ABSTRACT
Background: Insomnia is common in service members and associated with many mental and physical health problems. Recently, longitudinal data have been used to assess the impact of disturbed sleep on mental health outcomes. These studies have consistently shown relationships between sleep disturbance and development of mental illness.
Objective: The present study examined the longitudinal relationship between sleep disturbance and PTSD symptomatology in a cohort of Marines and Navy Corpsmen deployed to Iraq and Afghanistan (n = 2,404) assessed prior to deployment, as well as at −3 and 6 months post-deployment. Additionally, we aimed to investigate the extent to which these relationships are moderated by combat-stress severity, and to what extent these findings are replicated in a second, separate cohort of Marines and Navy corpsmen (n = 938) assessed with identical measures prior to deployment and within 3 months of return.
Method: The present study employed latent variable path models to examine the relationships between pre-deployment sleep disturbance and post-deployment re-experiencing symptoms. Initial cross-lagged path models were conducted on discovery and replication samples to validate the hypothesized predictive relationships. Follow up moderation path models were then conducted to include the effect of combat-stress severity on these relationships.
Results: Initial cross-lagged models supported a significant relationship between pre-deployment sleep disturbance and future re-experiencing PTSD symptoms at all time points. Initial moderation models showed a small moderator effect of combat-stress severity, though the main predictive relationship between pre-deployment sleep disturbance and PTSD symptoms remained significant. The moderator effect was not significant in the replication sample.
Conclusions: The results of this study support pre-deployment sleep disturbance as a risk factor for development of post-deployment PTSD symptoms. Interventions aimed at normalizing sleep may be important in preventive measures for PTSD.
HIGHLIGHTS
• This study examined the predictive relationships between sleep disturbance and PTSD symptoms in military personnel.• Results demonstrated that sleep disturbance prior to combat deployment is related to the development of PTSD symptoms following return home.• These results suggest that addressing sleep disturbance prior to deployment may be important in preventing development of PTSD in military service members.
Antecedentes: El insomnio es común en los miembros del servicio y está asociado con muchos problemas de salud mental y física. Recientemente, se han utilizado datos longitudinales para evaluar el impacto de las alteraciones del sueño en los resultados de salud mental. Estos estudios han demostrado consistentemente las relaciones entre las alteraciones del sueño y el desarrollo de enfermedades mentales.
Objetivo: El presente estudio examinó la relación longitudinal entre el trastorno del sueño y la sintomatología del TEPT en una cohorte de infantes y médicos de Marina desplegados en Irak y Afganistán evaluados antes del despliegue, así como a los 3 y 6 meses posteriores al despliegue. Además, nuestro objetivo fue investigar hasta qué punto estas relaciones son moderadas por la gravedad del estrés de combate, y en qué medida estos hallazgos se replican en una segunda cohorte separada de marinos y médicos de la Marina evaluados con medidas idénticas antes del despliegue y dentro de los 3 meses de regreso.
Método: El presente estudio empleó modelos de trayectorias de variables latentes para examinar las relaciones entre la alteración del sueño previa al despliegue y los síntomas de reexperimentación posterior al despliegue. Se llevaron a cabo modelos iniciales de trayectorias de retardo cruzadas en muestras de descubrimiento y replicación para validar las relaciones predictivas hipotéticas. Los modelos de seguimiento de las trayectorias de moderación se llevaron a cabo para incluir el efecto de la gravedad del estrés de combate en estas relaciones.
Resultados: Los modelos iniciales de retardo cruzado respaldaron una relación significativa entre la alteración del sueño previa al despliegue y los síntomas futuros de TEPT que se vuelven a experimentar en todos los momentos. Los modelos de moderación iniciales mostraron un efecto moderador pequeño de la gravedad del estrés de combate, aunque la principal relación predictiva entre la alteración del sueño previa al despliegue y los síntomas de TEPT se mantuvo significativa. El efecto moderador no fue significativo en la muestra de replicación.
Conclusiones: Los resultados de este estudio respaldan que la alteración del sueño previa al despliegue es un factor de riesgo para el desarrollo de síntomas de TEPT posterior al despliegue. Intervenciones dirigidas a normalizar el sueño pueden ser importantes en cuanto a medidas preventivas para el TEPT.
背景:失眠在服役人员中常见且与许多身心健康问题有关。最近,纵向数据被用于评估睡眠障碍对心理健康结果的影响。这些研究一致揭示了睡眠障碍与精神疾病发展的关系。
目的:本研究考查了被派遣到伊拉克和阿富汗的海军医护兵在部署前和部署后3个月、6个月的睡眠障碍和创伤后应激障碍症状之间的纵向关系。此外,我们旨在考查这些关系在多大程度上受到战斗应激严重程度的影响。并通过让另一个海军医护兵独立群体在其部署前和在回国后3个月内接受相同测评考查这些结果在多大程度上能够重复。
方法:本研究采用潜变量路径模型来考查部署前睡眠障碍与部署后再体验症状之间的关系。在探索样本和重复样本上应用初始交叉滞后路径模型以验证假设的预测关系。随后在调节路径模型中将战斗应激严重程度对这些关系的影响纳入。
结果:初始交叉滞后模型在所有时间点都支持了部署前睡眠障碍与未来出现再体验这一PTSD症状之间显著相关。虽然部署前睡眠障碍对创伤后应激障碍症状的主预测关系仍然显著,初始调节模型显示战斗应激严重程度的调节效应较小。调节效应在重复样本中不显著。
结论:本研究的结果支持部署前睡眠障碍作为部署后PTSD症状发展的一个风险因子。旨在使睡眠正常化的干预在创伤后应激障碍的预防措施中可能很重要。
PALABRAS CLAVES:
1. Introduction
Sleep is an essential, phylogenetically conserved biological function that is in part genetically determined, but that is impacted as well by an array of non-genetic environmental factors, such as shift work, long-distance travel, noise, physical and emotional stress (Lane et al., Citation2017; Stein et al., Citation2018). Individual variation in insomnia, and sleep-related traits such as sleep duration and quality, are of high medical interest, as sleep disturbance is both common and ubiquitous worldwide, and is associated with a range of negative physical and mental health outcomes, and with increased mortality (Bramoweth & Germain, Citation2013; Irwin & Opp, Citation2017; Roth et al., Citation2011). Sleep disturbance can be characterized by the presence of insomnia symptoms that are bothersome and result in daytime consequences (i.e. fatigue and depressed mood), and/or presence of dissatisfaction with quality or quantity of sleep.
