ABSTRACT
Background: Post-traumatic stress disorder (PTSD) is triggered by distinct events and is therefore amenable to studies of its early pathogenesis. Longitudinal studies during the year that follows trauma exposure revealed typical symptom trajectories leading to either recovery or protracted PTSD. Thezneurobehavioral correlates of early PTSD symptoms’ trajectories have not been longitudinally explored.
Objective: To present the rationale and design of a longitudinal study exploring the relationship between evolving PTSD symptoms and co-occurring cognitive functioning and structural and functional brain imaging parameters.
Method: Adult civilians consecutively admitted to a general hospital emergency room (ER) for traumatic injury will be screened for early PTSD symptoms suggestive of chronic PTSD risk, and consecutively evaluated 1, 6 and 14 months following the traumatic event. Consecutive assessments will include structured clinical interviews for PTSD and comorbid disorders, self-reported depression and anxiety symptoms, a web-based assessment of cognitive domains previously linked with PTSD (e.g., memory, executive functions, cognitive flexibility), high-resolution structural MRI of both grey and white matter, functional resting-state connectivity, and fMRI tasks examining emotional reactivity and regulation, as well as motivation processing and sensitivity to risk and reward. Data analyses will explore putative cognitive predictors of non-remitting PTSD, and brain structural and functional correlates of PTSD persistence or recovery.
Conclusion: This work will longitudinally document patterns of brain structures, connectivity, and functioning, predictive of (or associated with) emerging PTSD during the critical first year of after the traumatic event. It will thereby inform our understanding of the disorder’s pathogenesis and underlying neuropathology. Challenges to longitudinal MRI studies of recent survivors, and methodological choices used to optimize the study’s design are discussed.
HIGHLIGHTS
• The protocol of a multimodal longitudinal study of recent trauma survivors is presented.• The study evaluates the evolving relationships between PTSD symptoms, neurocognitive functioning, and brain imaging parameters (structural and functional).• The study rationale, methodology, and design are reported.• Technical and conceptual challenges to performing longitudinal multimodal studies of recent PTSD are discussed.• Study design elements that addresses these challenges (e.g., changes in PTSD diagnostic template, optimal assessments’ timing, and minimizing subject loss) are discussed.
Antecedentes: Los trastornos de estrés postraumático (TEPT) son desencadenados por distintos eventos y son por lo tanto susceptibles para estudios de su patogénesis temprana. Los estudios longitudinales durante el año que sigue la exposición al trauma revelan trayectorias de síntomas típicos que llevan tanto a la recuperación como al TEPT prolongado. Los correlatos neuroconductuales de las trayectorias de los síntomas tempranos del TEPT no han sido explorados longitudinalmente.
Objetivo: Se presenta la justificación y el diseño de un estudio longitudinal explorando la relación entre los síntomas del TEPT en evolución y la co-ocurrencia del funcionamiento cognitivo y los parámetros de las imágenes cerebrales estructurales y funcionales.
Método: Los adultos civiles ingresados consecutivamente a una sala de emergencia (ER en su sigla en inglés) de un hospital general por lesión traumática serán tamizados por los síntomas tempranos del TEPT sugerentes de riesgo de TEPT crónico, y evaluados consecutivamente a los uno, seis, y catorce meses luego del evento traumático. Las evaluaciones consecutivas incluirán entrevistas clínicas estructuradas para el TEPT y los trastornos comórbidos, auto-reporte de los síntomas de depresión y ansiedad, una evaluación online de los dominios cognitivos vinculados previamente con el TEPT (por ej., memoria, funciones cognitivas, flexibilidad cognitiva), MRI estructural de alta definición para tanto la materia blanca como para la gris, conectividad en estado de descanso funcional, y tareas de MRI funcional (fMRI en su sigla en inglés) examinando la reactividad emocional y la regulación, como también el procesamiento de la motivación y la sensibilidad al riesgo y a la recompensa. Los análisis de los datos explorarán supuestos predictores cognitivos del TEPT no remitidos, y los correlatos estructurales y funcionales del cerebro de la persistencia o recuperación del TEPT.
Conclusión: Este trabajo documentará longitudinalmente los patrones de las estructuras cerebrales, conectividad, y predicción funcional de, o asociado con TEPT emergente durante el primer año crítico, luego de un evento traumático. Así, informará nuestro entendimiento de la patogénesis del trastorno y la neuropatología de base. Se discuten los desafíos de los estudios longitudinales de MRI con sobrevivientes recientes, y las decisiones metodológicas usadas para optimizar el diseño del estudio.
