ensure the inclusion of unpublished studies. The title, abstract, and main text of each study were examined independently by two authors (JXK and RLvE), and exclusions of studies occurred at each stage of the process (see ).
2.2. Data extraction and analysis
Data of included studies were entered into a spreadsheet independently by two authors (AG and RLvE). For each study included in the meta-analysis, we coded sample and intervention characteristics. The primary outcome was the standardized mean difference (Hedges’ g) in PTSD symptom severity. Secondary outcomes included Hedges’ g for depressive and anxiety symptoms as assessed via various self-report measures in VRET and control conditions post-intervention. Means, standard deviations and sample sizes were retrieved and entered into a spreadsheet. The calculations of the effect sizes and the subsequent meta-analysis were then conducted using the package metafor for R (Viechtbauer, Citation2010). Following general convention (Cohen, Citation1988), an effect size an effect size of 0.20 or above was considered a small effect, 0.50 or above a medium effect, and 0.80 or above a large effect. Random effects models were applied to estimate aggregated effect sizes. Effect sizes were based on completer samples due to a lack of intention-to-treat data (k = 2, McLay et al., Citation2017; Reger et al., Citation2016). Heterogeneity across study outcomes was reported with I2 values, where 0% to 40% might not be important, 30% to 60% may represent moderate heterogeneity, and 50% to 90% may represent substantial heterogeneity (Higgins & Green, Citation2011). All data and codes are stored on a repository of the Open Science Framework (doi:10.17605/OSF.IO/Y8U6G).
3. Results
The initial search yielded 451 articles. The titles and abstracts were screened for eligibility and full-text papers were obtained where necessary to evaluate their suitability for inclusion in further analyses. After screening, nine studies – eight peer-reviewed journal articles and one book chapter – were identified and included in the present meta-analysis. See for study characteristics and measures of included studies.
Table 1. Characteristics of the nine studies included in the meta-analysis.
Gamito et al. (Citation2010) used both waitlist and imaginal exposure therapy as control conditions; however, as only one participant provided pre- and post-scores of trauma severity in the imaginal therapy condition (personal communication, 22 October 2018), we only included the waitlist condition (n = 3) as the control group in our meta-analysis. The study by Roy et al. (Citation2010) was eligible to be included in our meta-analysis, but the authors reported the results of the clinical trial, focusing on efficacy, in a separate paper (Roy, Costanzo, Blair, & Rizzo, Citation2014). Therefore, we included Roy et al. (Citation2014) and not Roy et al. (Citation2010) in the meta-analysis.
Roy et al. (Citation2010, but not Citation2014) reported a significant reduction of anxiety scores in the VR treatment group over time. However, it was not possible to retrieve sufficient data to compute efficacy as compared to the control group. Hence, this study could not be included in our analysis of anxiety scores.
3.1. Study characteristics
In total, nine controlled trials were included with a total of 296 participants (124 in VR treatment conditions, 172 in control groups).
Regarding the control groups, five studies used exposure therapy controls (Cárdenas-López, de la Rosa-Gómez, Durán-Baca, & Bouchard, Citation2015; McLay et al., Citation2017, Citation2011; Reger et al., Citation2016; Roy et al., Citation2014; N = 100), four studies were waitlist controls (Difede et al., Citation2007; Gamito et al., Citation2010; Miyahira et al., Citation2012; Reger et al., Citation2016; N = 68), and one study used a present-centred control group (Ready, Gerardi, Backscheider, Mascaro, & Rothbaum, Citation2010, N = 4). The dropout rate was 34% for the VR conditions (20% for control conditions; 24% for active control conditions, 13% for waitlist controls), and none of the studies reported any treatment-related adverse effects (e.g. worsening of symptoms or attempted suicide) over the course of the studies. Similarly, no data were provided regarding dropouts due to adverse effects.
Seven studies included military servicemen/women, either in active duty or with veteran status. Two studies (Cárdenas-López et al., Citation2015; Difede et al., Citation2007) reported results from civilian victims or witnesses of violent crime as well as firefighters, disaster relief workers, and civilians with PTSD related to the September 11 attacks. Seven studies were conducted in the USA, one in Mexico (Cárdenas-López et al., Citation2015), and one in Portugal (Gamito et al., Citation2010).
In all included studies, the reduction in PTSD symptom severity was operationalized by the Clinician-Administered PTSD Scale (CAPS, Blake et al., Citation1995). Gamito et al. (Citation2010) additionally measured PTSD symptoms with the Impact of Events Scale-Revised (IES-R, Weiss & Marmar, Citation1996), Miyahira et al. (Citation2012) with the PTSD Diagnostic Scale (Foa, Cashman, Jaycox, & Perry, Citation1997), Cárdenas-López et al. (Citation2015) with the Posttraumatic Stress Symptom Scale – Self-Report version (PSS-SR, Foa, Riggs, Dancu, & Rothbaum, Citation1993), and Reger et al. (Citation2016) with the PTSD Checklist – Civilian version (PCL-C; Weathers, Litz, Herman, Huska, & Keane, Citation1993). We chose only to include the CAPS score in our meta-analysis, as it is widely used and has been proven to be psychometrically sound (Weathers, Keane, & Davidson, Citation2001).