A growing body of research has shown that USA (US) military personnel are at elevated risk for insufficient sleep and poor sleep quality, with rates of sleep disturbance higher than rates in civilians (Klingaman, Brownlow, Boland, Mosti, & Gehrman, Citation2018; RAND Corporation; Citation2015). These research findings are consistent with results of sleep study in Canadian and European military cohorts, although mixed results have also been reported (Danker-Hopfe et al., Citation2017; Pettersson, Saers, Lindberg, & Janson, Citation2016; Reijnen, Rademaker, Vermetten, & Geuze, Citation2015; Richardson, Thompson, & King et al., Citation2017). Based on a recent study that assessed pre-deployment psychosocial variables and insomnia complaints (defined as an Insomnia Severity Index ≥15; Morin, Belleville, Belanger, & Ivers, Citation2011) in soldiers located at Ft. Hood, clinically significant sleep disturbance occurred in about one of five of the soldiers prior to their combat deployment and was associated with a wide array of psychosocial stressors and mental and physical health problems (Taylor et al., Citation2016). These findings are similar to those observed in soldiers with sleep disturbance in the larger Army STARRS study (n = 21,499) (Klingaman et al., Citation2018; Taylor et al., Citation2016). As noted in both studies, in comparison to civilians, military service members face a number of environmental stressors and challenges that may place them at increased risk for sleep disturbance including, but not limited to, frequent overnight and early-morning shift work, exposure to deployment stress, frequent changes in duty assignments, and in duty station (Klingaman et al., Citation2018; Taylor et al., Citation2016).
Over the past decade, longitudinal data have begun to be used prospectively to assess the impact of sleep disturbance on mental health outcomes. Millennium Cohort researchers showed that not only were insomnia complaints significantly associated with poorer physical health and poorer function (i.e. more lost work days, lower odds of deployment, earlier discharge, and more health-care utilization) in a broad range of active duty military personnel, but that the personnel with poorer pre-deployment sleep quality were more likely to (newly) screen positive for mental health disorders after a deployment (Gehrman et al., Citation2013; Seelig et al., Citation2016). Similarly, based on prospective evaluation of a National Guard cohort, Koffel et al., showed that pre-deployment daytime and nighttime sleep complaints significantly contributed to PTSD and depression severity up to 2 years after deployment. However, sleep disturbance did not predict substance use, which together led to suggestion that sleep disturbance is a risk factor for internalizing disorders (e.g. PTSD and depression) but not externalizing disorders (Koffel, Polusny, Arbisi, & Erbes, Citation2013). In 453 Dutch service members who were assessed before deployment to Afghanistan, nightmares, but not insomnia complaints, predicted PTSD at 6-months post-deployment (van Liempt, van Zuiden, Westenberg, Super, & Vermetten, Citation2013). However, given that the study researcher used six questions extracted from the Symptom Checklist (SCL-90) and the Self-Reported Inventory for posttraumatic stress disorder (SRIP) to assess the pre-deployment parameters, overlap in the pre-deployment comparator and outcome variables may have been a study limitation. However, more recently, a well-designed prospective analysis of soldier participants of the Army STARRS study (n = 4,645) showed pre-deployment insomnia to be a significant risk contributor to post-deployment PTSD and to suicidal ideation after controlling for relevant baseline and deployment-related factors (Wang et al., Citation2019). And, Biddle et al. used a prospective design in a general civilian population of sleep disturbance to control for study confounders (i.e. individual demographics, traits and past and current mental health as covariates in the analysis) in such a way as to disentangle the association between prior sleep disruption (insomnia) and subsequent development of mental illness (Biddle, Kelly, Hermens, & Glozier, Citation2018). The authors found that sleep disturbance maintained a consistent predictive relationship with future mental illness unaccounted for by its association with past and current mental illness symptoms.
Here we aimed to examine if deployment-related PTSD symptomology can be predicted prospectively by sleep disturbances soon after the return from deployment and/or even as early as before deployment, and to replicate currently published findings. To that end, the present study aim is to examine the longitudinal relationship between sleep disturbance and PTSD symptomatology in a cohort of Marines and Navy Corpsmen deployed to Iraq and Afghanistan who were assessed prior to deployment, as well as 3 and 6 months after their return home. Additionally, our aim is to investigate the extent to which these relationships are moderated by combat-stress severity. Further, we have sought to replicate these findings using a second, separate cohort of Marines and Navy corpsmen assessed with identical measures prior to deployment and within 5–7 months of return from combat. As sleep disturbance is a fundamental aspect of PTSD as a syndrome, prospective associations between sleep disturbance and future PTSD diagnosis or total symptom rating scores may contain an element of confound due to associations between sleep disturbance and related symptoms at both time points. To address this, we employed a principal components analysis as an empirical method to separate symptoms that were highly related to sleep disturbance from less related symptoms. Thus, here we focus on prediction of re-experiencing symptoms, which emerged as the PTSD symptom cluster most distinct from those symptoms associated with sleep.
2. Methods
2.1. Participants
Data were extracted from a prospective longitudinal study of Marines and Navy corpsmen stationed in southern California (data collected 2008–2013). For the initial analysis, 2,594 participants completed the relevant assessment measures. Of these, 190 were excluded due to elevated PTSD and/or depression symptoms prior to deployment (see below for symptom-based exclusion criteria). Thus, 2,404 participants were included in the analysis. All participants were recruited from Infantry battalions, which were all male at the time of data collection; the pre-deployment mean age was 22.77 (SD = 3.52). Assessments were gathered as part of a larger study aimed at identifying markers of risk and resilience for combat-stress injuries (Marine Resiliency Study (MRS); Baker, Nash, & Litz et al., Citation2012). Participants were assessed approximately 1 month prior to a 7-month deployment to Iraq or Afghanistan, and again at 3 and 6 months following return from deployment. For the replication analysis, data were collected from a separate cohort of 1,040 additional participants (2012 to 2013). Of these, 102 were excluded due to elevated PTSD or depression symptoms prior to deployment. Thus, 938 participants were included in the replication analysis. Being recruited from Infantry battalions, all participants were (again) male, with a mean age at pre-deployment of 21.87 (SD = 2.76). Longitudinal assessments for the replication cohort were assessed on average 4 weeks (SD = 4.9) prior to deployment and 22 weeks (SD = 22.4) weeks following deployment (Marine Resiliency Study II; Mooreet al., Citation2017) This study was approved by the institutional review boards of the University of California, San Diego; the Veterans Affairs San Diego Research Service; and the Naval Health Research Centre and written informed consent were obtained from all participants.