背景:创伤后应激障碍(PTSD)由不同事件引发,因此适合对其早期发病机制进行研究。创伤暴露后一年内的纵向研究揭示出典型的PTSD症状轨迹:恢复型PTSD或持续型PTSD。尚未有早期PTSD症状轨迹的神经行为方面相关的纵向研究。我们提出了一项纵向研究的理论和设计,探讨了不断发展的PTSD症状及并发的认知功能与脑结构和脑功能成像参数之间的关系。
方法:将对连续因创伤性损伤被送往综合医院急诊室(ER)的成人进行筛查,以发现提示其存在持续型PTSD风险的早期PTSD症状,并在创伤事件发生后的一个月, 六个月和十四个月时连续进行评估。连续的评估将包括:针对PTSD及其并发症的结构性临床访谈,自我报告式的抑郁和焦虑症状,针对先前与PTSD相关的认知领域(例如,记忆,执行功能,认知灵活性)的网络评估,包含灰质, 白质的高分辨率结构MRI,静息态功能连接及检测情绪反应和调节的fMRI任务,以及动机处理和对风险及奖赏的敏感性。数据分析将研究持续型PTSD推定的认知预测因素,及PTSD持续或恢复的脑结构和功能连接。
结论:这项工作将纵向记录在创伤事件发生后关键的第一年中脑结构, 脑连接及脑功能与PTSD的相关或预测作用。因此,它将有助于我们了解该疾病的发病机制及潜在的神经病理机制。讨论了针对近期创伤幸存者的纵向MRI研究面临的挑战及用于优化研究设计的方法选择。
1. Background
Post-Traumatic Stress Disorder (PTSD) is a common and severe mental disorder with profound public health impact due to its high prevalence, chronicity, associated functional impairment and frequent comorbidities (Kessler, Citation2000; Kessler, Sonnega, Bromet, Hughes, & Nelson, Citation1995; Shalev, Liberzon, & Marmar, Citation2017). Early PTSD symptoms, frequently observed shortly after trauma exposure, subside in most of those initially expressing them, persisting in about 30% of those who meet PTSD diagnostic criterial 1 month after trauma (Davidson, Stein, Shalev, & Yehuda, Citation2004; Freedman, Brandes, Peri, & Shalev, Citation1999a; Koren, Arnon, & Klein, Citation2001; O’Donnell, Creamer, Bryant, Schnyder, & Shalev, Citation2003; Peleg & Shalev, Citation2006; Perkonigg et al., Citation2005; Shalev, Citation1996; Shalev, Peri, Canetti, & Schreiber, Citation1996; Yehuda, McFarlane, & Shalev, Citation1998a). Additionally, some initially asymptomatic survivors develop delayed-onset PTSD (Shalev et al., Citation2019). The occurrence of chronic PTSD in a limited subset of trauma-exposed survivors, and the tenacity of the chronic condition, suggest a long-lasting neurobehavioral modification (Pitman et al., Citation2012). Longitudinal studies, therefore, classify early PTSD symptom into trajectories of non-remission, rapid remission, and delayed (often incomplete) remission (Marmar, Weiss, & Schlenger et al., Citation1994; Mayou, Tyndel, & Bryant, Citation1997; Stein, Walker, Hazen, & Forde, Citation1997; Yehuda, McFarlane, & Shalev, Citation1998b).
Symptom trajectories provide an observable dimension of PTSD development, or remission. To better understand the underlying neurobiology of PTSD pathogenesis and resilience, these trajectories must be linked with co-occurring developments in objectively measured cognitive functioning, brain structure, and brain function. Such studies must be large enough to allow an exploration of the resulting multimodal matrix. To date, however, large-scale longitudinal studies combining cognitive and neuroimaging with PTSD symptom trajectories are missing.
1.1. Cognitive Functioning
Several cognitive deficits have been previously linked with PTSD (Scott et al., Citation2015) including working memory, information processing speed, verbal learning, short-term and declarative memory (Johnsen & Asbjørnsen, Citation2008; Samuelson, Citation2011), attention, and executive functioning (Aupperle, Melrose, Stein, & Paulus, Citation2012; Polak, Witteveen, Reitsma, & Olff, Citation2012). PTSD has additionally been associated with poor response inhibition, attentional switching and flexibility (Casada & Roache, Citation2005; Hart et al., Citation2017; Koenen et al., Citation2001; Leskin & White, Citation2007; Vasterling, Brailey, Constans, & Sutker, Citation1998), and these features were hypothetically linked with PTSD patients’ difficulties to disengage from attending salient stimuli (Pineles, Shipherd, Mostoufi, Abramovitz, & Yovel, Citation2009).