Seven studies reported measures of depressive symptoms, with six studies operationalizing depressive symptoms using the Beck Depression Inventory (BDI; Beck, Ward, Mendelson, Mock, & Erbaugh, Citation1961) and one study (Gamito et al., Citation2010) using the depression subscale of the Symptom Checklist Revised (SCL-90-R; Derogatis, Citation1994). Three studies reported measures of anxiety symptoms, with Reger et al. (Citation2016) using the Beck Anxiety Inventory (BAI; Derogatis & Culpepper, Citation2004), Gamito et al. (Citation2010) the anxiety subscale of the SCL, and Cárdenas-López et al. (Citation2015) the state anxiety subscale of the State-Trait Anxiety Inventory (STAI; Spielberger, Citation1983; see for study characteristics and measures).
Three studies compared VR treatments to waitlist controls (Difede et al., Citation2007; Gamito et al., Citation2010; Miyahira et al., Citation2012) and five studies (Cárdenas-López et al., Citation2015; McLay et al., Citation2017, Citation2011; Ready et al., Citation2010; Roy et al., Citation2014) compared VR treatments to active control groups. One study (Reger et al., Citation2016) compared VR treatment to both a waitlist and active comparator; this study was thus included in both comparisons. In another study (Miyahira et al., Citation2012), participants in the control group received minimal attention, defined as ‘brief telephone contacts, every 2 weeks for 5 weeks to assess safety and to affirm their continued interest in study participation’ (p. 130). We therefore included this study in our comparisons to waitlist controls. Ready et al. (Citation2010) used present-centred therapy as a control condition, which does not comprise exposure therapy but rather involves nonspecific components of psychotherapy (e.g. psychoeducation, problem-solving techniques). McLay et al. (Citation2011) compared VR treatment to a treatment-as-usual control group, in which participants were able to choose freely from all services provided by Veterans Affairs facilities, including, for instance, prolonged exposure or cognitive-processing therapy. Another study (McLay et al., Citation2017) used a specific form of control exposure therapy, namely a treatment that is very similar to VR exposure but in which stimuli are viewed as still images on a computer and not via an immersive VR headset.
Sample sizes were very small across studies, with only two studies (McLay et al., Citation2017; Reger et al., Citation2016) reporting more than 10 participants having completed treatment in the VR condition. The participants’ mean age ranged from 28 to 64 years across studies. Included participants were predominantly male: Only one study had a sample that included 50% females (Cárdenas-López et al., Citation2015), while the remaining samples comprised 88% male participants or higher. Five studies (Cárdenas-López et al., Citation2015; Difede et al., Citation2007; Gamito et al., Citation2010; Ready et al., Citation2010; Reger et al., Citation2016) reported that medication of participants was held constant over the course of the treatments or that participants did not take any medication – the remainder of the studies did not report any details concerning medication. VRET treatments varied regarding the duration of individual sessions (min. 60 and max. 120 minutes) as well as the number of treatment sessions (see for study characteristics).
3.2. Efficacy of VRET regarding PTSD symptom severity
One hundred twenty-two participants (N = 54 in VR treatment, N = 68 in waitlist control groups, k = 4) were analysed to compare the efficacy of VRET and waitlist controls regarding PTSD symptom severity. The meta-analysis yielded an effect of g = 0.62 (CI 0.11 to 1.12) in favour of VR treatment over waitlist conditions. This effect was significant (p = .017) and heterogeneity was considered as not important (I2 = 29, Q(3) = 4.32, p = .229).
Furthermore, data of 204 participants (k = 6) were analysed to calculate the effect of VRET on PTSD symptom severity in comparison with active control groups (N = 100 in VRET conditions, N = 104 in active control conditions). The meta-analysis yielded a non-significant effect of g = 0.25 (CI −0.28 to 0.79, p = .356). Heterogeneity was significant and substantial for the six studies, I2 = 67 (Q(5) = 13.97, p = .016). A forest plot is provided in .
Figure 2. Forest plot of the standardized mean difference (Hedges’ g) in post-treatment CAPS scores of VR treatments compared to control conditions (waitlist and active control). A positive effect size indicates that the outcome was in favour of VR treatment. Average effect was calculated using a random-effects model.
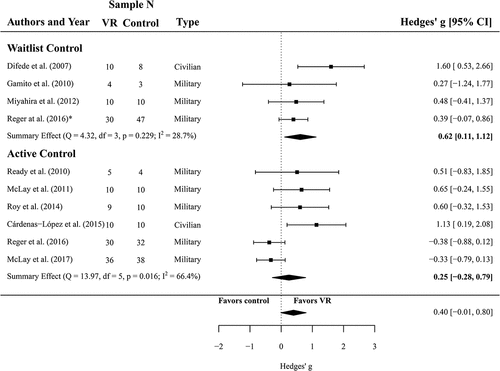
Figure 3. Forest plot of the standardized mean difference (Hedges’ g) in post-treatment depression scores of VR treatments compared to control conditions (waitlist and active control). A positive effect size indicates that the outcome was in favour of VR treatment. Average effect was calculated using a random-effects model.