2.2. Measures
2.2.1. All measures were completed at each time period
2.2.1.1. PTSD
Posttraumatic stress disorder symptoms were assessed using the Clinician Administered PTSD Scale, DSM-IV Version (CAPS; Blake et al., Citation1995). The CAPS is a structured interview used to make a categorical PTSD diagnosis with a total score scale ranging from 0 to 136. PTSD diagnosis at pre-deployment was assessed using the partial PTSD criteria articulated by Stein, Walker, Hazen, and Forde (Citation1997) and used in past research with this sample (Acheson et al., Citation2015). Partial criteria, rather than full diagnostic, criteria were used due to the relative health of the sample. Criteria for a diagnosis of ‘partial PTSD’ were the presence of at least 1 B symptom (generally re-experiencing), 2 C symptoms (generally avoidance), and 2 D symptoms (generally hyperarousal). Interrater reliability in the MRS sample was high for both CAPS total score (ICC = .99) and PTSD diagnosis (kappa = .714). All interviews were conducted by study personnel who were trained, certified, and supervised by a licenced psychiatrist (DGB; Baker et al., Citation2012).
2.2.1.2. Combat stress
Stressful experiences during combat were assessed at approximately 1-month following return from deployment with four scales from the Deployment Risk and Resilience Inventory-2 (DRRI-2; Vogt et al., Citation2013; Post-Battle Experiences, Combat Experiences, Deployment Concern, and Difficult Living and Work Environment). A composite score was created from standardized scores on each subscale, with a mean score approximating zero (Glenn et al., Citation2017).
2.2.1.3. Depression
Depression symptoms were assessed using the Beck Depression Inventory 2 (BDI-2; Beck, Steer, Ball, & Ranieri, Citation1996). The BDI-2 measures the presence of depressive symptoms during the past 2 weeks with a total score scale ranging from 0 to 63. Consistent with previous research using the MRS cohort (Acheson et al., Citation2015), pre-deployment depression was defined as a BDI-2 score greater than or equal to 20 to indicate the presence of at least moderate depression. For the analyses, the BDI insomnia/hypersomnia item was recoded so that only insomnia was scored.
2.2.1.4. Anxiety
Anxiety of symptoms was assessed using the Beck Anxiety Inventory (BAI; Beck & Steer, Citation1993). The BAI measures the presence of anxiety symptoms during the past week and effectively distinguishes between anxiety vs. depressive symptoms (Clark, Steer, & Beck, Citation1994).
2.2.1.5. Dissociative symptoms
Peritraumatic dissociative symptoms were assessed with the Peritraumatic Dissociative Experiences Scale (PDEQ; Agorastos et al., Citation2013). This measure has a range of 0–50, with higher score representing increased dissociation.
2.2.1.6. Affectivity
Affect was measured with the Positive and Negative Affect Schedule (PANAS; Watson, Clark, & Tellegen, Citation1988). The PANAS is a self-report questionnaire which measures both positive and negative affect over the past week. It has been assessed to have high reliability and validity (Crawford & Henry, Citation2004).
2.2.1.7. General health
The Short Form Health Survey is a reliable and valid short measure of general health (SF-12; Ware, Kosinski, & Keller, Citation1996). The questions are combined, scored, and weighted to give an overall picture of physical health, mental health, and overall health-related quality of life.
2.2.2. Item selection by principal components analysis
As sleep disturbance is a fundamental symptom of PTSD, assessments of the relationship between sleep and PTSD symptoms may be confounded due to variance associated with sleep being present in both constructs. Thus, it was necessary to assure that indicators of sleep disturbance and PTSD represented maximally distinct constructs. To this end, we employed a principal components analysis (PCA) on the initial sample with varimax rotation on all 102 individual measure items to identify those items which were indicators of PTSD symptoms but that did not load on the same component as items indicative of sleep disturbance.
Full details of the PCA are included in supplemental materials. In short, a nine-component solution was identified which accounted for 38% of variance in items. A six-item component was identified that we label ‘re-experiencing symptoms’ as this component contained all 5 items of the CAPS representing the re-experiencing cluster of symptoms as well as an item indexing efforts to avoid trauma reminders. No items indexing sleep disturbance loaded onto this component. The three items indexing sleep disturbance loaded onto a larger factor containing a number of items from different measures which all appeared to index domains of behavioural/emotional hyperactivation/arousal. As this component also included CAPS items indexing additional avoidance and hyperarousal symptoms, these items were not included in the current analysis in order to avoid conflating PTSD symptom and sleep disturbance constructs. Thus, we reduced our data set from the full 102 items to 3 items serving as indicators of the construct ‘sleep disturbance’ (BDI insomnia; CAPS average hours of sleep; CAPS difficulty falling or staying asleep), and 6 items from the CAPS serving as indicators of the construct ‘re-experiencing symptoms’ (B1: intrusive thoughts; B2: nightmares; B3 flashbacks; B4: cue distress; B5: cue reactivity; C1: avoidance of thoughts/feelings/conversations). Thus, only these items were examined in our predictive models given the results of the PCA and the scope of our analytic aims. Of note, the constructs applied to our predictive models represented continuous self-report ratings of symptoms, and DSM-derived diagnosis of PTSD or Insomnia Disorder was not determined.
2.3. Data analysis
2.3.1. Cross-lagged panel model
Structural Equation Modelling (SEM) was employed to build an autoregressive cross-lagged panel model exploring the longitudinal relationships among constructs (Sleep Disturbance and Re-Experiencing Symptoms) using latent variables. Model parameters were estimated using SPSS AMOS software. Individual measure items identified via PCA were used as indicators of latent constructs. Model fit was assessed using the Comparative Fit Index (CFI) as well as the Root Mean Square Error of Approximation (RMSEA) criteria. CFI values in the range of .9 to .95 and above, and RMSEA values of .08 to .06 and below are generally considered indicators of acceptable model fit (Hooper, Coughlan, & Mullen, Citation2008).
2.3.2. Moderation analyses
In response to the results of our original cross-lagged panel model, two additional path models were constructed to examine the effect of pre-deployment sleep on re-experiencing symptoms at both 3 and 6 months post-deployment separately. Additionally, the moderating effect of combat-stress severity was included in these models using established guidelines for constructing interaction terms in SEM (Little, Bovaird, & Widaman, Citation2006). The replication model included pre and post-deployment time points. CFI and RMSEA criteria were again used to assess model fit.