1.2. Brain Structure
Several structural brain abnormalities have been associated with chronic PTSD (Bonne et al., Citation2001; Gilbertson et al., Citation2002), including reduced volume of the hippocampus, medial prefrontal cortex (mPFC) and anterior cingulate cortex (ACC), as well as thinner dorsolateral prefrontal cortex (dlPFC) (Bremner, Elzinga, Schmahl, & Vermetten, Citation2007; Ferrari, Busatto, McGuire, & Crippa, Citation2008; Gilbertson et al., Citation2002; Kasai et al., Citation2008). Alterations in white matter have also been implicated in top-down regulation deficits in PTSD patients (Abe et al., Citation2006; Admon et al., Citation2013; Kim et al., Citation2007; Schuff et al., Citation2011). Diffusion tensor imaging (DTI) studies of PTSD have documented changes in fractional anisotropy in the posterior cingulum bundles, connecting cingulate and entorhinal cortices (Fani et al., Citation2012; Kim et al., Citation2007; Zhang et al., Citation2010), as well as decreased white matter integrity in the uncinate fasciculus (Ayling, Aghajani, Fouche, & van der Wee, Citation2012; Phan et al., Citation2009).
1.3. Brain Function
Functional neural systems associated with PTSD include fear learning, threat detection, executive function and emotion regulation, and contextual processing (Shalev et al., Citation2017). Altered activity in the neural circuits mediating these systems (i.e., the amygdala, ventromedial prefrontal cortex (vmPFC), dorsal anterior cingulate cortex (dACC), hippocampus, and insula) have been found in PTSD (reviewed in (Liberzon & Sripada, Citation2007; Pitman et al., Citation2012; Shin, Rauch, & Pitman, Citation2006)). Finally, resting-state connectivity studies in PTSD showed reduced coupling within the default mode network (DMN), greater coupling within the salience network (SN), and decreased segregation of the DMN and SN (Koch et al., Citation2016; Sripada et al., Citation2012). These connectivity patterns suggest possible biomarkers for PTSD and its dissociative subtype.
Studies outlined above have lead to several mechanistic hypotheses of PTSD pathogenesis (Pitman et al., Citation2012; Shalev et al., Citation2017). Testing these pathogenic hypotheses, however, requires longitudinal studies, optimally carried out during the early aftermath of traumatic events, simultaneously exploring multimodal dimensions of the response to trauma (e.g., symptoms, cognitive functions, brain structure and functioning). Such studies are challenging: their design must accommodate subjects’ burden and expected attrition; their size should allow statistical inference; to truly assess PTSD pathogenesis, they should remain with a large enough number of PTSD patients at endpoint; Finally, their contents and timing should address the critical stages in PTSD longitudinal development. Given such constraints, designing multimodal studies of PTSD development requires methodological choices and trade-offs, as well as a clear delineation of theoretically pertinent, feasible and attainable goals.
Exemplifying such constraints and choices, we present here the design of a multimodal longitudinal study of neurobehavioral moderators of PTSD trajectories, that concurrently documents PTSD symptom trajectories, cognitive functioning, brain structure and theoretically compelling brain functions.
The study’s stated aims and hypothesis are the following: (1) to document PTSD symptoms trajectories during the year that follows trauma exposure, and evaluate the contribution of putative covariates (e.g., co-occurring depression and anxiety; trauma type, age, gender) to these trajectories. Following previous studies (Galatzer-Levy et al., Citation2013) we expected to uncover distinct longitudinal trajectories hypothetically encompassing rapid remission, slow or delayed remission or non-remission. (2) To identify cognitive mechanisms that predict or accompany PTSD symptom trajectories, using a comprehensive battery of cognitive functioning described below. (3) To identify neural mechanisms that predict or accompany PTSD symptom trajectories, using functional MRI (fMRI) of resting-state functional connectivity and specific cognitive-emotional probes described below. (4) To identify structural brain alterations associated with PTSD symptom trajectories, using high-resolution structural MRI and Diffusion Kurtosis Imaging (DKI). We hypothesized that initial structural abnormalities of grey matter (e.g. smaller hippocampus) and white matter (e.g. reduced white matter integrity of the uncinate fasciculus) would be associated with the non-remission symptom trajectory. (5) To explore the multi-level integration of cognitive and neural variables associated with PTSD symptom trajectories (exploratory aim).