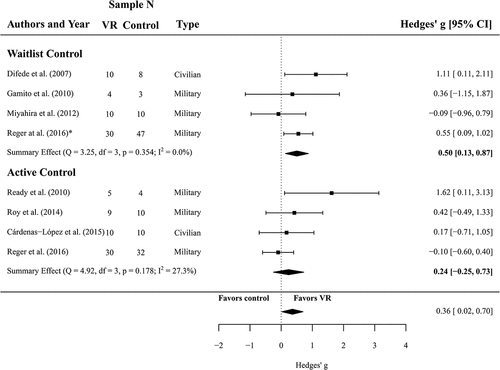
3.3. Efficacy of VRET regarding depressive symptoms
For the comparison between VRET and waitlist controls (N = 54 and N = 68, respectively, k = 4), we found a significant summary effect of g = 0.50 (CI 0.13 to 0.87, p = .008) and low, non-significant heterogeneity of I2 = 0 (Q(3) = 3.25, p = .354), all in favour of VRET. Four effect sizes from the data of 110 participants were analysed to compare the efficacy of VR treatment against active controls with respect to the reduction of depressive symptoms (N = 54 in VR treatment, N = 56 active controls). The meta-analysis yielded an effect of g = 0.24 (CI −0.25 to 0.39) and this comparison was not significant (p = .340). Heterogeneity was considered as not important and non-significant (I2 = 27, Q(3) = 4.92, p = .178) for these studies. A forest plot is provided in .
3.4. Efficacy of VRET regarding anxiety symptoms
Based on the data of 84 participants (N = 34 in VR treatment, N = 50 controls, k = 2) we compared VRET with waitlist controls regarding the reduction of anxiety symptoms. The meta-analysis revealed a non-significant effect of g = 0.47 (CI −0.60 to 1.54, p = .387).
For the comparison of VRET and active controls, the data of 82 participants (N = 40 in VR treatment, N = 42 controls, k = 2) was analysed to compare anxiety symptoms after VRET and active treatment. Again, no significant effect was found in this comparison: g = −0.26 (CI −0.70 to 0.18, p = .241).
3.5. Sensitivity analysis
To evaluate a potential sampling bias, we excluded the two studies that investigated civilian samples and re-analysed our main outcome (reduction of PTSD symptom severity) with the more homogenous samples of only military service members. This did not change any of our results, irrespective of control comparison (PTSD symptom severity: VRET vs. waitlist, k = 3, N = 132, g = 0.40, CI 0.01 to 0.80, p < .05; VRET vs. active control, k = 5, N = 243, g = 0.06, CI −0.43 to 0.54, p > .05). We further re-analysed our active control groups without the participants of Ready et al. (Citation2010) and McLay et al. (Citation2011), since these studies did not use exposure therapy in their control group and instead employed present-centred therapy and several other standard treatments. Likewise, this new analysis (with k = 4 and N = 223) did not significantly change the direction or magnitude of our results (g = 0.16, CI −0.54 to 0.86, p > .05).
3.6. Publication bias
Due to the small sample size of most preliminary or pilot studies evaluating the efficacy of VRET, the probability of traditional dissemination bias (i.e. non-publication of entire studies) should not be dismissed. The fact that most studies did not find significant effects in favour of VRET makes the occurrence of publication bias less likely. Furthermore, guidelines for conducting funnel plot asymmetry tests recommend the inclusion of at least 10 studies to maintain sufficient power for distinguishing chance from real asymmetry (Egger, Smith, Schneider, & Minder, Citation1997; Sterne et al., Citation2011). While it was therefore unfeasible to conduct Egger’s regression test (Sterne & Egger, Citation2005) or trim-and-fill analysis (Duval & Tweedie, Citation2000) in our analysis, it should be noted that the omission of results and selective reporting are well-documented (Dwan et al., Citation2008; Ioannidis, Munafo, Fusar-Poli, Nosek, & David, Citation2014) and are expected to be the main reason for bias in our meta-analysis.