3. Results
3.1. Initial cross-lagged panel model
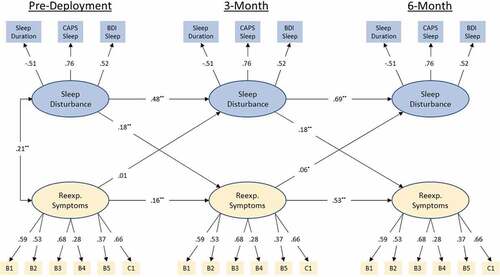
The cross-lagged panel model with standardized path coefficients can be seen in . Model fit was good with a CFI = .97 and RMSEA = .025 (90%CI .022 – .028). Sleep disturbance at baseline was a significant predictor of re-experiencing symptoms at 3-month and sleep disturbance at 3-month was a significant predictor of re-experiencing symptoms at 6-month (βs = .18; ps<.001). Re-Experiencing symptoms at pre-deployment were not significant predictors of sleep disturbance at 3-month, though re-experiencing symptoms at 3-month did predict sleep disturbance at 6-month. However, this effect was relatively small (βs<.07; p < .05).
Figure 1. Standardized coefficients for autoregressive cross-lagged panel model on the initial (MRS I) sample. Observed variables are indicated by rectangular nodes, while latent constructs are indicated by oval-shaped nodes. Observed variables B1-C1 indicate specific items from Clinician Administered PTSD Scale (CAPS), DSM IV version (see PCA results for specific items). Modelled correlated error for repeated measures has been omitted for clarity of presentation (values range .04 – .33). Modelled correlated error between latent constructs at 3 and 6-month time points has likewise been omitted for clarity (values .4 and .33, respectively). * = p < .05, ** = p < .001.
3.2. Replication of cross-lagged panel model
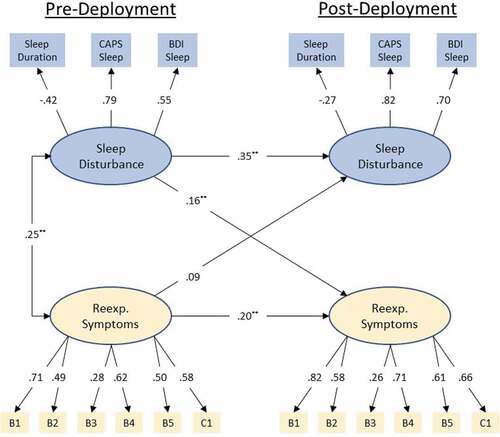
The replicated cross-lagged panel model with standardized path coefficients can be seen in . Model fit was again good with a CFI = .95 and RMSEA = .04 (90%CI .035 – .046). Sleep disturbance at Pre-Deployment was a significant predictor of re-experiencing symptoms at Post-Deployment (β = .17; p < .001). Re-Experiencing symptoms at pre-deployment were not a significant predictor of sleep disturbance Post-Deployment (βs = .08; p < .06).
Figure 2. Standardized coefficients for the replicated (MRS II) autoregressive cross-lagged panel model. Observed variables are indicated by rectangular nodes, while latent constructs are indicated by oval-shaped nodes. Observed variables B1-C1 indicate specific items from Clinician Administered PTSD Scale (CAPS) DSM IV version (see PCA results for specific items). Modelled correlated error for repeated measures has been omitted for clarity of presentation (values range −.03 – .43). Modelled correlated error between latent constructs at post-deployment has likewise been omitted for clarity (.35). ** = p < .001.
3.3. Initial moderation models
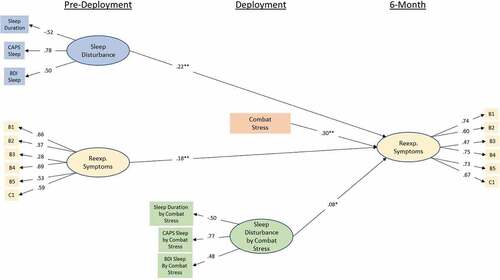
The moderation path models can be seen in and . Model fit was good for both models with CFIs of .96 and .94 and RMSEAs of .03 (90%CI .027 – .033) and .032 (90%CI .029 – .035). For the 3-month model, pre-deployment sleep disturbance continued to be a significant predictor of re-experiencing symptoms at 3-month (β = .15; p < .001), and combat-stress severity was also a significant predictor of symptoms (β = .39; p < .001). Additionally, there was a significant effect of the interaction terms such that the relationship between sleep disturbance re-experiencing symptoms was stronger in the presence of greater combat-stress severity (β = .06; p < .04). A similar pattern of results was obtained in the 6-month model, with pre-deployment sleep disturbance (β = .22; p < .001) and combat-stress severity (β = .3; p < .001) predicting re-experiencing symptoms at 6-month. Further, the moderating effect of combat-stress severity on the relationship between pre-deployment sleep disturbance and re-experiencing symptoms at 6-month remained (β = .08; p < .02).
Figure 3. Standardized coefficients for the initial (MRS I) moderation model-predicting re-experiencing symptoms at 3-month from re-experiencing symptoms at pre-deployment, sleep disruption at pre-deployment, combat-stress experience, and the interaction between pre-deployment sleep disruption and combat stress. Observed variables are indicated by rectangular nodes, while latent constructs are indicated by oval-shaped nodes. Observed variables B1-C1 indicate specific items from Clinician Administered PTSD Scale (CAPS) DSM IV version (see PCA results for specific items). Indicators of the interaction construct represent the residual of the product of the two items multiply regressed on both individual items in order to isolate variance accounted for by the interaction. * = p < .05, ** = p < .001.
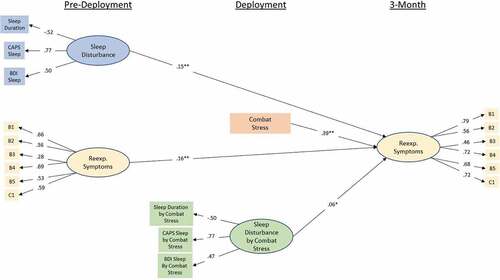
Figure 4. Standardized coefficients for the initial (MRS I) moderation model-predicting re-experiencing symptoms at 6-month from re-experiencing symptoms at pre-deployment, sleep disruption at pre-deployment, combat-stress experience, and the interaction between pre-deployment sleep disruption and combat stress. Observed variables are indicated by rectangular nodes, while latent constructs are indicated by oval-shaped nodes. Observed variables B1-C1 indicate specific items from Clinician Administered PTSD Scale (CAPS) DSM IV version (see PCA results for specific items). Indicators of the interaction construct represent the residual of the product of the two items multiply regressed on both individual items in order to isolate variance accounted for by the interaction. * = p < .05, ** = p < .001.