4. Discussion
Given the need for stronger evidence regarding the added value of using VR for PTSD treatment, the current meta-analysis set out to systematically review relevant controlled trials and to analyse the efficacy of VRET in relation to waitlist controls and active controls. Our meta-analysis reveal encouraging preliminary findings regarding the efficacy of VRET compared to waitlist controls in terms of improving PTSD symptom severity, as well as symptoms of depression, but not anxiety. Effect sizes for PTSD symptom reduction in favour of VRET were medium (g = 0.62 for PTSD symptoms; g = 0.50 for depressive symptoms), and effects for treatment-related changes in anxiety symptoms were non-significant (g = 0.47, p = .387). These results are in line with previous research (Carl et al., Citation2019), but depict better outcomes than another meta-analysis (Fodor et al., Citation2018). Furthermore, our findings suggest that there is no significant difference between VRET and active controls regarding the improvement of PTSD symptoms (g = 0.25) and depressive symptoms (g = 0.24). Given that exposure therapy is suggested as a first-line treatment for PTSD (APA Guidelines, Citation2017; ISTSS Guidelines, Citation2018), VRET has been proposed as a potentially efficacious tool for exposure therapy in PTSD (Gonçalves et al., Citation2012; Opriş et al., Citation2012). However, our results illustrate that VRET has only medium effects or non-significant effects when compared to waitlist and active controls. In contrast, recent meta-analytic research (Cusack et al., Citation2016) found large effects for specific manualized therapies (e.g. CBT exposure therapy, CPT/CT, EMDR) when compared to waitlist (CBT exposure therapy: d = 1.16; CPT/CT: d = 1.56–1.61; EMDR: d = 1.37) as well as active controls (CBT exposure therapy: d = 1.79; CPT/CT: d = 0.60). This suggests a greater efficacy of traditional exposure therapy, CPT/CT or EMDR compared to VRET with respect to PTSD symptom severity improvement. In light of these findings, it seems appropriate to infer that these well-controlled therapies should be the first choice of treatment whenever feasible, with VRET providing an alternative in scenarios in which the opportunity for in vivo treatment, for instance, is lacking.
4.1. Applicability of findings
The generalizability and applicability of the current meta-analysis is limited by a number of factors. For instance, most studies examined active-duty military members or veteran samples with combat-related trauma. Only two (Cárdenas-López et al., Citation2015; Difede et al., Citation2007) of the nine studies included in this meta-analysis considered civilians, and among these, one study (Difede et al., Citation2007) had a mixed sample consisting not only of civilians but also of professionals such as firefighters and disaster relief workers who are all systematically trained to handle disaster scenarios. This constitutes a source of bias and prevents wide-ranging conclusions regarding the efficacy of VRET for PTSD, as it is unclear whether these results are applicable to all adults with PTSD. Military personnel have been observed to be less responsive to (traditional) PTSD treatment than other PTSD samples, with some studies showing null effects for therapy gains or even indicating a reverse outcome with an exacerbation of trauma-related symptoms post-intervention (Hammarberg & Silver, Citation1994; Rothbaum, Meadows, Resick, & Foy, Citation2000).
Additionally, these associations seem to be impacted by another factor which potentially shapes the treatment outcome: In our meta-analysis, participants’ age (MVR = 39.3; Mcontrol = 38.9) ranged widely, from 28 years (active-duty service members, McLay et al., Citation2011) to 63 years (veterans, Gamito et al., Citation2010). It has been speculated that younger adults – as representatives of the digital generation – may be more willing to try an innovative intervention approach like VR than older groups (Rizzo & Shilling, Citation2018). Hence, age should be investigated more carefully in future controlled studies in relation to its possible impact on treatment adherence and attrition. Similarly, patients’ preferences for certain interventions should be considered when evaluating the efficacy of PTSD treatments in general and of VRET in particular (Tarrier, Liversidge, & Gregg, Citation2006).
Another factor which might affect the generalizability of findings is gender. Studies included in this review predominantly included male participants. This is all the more relevant since female gender has consistently been shown to be a significant risk factor across different trauma types (Brewin, Andrews, & Valentine, Citation2000) and has been reported to be associated with higher PTSD-related health care costs compared to male gender (Walker et al., Citation2003). Moreover, the probability of being diagnosed with PTSD is twice as high for women than for men (Koenen et al., Citation2017). Hence, gender should be considered in the design of future trials.
Finally, regarding the effect of VRET on PTSD symptom severity, a high heterogeneity between active control trials was detected. A possible explanation for these heterogeneous findings might be that different active interventions were included in our analysis (e.g. stand-alone imaginal exposure, prolonged exposure, EMDR, CBT exposure and present-centred therapy). Furthermore, the included studies showed a high variability regarding the number of sessions and the duration of individual sessions. Due to small sample sizes, we were unable to control for the specific method applied, and had to subsume all of these interventions under the active control condition. Nevertheless, all of the studies in the active control condition – except for two (present-centred therapy, Ready et al., Citation2010; various treatment-as-usual; McLay et al., Citation2011) – used types of exposure therapy. Excluding the two studies using other treatments did not have a significant impact on the direction or magnitude of the effects. Notably, no study included in vivo exposure as an active comparator.
4.2. Limitations
One of the main limitations of the present meta-analysis pertains to the small sample sizes within the included studies and the restricted number of controlled trials eligible for meta-analysis. This limits our ability to draw any firm conclusions and is a major limitation. Furthermore, only one study (Difede et al., Citation2007) specified the type of concurrent medication, whereas the other studies either did not report any information about medication at all or did not provide details on the type, dosage, or duration. However, four studies (Difede et al., Citation2007; Gamito et al., Citation2010; Ready et al., Citation2010; Reger et al., Citation2016) did report that medication was stable during the intervention. Medication is considered as a next-line treatment by most guidelines on PTSD treatment, but may serve as a reasonable initial option if no first-line treatment is available. It should be noted that the pharmacological treatment of PTSD, especially in combination with a comorbid depressive disorder, is complex, and there are not yet any specific guidelines on this issue (e.g. American Psychological Association, Citation2017; for an overview see Cusack et al., Citation2016).
Similarly, none of the included studies provided any information about adverse treatment effects. This is a key issue as reporting adverse effects, and dropouts due to adverse effects, may foster the understanding not only of treatment efficacy but also of its effectiveness. In addition, some trials (e.g. McLay et al., Citation2011) examined participants with a long history of failed treatment approaches – both medical and psychological. On the one hand, this may limit cross-sample comparisons, while on the other hand, the reported positive treatment outcomes may suggest that treatment-resistant patients might particularly benefit from VRET (Difede et al., Citation2014). However, due to small sample sizes, it was not possible to consider these sample-specific factors in our analyses. Further studies are needed in order to evaluate a possible added value of VR-based approaches for treatment-resistant PTSD cohorts. Moreover, the included studies did not report long term follow-ups, which further limits the ability to draw conclusions about the treatment`s effectiveness.
Finally, due to the different control conditions and the low number of studies, we are unable to rule out or confirm the presence of a possible publication bias, as assessments of funnel plots and more advanced regression-based assessments are unfeasible in reviews with 10 or fewer studies (Dalton, Bolen, & Mascha, Citation2016). Therefore, the results of this meta-analysis have to be regarded as preliminary in nature. Furthermore, some papers using keywords outside of our search strategy, or grey literature published outside of peer-reviewed journals, may have been missed. A further limitation pertains to the substantial amount of heterogeneity within active controls regarding PTSD symptom severity (I2 = 66%). We were unable to analyse potential confounders (e.g. via meta-regression) due to the small sample of eligible studies and the resulting low power (Hempel et al., Citation2013).
4.3. Conclusion and future directions
This meta-analysis revealed a medium sized effect for VRET over waitlist controls and at least no significant difference between VRET and active interventions in terms of reducing PTSD symptom severity and depressive symptoms in adults with PTSD. However, VRET did not differ from controls in reducing anxiety symptoms, either for waitlist or for active comparators. Furthermore, VRET showed a lower efficacy in comparison to other meta-analyses (e.g. Cusack et al., Citation2016) which investigated improvement of PTSD symptoms due to in vivo exposure therapy. Past studies show that individuals differ in their ability to become immersed in or engaged with the virtual environment (e.g. Sacau, Laarni, & Hartmann, Citation2008; Weibel, Wissmath, & Mast, Citation2010), which may hinder positive treatment outcome. Hence, future studies should further investigate specific user characteristics such as personality traits (i.e. openness to experience or neuroticism) as they could potentially mediate treatment outcome in VRET.
In general, VRET constitutes an ecologically valid, safe and controlled environment for the induction of emotional, cognitive and behavioural as well as physiological reactions which are equivalent to those found in comparable in vivo surroundings (e.g. Dibbets, Citation2019; Kothgassner et al., Citation2016). In the past, high costs for the hardware and software of the respective systems may have limited the access to this technology. Nevertheless, as the affordability of VR systems has been increasing over the last decade (Rizzo & Shilling, Citation2018), clinical researchers may be more inclined to include and evaluate this tool in their routine therapy and thus improve accessibility for affected samples. It is, however, essential to note that VRET should not be used as a stand-alone tool and a trained therapist is always necessary to guide the process. By drawing on pre-programmed environments and stimuli, VRET has the potential for treatment standardization. This could be an interesting option to increase comparability across multiple trials in future studies.
Moreover, due to the rise of personalized medicine, future research should be encouraged to achieve a better understanding of the effect of moderating factors (e.g. age, gender) on the efficacy of VRET and to enable analyses of specific samples. In particular, children and adolescents with considerably high lifetime prevalence rates of PTSD should be included in trials evaluating VRET (Meiser-Stedman, Smith, Yule, Glucksman, & Dalgleish, Citation2017; Merikangas et al., Citation2010).
In sum, the evidence from the present meta-analysis was insufficient to assume efficacy of VRET for particular trauma types. While a considerable body of research supports the efficacy of VRET for the treatment of specific phobias (for an overview, see Carl et al., Citation2019; Fodor et al., Citation2018; Opriş et al., Citation2012), only a small number of controlled studies have investigated the efficacy of VRET in patients with PTSD. As such, we have to state that despite the moderately positive results of the present meta-analysis, more research is required to determine whether VRET constitutes a valuable tool for PTSD treatment.
Acknowledgments
The authors thank Sarah Mannion de Hernandez for correcting the English manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Studies included in the meta-analysis are denoted with an asterisk.
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Washington, DC: American Psychiatric Association.
- American Psychological Association. (2017). Clinical practice guideline for the treatment of posttraumatic stress disorder (PTSD). Retrieved from https://www.apa.org/ptsd-guideline/ptsd.pdf
- Atwoli, L., Stein, D. J., Koenen, K. C., & McLaughlin, K. A. (2015). Epidemiology of posttraumatic stress disorder: Prevalence, correlates and consequences. Current Opinion in Psychiatry, 28(4), 307–13.
- Beck, A. T., Ward, C. H., Mendelson, M., Mock, J., & Erbaugh, J. (1961). An inventory for measuring depression. Archives of General Psychiatry, 4, 561–571.