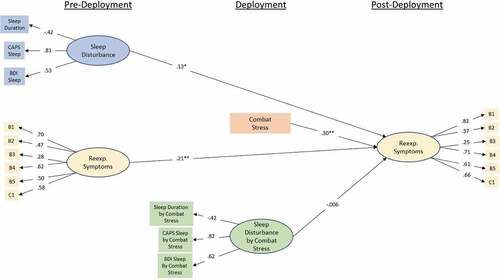
3.4. Replication of moderation model
The replication moderation path model can be seen in . Model fit was good with CFI of .91 and RMSEA of .05 (90%CI .041 – .051). Pre-deployment sleep disturbance was a significant predictor of re-experiencing symptoms at post-deployment (β = .13; p < .002), and combat-stress severity was also a significant predictor of symptoms (β = .30; p < .001). However, there was not a significant effect of the interaction term in the replication model (β = −.006; p = .89).
Figure 5. Standardized coefficients for the replicated (MRS II) moderation model predicting re-experiencing symptoms at post-deployment from re-experiencing symptoms at pre-deployment, sleep disruption at pre-deployment, combat-stress experience, and the interaction between pre-deployment sleep disruption and combat stress. Observed variables are indicated by rectangular nodes, while latent constructs are indicated by oval-shaped nodes. Observed variables B1-C1 indicate specific items from Clinician Administered PTSD Scale (CAPS) DSM IV version (see PCA results for specific items). Indicators of the interaction construct represent the residual of the product of the two items multiply regressed on both individual items in order to isolate variance accounted for by the interaction. ** = p < .001.
4. Discussion
The present large-scale study builds upon recent work to further elucidate the longitudinal sleep disturbance-PTSD symptomology relationship among military personnel. Our findings illustrate that sleep disturbance prior to potential trauma exposure (i.e. military combat deployment) predicts re-experiencing symptoms at 3-months post-deployment, and that sleep disturbance at 3 months predicts re-experiencing symptoms 6 months post-deployment. In the larger cohort, the relationship between pre-deployment sleep disturbance and future re-experiencing symptoms at both time points was moderated by the severity of combat exposure, though this effect was modest. However, this moderator effect was not found in our replication sample. The lack of a moderator effect in the replication sample may have been due to either its smaller size (n = 2,404 vs. 1,040) or to differences in the post-deployment assessment time points (3 and 6 months post-deployment vs. about 5–7 months post-deployment). Our findings failed to support a robust relationship between re-experiencing symptoms and future sleep disturbance at any time point, thus illustrating sleep disturbance to be a fundamental mechanism in PTSD emergence which is only moderately altered by subsequent trauma exposure.
Our findings demonstrate that pre-deployment sleep disturbance predicts subsequent re-experiencing symptoms and, thus, may be implicated in the development of core PTSD symptoms. Our findings align with those of extant studies investigating pre-trauma sleep impairment and subsequent development of psychiatric illnesses, such as PTSD (Biddle et al., Citation2018; Bryant, Creamer, O’Donnell, Silove, & McFarlane, Citation2010; Gehrman et al., Citation2013; Wang et al., Citation2019). As per the diathesis-stress conceptualization of PTSD (McKeever & Huff, Citation2003), pre-deployment sleep disturbance may thus predispose to post-deployment development of re-experiencing symptoms following onset of trauma experienced during military combat.
The genetic and biological mechanisms responsible, and their relationship to, pre-existing sleep disturbance are yet to be fully elucidated. GWAS studies based on self-report sleep quality and accelerometer data confirm the heritability of sleep disturbance and reveal a number of candidate risk loci (Hill, O’Connor, & Shirasu-Hiza, Citation2018; Joiner, Citation2018; Jones et al., Citation2019; Lane et al., Citation2017; Stein et al., Citation2018). These specific risk-genes and their physiological contributions to PTSD risk are yet to be adequately investigated, although genes identified in GWAS-sleep studies are currently being studied for their role in physiological contribution to fear learning and PTSD-risk (Feusner et al., Citation2001; Krzyzewska et al., Citation2018; Li et al., Citation2019; Soya & Sakurai, Citation2018).
While risk-genes may ultimately be shown to contribute to polygenic risk for PTSD development in poor sleepers and their mechanistic contributions to physiology clarified, inflammation, shown to contribute to development and maintenance of mental health disorders may be an important mechanism for PTSD development in sleep-disturbed service members, although persistent disturbances of sleep appear to be necessary for inflammatory signalling to be translated into an increase in measurable systemic markers of inflammation (Irwin, Olmstead, & Carroll, Citation2016; Irwin & Opp, Citation2017). Both sleep disturbance and shorter sleep duration (but not the extreme of short sleep) are associated with higher levels of inflammatory signalling (i.e. increased C-reactive protein; CRP, interleukin-6; IL-6) (Irwin et al., Citation2016). There is already considerable evidence for a significant association between inflammation and PTSD diagnosis (Breen et al., Citation2018; Brudluy et al., Citation2015; Michopoulos et al., Citation2015; Miller et al., Citation2018; Spitzer et al., Citation2010). Moreover, there is prospective evidence that inflammation may be a risk factor for PTSD emergence (Breen et al., Citation2015; Eraly et al., Citation2014) and that it affects fear circuits and processes (Deslauriers, Powell, & Risbrough, Citation2017). Taken together with findings of the present study, it is thus conceivable that pre-deployment sleep disturbance among military personnel may have resulted in an increase in inflammatory signalling which predisposed to the development of re-experiencing symptoms following trauma exposure (Besedovsky, Lange, & Haack, Citation2019). As pre-deployment sleep disruption is prevalent among military personnel (Xue et al., Citation2015), our findings demonstrate the importance of achieving adequate sleep prior to combat exposure. Facilitating improvements in the sleep of military personnel may thus help protect those deployed to combat situations from subsequent development of PTSD.
The finding that sleep disturbance 3 months post-deployment predicts re-experiencing symptoms 6 months post-deployment illustrates the role of sleep disturbance in the maintenance of PTSD symptomology. The impaired ability to inhibit learned fear experienced during combat deployment may be one mechanism that contributes to the maintenance of re-experiencing symptoms. Fear inhibition processes, including fear extinction and safety learning, are crucial to recovery following trauma exposure (Careaga, Girardi, & Suchecki, Citation2016; Jovanovic, Kazama, Bachevalier, & Davis, Citation2012). Thus, these processes comprise a critical aspect of clinical interventions, such as exposure therapy (Foa, Hembree, & Rothbaum, Citation2007). Studies have demonstrated a relationship between poor sleep consolidation and fear inhibition ability. In particular, rapid eye movement (REM) sleep, a stage of sleep is implicated in the regulation of emotional-laden memories (Abel, Havekes, Saletin, & Walker, Citation2013). For example, subjects who had disruption of REM sleep subsequently exhibit lower levels of fear extinction memory recall (Menz, Rihm, & Buchel, Citation2016; Straus, Acheson, Risbrough, & Drummond, Citation2017). Additionally, safety learning memory recall is dependent on the consolidation of REM sleep acquired across the previous night (Marshall, Acheson, Risbrough, Straus, & Drummond, Citation2014). In the present study, subject’s reported sleep disturbance 3 months post-deployment may indicate insufficient consolidation of REM sleep. Lack of REM sleep, then, maybe impairing fear extinction and safety learning processes and consequently facilitating the persistence of re-experiencing symptoms 6 months post-deployment. To this end, the adequate consolidation of sleep, especially REM sleep, may be crucial for inhibition of fear learned during combat deployment. However, polysomnographic sleep measurement was not employed in the present study, thereby limiting our ability to definitively determine whether poor REM sleep consolidation was indeed a contributing factor.
Finally, our findings reveal that the relationship between sleep disturbance and re-experiencing symptoms may be moderately dependent on severity of trauma encountered. This finding is anticipated, given that severity of trauma exposure is a robust risk factor for development of PTSD among military personnel. Similarly, a study of the New York population 6 months following September 11 demonstrated a higher prevalence of PTSD among those directly involved (Galea, Citation2003), thus suggesting proximity to trauma may also contribute to the subsequent development of PTSD. Thus, while sleep disturbance may be implicated in the development and maintenance of re-experiencing symptoms in military personnel (e.g. via inflammatory processes and fear inhibition impairment, respectively), development of such symptomology may depend on the precipitating influence of the traumatic event. The link between sleep disturbance and re-experiencing symptoms, then, maybe of particular concern to individuals who’s occupation places them at risk of experiencing high-severity trauma, such as military personnel (Richardson, Frueh, & Acierno, Citation2010) and those employed within the emergency services (Berger et al., Citation2012). Emphasizing the importance of sufficient sleep consolidation and screening for sleep difficulties in individuals at-risk for high-severity trauma is thus particularly critical for the prevention as well as treatment of PTSD within these high-risk populations.
The current study has a number of limitations that should be mentioned. The studies from which these data were extracted did not include a specific sleep quality measure, such as the Insomnia Severity Index (ISI; Morin et al., Citation2011). Thus, we had to infer sleep disturbance from items embedded in other measures (CAPS, BDI). However, it is important to note that the results of this study are consistent with other studies demonstrating a predictive link between sleep disturbance and development of psychiatric symptoms which did use explicit sleep measures (Taylor et al., Citation2016). The lack of an explicit sleep measure in these studies also necessitated the somewhat unconventional use of a principal components analysis to select latent variable indicator items as sleep items from the CAPS at baseline would have been confounded with PTSD total score. While this method may not be perfect in eliminating overlap between sleep disturbance and PTSD symptoms, it provided a data-driven method of maximizing the distinction, and we have further attempted to address this by including the baseline correlation between latent variables in our models.
5. Conclusion
The present study provides longitudinal evidence to support the critical predisposing and perpetuating influence of sleep disturbance pre and post-deployment, respectively, in subsequent development of PTSD symptomology (i.e. in this case, re-experiencing symptoms). Thus, improvement of sleep pre and post-potential trauma exposure may aid in the prevention of symptoms associated with PTSD among military personnel. This notion is encouraging, as sleep is generally considered a modifiable variable, especially when clinical interventions are sought (e.g. cognitive behavioural therapy for insomnia (van Straten et al., Citation2017). Improved understanding of the mechanisms of sleep disturbance and the implementation of sleep-specific interventions and treatment may thus be of critical importance to military personnel in the prevention and treatment of PTSD.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Abel, T., Havekes, R., Saletin, J. M., & Walker, M. (2013). Sleep, plasticity and memory from molecules to whole brain networks. Current Biology, 23, 774–12.
- Acheson, D. T., Geyer, M. A., Baker, D. G., Nievergelt, C. M., Yurgil, K., & Risbrough, V. B. (2015). Conditioned fear and extinction learning performance and its association with psychiatric symptoms in active duty Marines. Psychoneuroendocrinology, 51, 495–505.
- Agorastos, A., Nash, W. P., Nunnink, S., Yurgil, K. A., Goldsmith, A., Litz, B. T., … Baker, D. G. (2013). The peritraumatic behavior questionnaire: Development and initial validation of a new measure for combat-related peritraumatic reactions. BMC Psychiatry, 13, 9.
- Baker, D. G., Nash, W. P., Litz, B. T., Geyer, M. A., Risbrough, V. B., Niervergelt, C. M., … MRS Team. (2012). Predictors of risk and resilience for posttraumatic stress disorder among ground combat marines: Methods of the marine resiliency study. Preventing Chronic Disease, 9, 110134.
- Beck, A. T., & Steer, R. A. (1993). Beck anxiety inventory manual. San Antonio, TX: Harcourt Brace and Company.
- Beck, A. T., Steer, R. A., Ball, R., & Ranieri, W. (1996). Comparison of beck depression inventories-IA and –II in psychiatric outpatients. Journal of Personality Assessment, 67, 588–597.
- Berger, W., Coutinho, E. S. F., Figueira, I., Marques-Portella, C., Luz, M. P., Neylan, T. C., … Mendlowicz, M. V. (2012). Rescuers at risk: A systematic review and meta-regression analysis of the worldwide current prevalence and correlates of PTSD in rescue workers. Social Psychiatry and Psychiatric Epidemiology, 47, 1001–1011.
- Besedovsky, L., Lange, T., & Haack, M. (2019). The sleep-immune crosstalk in health and disease. Physiological Reviews, 99(3), 1325–1380.
- Biddle, D. J., Kelly, P. J., Hermens, D. F., & Glozier, N. (2018). The association of insomnia with future mental illness: Is it just residual symptoms? Sleep Health, 4(4), 352–359.
- Blake, D. D., Weathers, F. W., Nagy, L. M., Kaloupek, D. G., Gusman, F. D., Charney, D. S., & Keane, T. M. (1995). The development of a clinician-administered PTSD scale. Journal of Traumatic Stress, 8(1), 75–90.
- Bramoweth, A. D., & Germain, A. (2013). Deployment-related insomnia in military personnel and veterans. Current Psychiatry Reports, 15(10), 401.
- Breen, M. S., Maihofer, A. X., Glatt, S. J., Tylee, D. S., Chandler, S. D., Tsuang, M. T., … Woelk, C. H. (2015). Gene networks specific for innate immunity define post-traumatic stress disorder. Molecular Psychiatry, 20(12), 1538–1545.
- Breen, M. S., Tylee, D. S., Maihofer, A. X., Neylan, T. C., Mehta, D., Binder, E. B., … Glatt, S. J. (2018). PTSD blood transcriptome mega-analysis: Shared inflammatory pathways across biological sex and modes of trauma. Neuropsychopharmacology, 43(3), 469–481.