- Blake, D. D., Weathers, F. W., Nagy, L. M., Kaloupek, D. G., Gusman, F. D., Charney, D. S., & Keane, T. M. (1995). The development of a clinician-administered PTSD scale. Journal of Traumatic Stress, 8(1), 75–90.
- Bohil, C. J., Alicea, B., & Biocca, F. A. (2011). Virtual reality in neuroscience research and therapy. Nature Reviews Neuroscience, 12(12), 752–762.
- Brewin, C. R., Andrews, B., & Valentine, J. D. (2000). Meta-analysis of risk factors for posttraumatic stress disorder in trauma-exposed adults. Journal of Consulting and Clinical Psychology, 68(5), 748–766.
- *Cárdenas-López, G., de la Rosa-Gómez, A., Durán-Baca, X., & Bouchard, S. (2015). Virtual reality PTSD treatment program for civil victims of criminal violence. In P. Cipresso & S. Seriono (Eds.), Virtual reality: Technologies, medical applications and challenges (pp. 269–289). New York, NY: Nova Science.
- Carl, E., Stein, A., Levihn-Coon, A., Pogue, J., Rothbaum, B., Emmelkamp, P., … Powers, M. (2019). Virtual reality exposure therapy for anxiety and related disorders: A meta-analysis of randomized controlled trials. Journal of Anxiety Disorders, 61, 27–36.
- Cohen, J. (1988). Statistical power analysis for the behavioral sciences (2nd ed.). Hillsdale, NJ: Lawrence Erlbaum Associates.
- Cusack, K., Jonas, D. E., Forneris, C. A., Wines, C., Sonis, J., Middleton, J. C., … Gaynes, B. N. (2016). Psychological treatments for adults with posttraumatic stress disorder: A systematic review and meta-analysis. Clinical Psychology Review, 43, 128–141.
- Dalton, J. E., Bolen, S. D., & Mascha, E. J. (2016). Publication bias: The elephant in the review. Anesthesia and Analgesia, 123(4), 812–813.
- Derogatis, L. R. (1994). SCL-90-R: Administration, scoring and procedures manual. Minneapolis, MN: National Computer Systems.
- Derogatis, L. R., & Culpepper, W. J. (2004). Beck anxiety inventory. In M. E. Maruish (Ed.), The use of psychological testing for treatment planning and outcomes assessment: Instruments for adults (3rd ed., pp. 59–100). Mahwah, NJ: Lawrence Erlbaum.
- Dibbets, P. (2019). A novel virtual reality paradigm: Predictors for stress-related intrusions and avoidance behavior. Journal of Behavior Therapy and Experimental Psychiatry. doi:10.1016/j.jbtep.2019.01.001
- *Difede, J., Cukor, J., Jayasinghe, N., Patt, I., Jedel, S., Spielman, L., … Hoffman, H. G. (2007). Virtual reality exposure therapy for the treatment of posttraumatic stress disorder following September 11, 2001. Journal of Clinical Psychiatry, 68(11), 1639–1647.
- Difede, J., Cukor, J., Wyka, K., Olden, M., Hoffman, H., Lee, F. S., & Altemus, M. (2014). D-cycloserine augmentation of exposure therapy for post-traumatic stress disorder: A pilot randomized clinical trial. Neuropsychopharmacology, 39(5), 1052–1058.
- Duval, S., & Tweedie, R. (2000). A nonparametric „trim and fill” method of accounting for publication bias in meta-analysis. Journal of the American Statistical Association, 95, 89–98.
- Dwan, K., Altman, D. G., Arnaiz, J. A., Bloom, J., Chan, A. W., Cronin, E., … Williamson, P. R. (2008). Systematic review of the empirical evidence of study publication bias and outcome reporting bias. PLoS One, 3(8), e3081.
- Egger, M., Smith, G. D., Schneider, M., & Minder, C. (1997). Bias in meta-analysis detected by a simple, graphical test. BMJ, 315, 629–634.
- Foa, E., Cashman, L., Jaycox, L., & Perry, K. (1997). The validation of a self-report measure of PTSD: The posttraumatic diagnostic scale. Psychological Assessment, 9, 445–451.
- Foa, E. B., Huppert, J. D., & Cahill, S. (2006). Emotional processing theory: An update. In B. O. Rothbaum (Ed.), Pathological anxiety: Emotional processing in etiology and treatment (pp. 3–24). New York: Guilford Press.
- Foa, E. B., & Kozak, M. J. (1986). Emotional processing of fear: Exposure to corrective information. Psychological Bulletin, 99, 20–35.
- Foa, E. B., Riggs, D. S., Dancu, C. V., & Rothbaum, B. O. (1993). Reliability and validity of a brief instrument for assessing post-traumatic stress disorder. Journal of Traumatic Stress, 6, 459–473.
- Fodor, L. A., Coteț, C. D., Cuijpers, P., Szamoskozi, Ș., David, D., & Cristea, I. A. (2018). The effectiveness of virtual reality based interventions for symptoms of anxiety and depression: A meta-analysis. Scientific Reports, 8(1), 10323.
- *Gamito, P., Oliveira, J., Rosa, P., Morais, D., Duarte, N., Oliveira, S., & Saraiva, T. (2010). PTSD elderly war veterans: A clinical controlled pilot study. Cyberpsychology, Behavior, and Social Networking, 13(1), 43–48.
- Gonçalves, R., Pedrozo, A. L., Coutinho, E. S. F., Figueira, I., & Ventura, P. (2012). Efficacy of virtual reality exposure therapy in the treatment of PTSD: A systematic review. PLoS One, 7(12), e48469.
- Hammarberg, M., & Silver, S. M. (1994). Outcome of treatment for post‐traumatic stress disorder in a primary care unit serving Vietnam veterans. Journal of Traumatic Stress, 7(2), 195–216.
- Hempel, S., Miles, J. N., Booth, M. J., Wang, Z., Morton, S. C., & Shekelle, P. G. (2013). Risk of bias: A simulation study of power to detect study-level moderator effects in meta-analysis. Systematic Reviews, 2, 107.
- Higgins, J. P. T., & Green, S. (2011). Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. The Cochrane Collaboration. Retrieved from http://handbook.cochrane.org
- International Society for Traumatic Stress Studies. (2018). Posttraumatic stress disorder prevention and treatment guidelines: Methodology and recommendations. Retrieved from http://www.istss.org/treating-trauma/new-istss-prevention-and-treatment-guidelines.aspx
- Ioannidis, J. P., Munafo, M. R., Fusar-Poli, P., Nosek, B. A., & David, S. P. (2014). Publication and other reporting biases in cognitive sciences: Detection, prevalence, and prevention. Trends in Cognitive Sciences, 18, 235–241.
- Kilmer, B., Eibner, C., Ringel, J. S., & Pacula, R. L. (2011). Invisible wounds, visible savings? Using microsimulation to estimate the costs and savings associated with providing evidence-based treatment for PTSD and depression to veterans of operation enduring freedom and operation Iraqi freedom. Psychological Trauma: Theory, Research, Practice, and Policy, 3(2), 201–211.
- Koenen, K. C., Ratanatharathorn, A., Ng, L., McLaughlin, K. A., Bromet, E. J., Stein, D. J., … Kessler, R. C. (2017). Posttraumatic stress disorder in the world mental health surveys. Psychological Medicine, 47(13), 2260–2274.
- Kothgassner, O. D., Felnhofer, A., Hlavacs, H., Beutl, L., Palme, R., Kryspin-Exner, I., & Glenk, L. M. (2016). Salivary cortisol and cardiovascular reactivity to a public speaking task in a virtual and real-life environment. Computers in Human Behavior, 62, 124–135.
- *McLay, R. N., Baird, A., Webb-Murphy, J., Deal, W., Tran, L., Anson, H., … Johnston, S. (2017). A randomized, head-to-head study of virtual reality exposure therapy for posttraumatic stress disorder. Cyberpsychology, Behavior, and Social Networking, 20, 218–224.
- *McLay, R. N., Wood, D. P., Webb-Murphy, J. A., Spira, J. L., Wiederhold, M. D., Pyne, J. M., & Wiederhold, B. K. (2011). A randomized, controlled trial of virtual reality-graded exposure therapy for post-traumatic stress disorder in active duty service members with combat-related post-traumatic stress disorder. Cyberpsychology, Behavior, and Social Networking, 14, 223–229.
- Meiser-Stedman, R., Smith, P., Yule, W., Glucksman, E., & Dalgleish, T. (2017). Posttraumatic stress disorder in young children three years post-trauma: Prevalence and longitudinal predictors. The Journal of Clinical Psychiatry, 78(3), 334–339.
- Merikangas, K. R., He, J. P., Burstein, M., Swanson, S. A., Avenevoli, S., Cui, L., … Swendsen, J. (2010). Lifetime prevalence of mental disorders in US adolescents: Results from the national comorbidity survey replication–adolescent supplement (NCS-A). Journal of the American Academy of Child & Adolescent Psychiatry, 49(10), 980–989.
- Miloff, A., Lindner, P., Hamilton, W., Reuterskiöld, L., Andersson, G., & Carlbring, P. (2016). Single-session gamified virtual reality exposure therapy for spider phobia vs. traditional exposure therapy: Study protocol for a randomized controlled non-inferiority trial. Trials, 17(1), 60.
- *Miyahira, S. D., Folen, R. A., Hoffman, H. G., Garcia-Palacios, A., Spira, J. L., & Kawasaki, M. (2012). The effectiveness of VR exposure therapy for PTSD in returning warfighters. Studies in Health Technology and Informatics, 181, 128–132.
- Morina, N., Ijntema, H., Meyerbröker, K., & Emmelkamp, P. M. (2015). Can virtual reality exposure therapy gains be generalized to real-life? A meta-analysis of studies applying behavioral assessments. Behaviour Research and Therapy, 74, 18–24.
- Opriş, D., Pintea, S., García-Palacios, A., Botella, C., Szamosközi, Ş., & David, D. (2012). Virtual reality exposure therapy in anxiety disorders: A quantitative meta-analysis. Depression and Anxiety, 29(2), 85–93.