- Brudluy, C., Park, J., Wiaderkiewicz, J., Kobayashi, I., Mellman, T. A., & Marvar, P. J. (2015). Autonomic and inflammatory consequences of posttraumatic stress disorder and the link to cardiovascular disease. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 309(4), 315–321.
- Bryant, R. A., Creamer, M., O’Donnell, M., Silove, D., & McFarlane, A. C. (2010). Sleep disturbance immediately prior to trauma predicts subsequent psychiatric disorder. Sleep, 33(1), 69–74.
- Careaga, M. B. L., Girardi, C. E. N., & Suchecki, D. (2016). Understanding posttraumatic stress disorder through fear conditioning, extinction and reconsolidation. Neuroscience and Biobehavioral Reviews, 71, 48–57.
- Clark, D. A., Steer, R. A., & Beck, A. T. (1994). Common and specific dimensions of self-reported anxiety and depression: Implications for the cognitive and tripartite models. Journal of Abnormal Psychology, 103, 645–654.
- Crawford, J. R., & Henry, J. D. (2004). The positive and negative affect schedule (PANAS): Construct validity, measurement properties and normative data in a large non-clinical sample. The British Journal of Clinical Psychology, 43(3), 245–265.
- Danker-Hopfe, H., Sauter, C., Kowalski, J. T., Kropp, S., Ströhle, A., Wesemann, U., & Zimmermann, P. L. (2017). Sleep quality of German soldiers before, during and after deployment in Afghanistan-a prospective study. Journal of Sleep Research, 26(3), 353–363.
- Deslauriers, J., Powell, S., & Risbrough, V. B. (2017). Immune signalling mechanisms of PTSD risk and symptom development: Insights from animal models. Current Opinion in Behavioral Sciences, 14, 123–132.
- Eraly, S. A., Nievergelt, C. M., Maihofer, A. X., Barkauskas, D. A., Biswas, N., Agorastos, A., … Baker, D. G. (2014). Assessment of plasma c-reactive protein as a biomarker of PTSD risk. JAMA Psychiatry, 71, 421–431.
- Feusner, J., Ritchie, T., Lawford, B., Young, R. M., Kann, B., & Noble, E. P. (2001). GABA(A) receptor beta 3 subunit gene and psychiatric morbidity in a post-traumatic stress disorder population. Psychiatry Research, 104(2), 109–117.
- Foa, E. B., Hembree, E., & Rothbaum, B. O. (2007). Prolonged exposure therapy for PTSD: Emotional processing of traumatic experiences therapist guide. New York, NY: Oxford University Press.
- Galea, S. (2003). Trends of probable post-Traumatic stress disorder in New York city after the September 11 terrorist attack. American Journal of Epidemiology, 158, 514–524.
- Gehrman, P., Seelig, A. D., Jacobson, I. G., Boyko, E. J., Hooper, T. I., Gackstetter, G. D., … Smith, T. C. (2013). Predeployment sleep duration and insomnia symptoms as risk factors for new-onset mental health disorders following military deployment. Sleep, 36(7), 1009–1018.
- Glenn, D. E., Acheson, D. T., Geyer, M. A., Nievergelt, C. M., Baker, D. G., & Risbrough, V. B. (2017). Fear learning alterations after traumatic brain injury and their role in development of posttraumatic stress symptoms. Depression and Anxiety, 34(8), 723–733.
- Hill, V. M., O’Connor, R. M., & Shirasu-Hiza, M. (2018, October 8). Tired and stressed: Examining the need for sleep. The European Journal of Neuroscience. [E-pub ahead of print].
- Hooper, D., Coughlan, J., & Mullen, M. R. (2008). Structural equation modelling: Guidelines for determining model fit. Electronic Journal of Business Research Methods, 6(1), 53–60.
- Irwin, M. R., Olmstead, R., & Carroll, J. E. (2016). Sleep disturbance, sleep duration, and inflammation: A systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biological Psychiatry, 80(1), 40–52.
- Irwin, M. R., & Opp, M. R. (2017). Sleep health: Reciprocal regulation of sleep and innate immunity. Neuropsychopharmacology, 42(1), 129–155.
- Joiner, W. J. (2018). The neurobiological basis of sleep and sleep disorders. Physiology, 33(5), 317–327.
- Jones, S. E., Lane, J. M., Wood, A. R., van Hees, V. T., Tyrrell, J., Beaumont, R. N., … Weedon, M. N. (2019). Genome-wide association analyses of chronotype in 697,828 individuals provides insights into circadian rhythms. Nature Communications, 10, 343.
- Jovanovic, T., Kazama, A., Bachevalier, J., & Davis, M. (2012). Impaired safety signal learning may be a biomarker of PTSD. Neuropharmacology, 62, 695–704.
- Klingaman, E. A., Brownlow, J. A., Boland, E. M., Mosti, C., & Gehrman, P. R. (2018). Prevalence, predictors and correlates of insomnia in US army soldiers. Journal of Sleep Research, 27(3), e12612.
- Koffel, E., Polusny, M. A., Arbisi, P. A., & Erbes, C. R. (2013). Pre-deployment daytime and nighttime sleep complaints as predictors of post-deployment PTSD and depression in National Guard troops. Journal of Anxiety Disorders, 27(5), 512–519.
- Krzyzewska, I. M., Ensink, J. B. M., Nawijn, L., Mul, A. N., Koch, S. B., Venema, A., … Henneman, P. (2018). Genetic variant in CACNA1C is associated with PTSD in traumatized police officers. European Journal of Human Genetics, 26(2), 247–257.
- Lane, J. M., Liang, J., Vlasac, I., Anderson, S. G., Bechtold, D. A., Bowden, J., … Saxena, R. (2017). Genome-wide association analyses of sleep disturbance traits identify new loci and highlight shared genetics with neuropsychiatric and metabolic traits. Nature Genetics, 49(2), 274–281.
- Li, G., Zhang, K., Wang, L., Cao, C., Fang, R., Liu, P., … Liberzon, I. (2019). The preliminary investigation of orexigenic hormone gene polymorphisms on posttraumatic stress disorder symptoms. Psychoneuroendocrinology, 100, 131–136.
- Little, T. D., Bovaird, J. A., & Widaman, K. F. (2006). On the merits of orthogonalizing powered and product terms: Implications for modelling interactions among latent variables. Structural Equation Modeling, 13(4), 497–519.
- Marshall, A. J., Acheson, D. T., Risbrough, V. B., Straus, L. D., & Drummond, S. P. A. (2014). Fear conditioning, safety learning, and sleep in humans. The Journal of Neuroscience, 24, 11754–11760.