- Parsons, T. D., & Rizzo, A. A. (2008). Affective outcomes of virtual reality exposure therapy for anxiety and specific phobias: A meta-analysis. Journal of Behavior Therapy and Experimental Psychiatry, 39(3), 250–261.
- Powers, M. B., & Emmelkamp, P. M. (2008). Virtual reality exposure therapy for anxiety disorders: A meta-analysis. Journal of Anxiety Disorders, 22(3), 561–569.
- *Ready, D. J., Gerardi, R. J., Backscheider, A. G., Mascaro, N., & Rothbaum, B. O. (2010). Comparing virtual reality exposure therapy to present-centered therapy with 11 U.S. Vietnam Veterans with PTSD. Cyberpsychology, Behavior, and Social Networking, 13, 49–54.
- *Reger, G. M., Koenen-Woods, P., Zetocha, K., Smolenski, D. J., Holloway, K. M., Kevin, M., … Gahm, G. A. (2016). Randomized controlled trial of prolonged exposure using imaginal exposure vs. virtual reality exposure in active duty soldiers with deployment-related posttraumatic stress disorder (PTSD). Journal of Consulting and Clinical Psychology, 84, 946–959.
- Rizzo, A. S., & Shilling, R. (2018). Clinical virtual reality tools to advance the prevention, assessment, and treatment of PTSD. European Journal of Psychotraumatology, 8, 1414560.
- Rothbaum, B. O., Hodges, L., Alarcon, R., Ready, D., Shahar, F., Graap, K., … Baltzell, D. (1999). Virtual reality exposure therapy for PTSD Vietnam veterans: A case study. Journal of Traumatic Stress, 12(2), 263–271.
- Rothbaum, B. O., Hodges, L. F., Ready, D., Graap, K., & Alarcon, R. D. (2001). Virtual reality exposure therapy for Vietnam veterans with posttraumatic stress disorder. The Journal of Clinical Psychiatry, 62, 617–622.
- Rothbaum, B. O., Meadows, E. A., Resick, P., & Foy, D. W. (2000). Cognitive-behavioral therapy. In F. B. Foa, T. M. Keane, & M. Friedman (Eds.), Effective treatments for PTSD: Practice guidelines from the international society for traumatic stress studies (pp. 60–83). New York: Guilford.
- *Roy, M. J., Costanzo, M. E., Blair, J. R., & Rizzo, A. A. (2014). Compelling evidence that exposure therapy for PTSD normalizes brain function. Studies in Health Technology and Informatics, 199, 61–65.
- Roy, M. J., Francis, J., Friedlander, J., Banks-Williams, L., Lande, R. G., Taylor, P., … Rothbaum, B. (2010). Improvement in cerebral function with treatment of posttraumatic stress disorders. Annals of the New York Academy of Sciences, 1208, 142–149.
- Sacau, A., Laarni, J., & Hartmann, T. (2008). Influence of individual factors on presence. Computers in Human Behavior, 24(5), 2255–2273.
- Spielberger, C. D. (1983). Manual for the State–trait anxiety inventory. Palo Alto, CA: Mind Garden.
- Sterne, J. A., Sutton, A. J., Ioannidis, J. P., Terrin, N., Jones, D. R., Lau, J., … Higgins, J. P. (2011). Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ, 343, d4002.
- Sterne, J. A. C., & Egger, M. (2005). Regression methods to detect publication and other bias in meta-analysis. In H. R. Rothstein, A. J. Sutton, & M. Borenstein (Eds.), Publication bias in meta-analysis: Prevention, assessment and adjustments (pp. 99–110). New York, NY: Wiley.
- Tarrier, N., Liversidge, T., & Gregg, L. (2006). The acceptability and preference for the psychological treatment of PTSD. Behaviour Research and Therapy, 44(11), 1643–1656.
- Viechtbauer, W. (2010). Conducting meta-analyses in R with the metafor package. Journal of Statistical Software, 36, 1–48.
- Walker, E. A., Katon, W., Russo, J., Ciechanowski, P., Newman, E., & Wagner, A. W. (2003). Health care costs associated with posttraumatic stress disorder symptoms in women. Archives of General Psychiatry, 60(4), 369–374.
- Weathers, F., Litz, B., Herman, D., Huska, J., & Keane, T. (1993, October). The PTSD checklist (PCL): Reliability, validity, and diagnostic utility. Paper presented at the Annual Convention of the International Society for Traumatic Stress Studies, San Antonio, TX.
- Weathers, F. W., Keane, T. M., & Davidson, R. T. J. (2001). Clinician-administered PTSD scale: The first ten years of research. Depression & Anxiety, 13, 132–156.
- Weibel, D., Wissmath, B., & Mast, F. W. (2010). Immersion in mediated environments: The role of personality traits. Cyberpsychology, Behavior, and Social Networking, 13(3), 251–256.
- Weiss, D. S., & Marmar, C. R. (1996). The impact of event scale–revised. In J. Wilson & T. M. Keane (Eds.), Assessing psychological trauma and PTSD (pp. 399–411). New York, NY: Guilford.