- McKeever, V. M., & Huff, M. E. (2003). A diathesis-stress model of posttraumatic stress disorder: Ecological, biological, and residual stress pathways. Review of General Psychology, 7(3), 237–250.
- Menz, M. M., Rihm, J. S., & Buchel, C. (2016). REM sleep is causal to successful consolidation of dangerous and safety stimuli and reduces return of fear after extinction. The Journal of Neuroscience, 36, 2148–2160.
- Michopoulos, V., Rothbaum, A. O., Jovanovic, T., Almli, L. M., Bradley, B., Rothbaum, B. O., … Ressler, K. J. (2015). Association of CRP genetic variation and CRP level with elevated PTSD symptoms and physiological responses in a civilian population with high levels of trauma. The American Journal of Psychiatry, 172(4), 353–362.
- Miller, M. W., Maniates, H., Wolf, E. J., Logue, M. W., Schichman, S. A., Stone, A., … McGlinchey, R. (2018). CRP polymorphisms and DNA methylation of the AIM2 gene influence associations between trauma exposure, PTSD, and C-reactive protein. Brain, Behavior, and Immunity, 67, 194–202.
- Moore, T. M., Risbrough, V. B., Baker, D. G., Larson, G. E., Glenn, D. E., Nievergelt, C. M., … Gur, R. C. (2017). Effects of military service and deployment on clinical symptomatology: The role of trauma exposure and social support. Journal of Psychiatric Research, 95, 121–128.
- Morin, C. M., Belleville, G., Belanger, L., & Ivers, H. (2011). The insomnia severity index: Psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep, 34(5), 601–608.
- Pettersson, K., Saers, J., Lindberg, E., & Janson, C. (2016). Sleep disturbances among Swedish soldiers after military service abroad. Upsala Journal of Medical Sciences, 121(1), 65–69.
- RAND Corporation. (2015). Sleep in the military: Promoting healthy sleep among U.S. Service members. Santa Monica: Author.
- Reijnen, A., Rademaker, A. R., Vermetten, E., & Geuze, E. (2015). Prevalence of mental health symptoms in Dutch military personnel returning from deployment to Afghanistan: A 2-year longitudinal analysis. European Psychiatry, 30, 341–346.
- Richardson, J. D., Thompson, A., King, L., Corbett, B., Schnaider, P., St Cyr, K., … Zamorski, M. (2017). Insomnia, psychiatric disorders and suicidal ideation in a National representative sample of active Canadian Forces members. BMC Psychiatry, 17(1), 211.
- Richardson, L., Frueh, B., & Acierno, R. (2010). Prevalence estimates of combat-related post-traumatic stress disorder: Critical review. Australian & New Zealand Journal of Psychiatry, 44, 4–19.
- Roth, T., Coulouvrat, C., Hajak, G., Lakoma, M. D., Sampson, N. A., Shahly, V., … Kessler, R. C. (2011). Prevalence and perceived health associated with insomnia based on DSM-IV-TR; international statistical classification of diseases and related health problems, tenth revision; and research diagnostic criteria/international classification of sleep disorders, Second Edition criteria: Results from the America insomnia survey. Biological Psychiatry, 69(6), 592–600.
- Seelig, A. D., Jacobson, I. G., Donoho, C. J., Trone, D. W., Crum-Cianflone, N. F., & Balkin, T. J. (2016). Sleep and health resilience metrics in a large military cohort. Sleep, 39(5), 1111–1120.
- Soya, S., & Sakurai, T. (2018). Orexin as a modulator of fear-related behavior: Hypothalamic control of noradrenaline circuit. Brain Research, S0006–8993(18), 30598–5.
- Spitzer, C., Barnow, S., Völzke, H., Wallaschofski, H., John, U., Freyberger, H. J., … Grabe, H. J. (2010). Association of posttraumatic stress disorder with low-grade elevation of C-reactive protein: Evidence from the general population. Journal of Psychiatric Research, 44(1), 15–21.
- Stein, M. B., McCarthy, M. J., Chen, C. Y., Jain, S., Gelernter, J., He, F., … Ursano, R. J. (2018). Genome-wide analysis of insomnia disorder. Molecular Psychiatry, 23(11), 2238–2250.
- Stein, M. B., Walker, J. R., Hazen, A. L., & Forde, D. R. (1997). Full and partial posttraumatic stress disorder: Findings from a community survey. The American Journal of Psychiatry, 154, 1114–1119.
- Straus, L. D., Acheson, D. T., Risbrough, V. B., & Drummond, S. P. A. (2017). Sleep deprivation disrupts recall of conditioned fear extinction. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 2, 123–129.
- Taylor, D. J., Pruiksma, K. E., Hale, W. J., Kelly, K., Maurer, D., Peterson, A. L., … Williamson, D. E. (2016). Prevalence, correlates, and predictors of insomnia in the US army prior to deployment. Sleep, 39(10), 1795–1806.
- van Liempt, S., van Zuiden, M., Westenberg, H., Super, A., & Vermetten, E. (2013). Impact of impaired sleep on the development of PTSD symptoms in combat veterans: A prospective longitudinal cohort study. Depression and Anxiety, 30(5), 469–474.
- van Straten, A., Van der Zweerde, T., Kleiboer, A., Cuijpers, P., Morin, C. M., & Lancee, J. (2017). Cognitive and behavioural therapies in the treatment of insomnia: A meta-analysis. Sleep Medicine Reviews, 38, 3–16.
- Vogt, D., Smith, B. N., King, L. A., King, D. W., Knight, J., & Vasterling, J. J. (2013). Deployment risk and resilience inventory-2 (DRRI-2): An updated tool for assessing psychosocial risk and resilience factors among service members and veterans. Journal of Traumatic Stress, 26(6), 710–717.
- Wang, H. E., Campbell-Sills, L., Kessler, R. C., Sun, X., Heeringa, S. G., Nock, M. K., … Stein, M. B. (2019). Pre-deployment insomnia is associated with post-deployment post-traumatic stress disorder and suicidal ideation in US army soldiers. Sleep, 42(2), 229.
- Ware, J., Kosinski, M., & Keller, S. D. (1996). A 12-item short-form health survey: Construction of scales and preliminary tests of reliability and validity. Medical Care, 34, 220–233.
- Watson, D., Clark, L. A., & Tellegen, A. (1988). Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology, 54(6), 1063–1070.
- Xue, C., Ge, Y., Tang, B., Liu, Y., Kang, P., Wang, M., … Schmahl, C. (2015). A meta-analysis of risk factors for combat-related PTSD among military personnel and veterans. PLoS One, 10(3), 1–21.