Following previous success in conducting longitudinal studies of PTSD (Marmar et al., Citation1994; Mayou et al., Citation1997; Stein et al., Citation1997; Yehuda et al., Citation1998b), we opted to study adult survivors of traumatic events admitted to a general hospital ER following trauma exposure, and used previous work (Bonne et al., Citation2001; Shalev et al., Citation2012a; Shalev, Ankri, Peleg, Israeli-Shalev, & Freedman, Citation2011) to infer the expected sample size, attrition and PTSD prevalence at endpoint. Aiming to capture at least 1 year of PTSD early development, and attempting to avoid the 12 months ‘anniversary reaction’, we followed the participants for a period of 14 months.
2. Methods
2.1. Study design
The following study is designed as an observational, prospective panel study of consecutive trauma survivors admitted to a general hospital emergency room (ER) following traumatic incidents. To reveal and document the neurobehavioral moderators of PTSD symptom trajectories, this work will repeatedly and simultaneously evaluate trauma survivors’ clinical symptoms, cognitive performance, brain structure and neural functioning at 1, 6, and 14 months following trauma exposure (Time Point 1, Time Point 2 and Time Point 3; TP1, TP2, and TP3, respectively).
2.2. Participant eligibility (inclusion and exclusion criteria)
Participants will constitute adult survivors of traumatic events, admitted to Tel Aviv Sourasky Medical Centre’s ER. Individuals will be considered for telephone screening interview if they meet the following inclusion criteria: (i) Age 18–65 years (ii) Able to read and comprehend Hebrew; (iii) Arrived in the ER after experiencing one of the following traumatic events: motor-vehicle accident (MVA), bicycle accident, work accident, terror attack, hostilities, physical assault, electric shock, fire, robbery, drowning or large-scale disaster.
Eligible individuals will not be included in the study if they sustained severe head injury or were a coma upon ER arrival; have known medical condition that interfere with their ability to give informed consent, cooperate with screening and/or treatment; suspected or diagnosed with chronic PTSD; have a lifetime history of psychotic illness; have current substance abuse disorder; express suicidal ideations or have other medical or psychological conditions that constitute treatment priority upon potential enrolment. Individuals who are not eligible for an MRI scan will be similarly excluded (e.g. those with pacemakers, implants that are not MRI-compatible, permanent makeup or large tattoos, and those known to suffer from claustrophobia). Due to the Ministry of Defence regulations, active service members of the Israel Defence Force (IDF) will not be included in this study.
2.3. Clinical instruments
Clinician-Administered PTSD Scale (CAPS) IV and 5 (Blake et al., Citation1995; Weathers, Bovin, & Lee et al., Citation2018). Structured clinical interview evaluating DSM-IV and DSM-5 PTSD symptom criteria on dimensions of frequency, intensity, and severity. An instrument combining both CAPS-IV and CAPS-5 will be used in order to keep continuity, after the DSM criteria changed. The CAPS contain explicit, behaviourally anchored probes for each PTSD symptom criteria and rating scale descriptors to enhance reliability. It yields both categorical notation of each symptom (present/absent) and continuous symptom severity score. The CAPS total score will be obtained by summing all individual items’ scores, serving as the main outcome measure of this study. CAPS-5 includes items assessing the dissociative subtype of PTSD (depersonalization and derealization severity). The Hebrew version which will be used in this work was cross-translated and compared with the original English instruments. CAPS-5 demonstrates high internal consistency (α = 0.88) and high test–retest reliability (ICC = 0.78), as well as good convergent validity with CAPS-IV (r = 0.83) and PTSD Checklist for DSM–5 (r = 0.66) (Weathers et al., Citation2018).
Structured clinical interview for DSM-IV (SCID-IV) (First, Citation1995). Structured clinical interview evaluating current and lifetime (pre-event) Axis I mental disorders. The official Hebrew version of SCID has been used extensively in the previous PTSD research (Hirschfeld et al., Citation2000; Rohde, Lewinsohn, & Seeley, Citation1997).
PTSD Checklist for DSM-IV (PCL) (Weathers, Litz, Herman, Huska, & Keane, Citation1993). A 17-item self-report questionnaire, corresponding to the DSM-IV symptom criteria for PTSD. PCL measures the distress caused from each PTSD symptom over the past month, using a 5‐point scale ranging from 1 = not at all to 5 = extremely. PCL item scores will be summed to yield a secondary continuous measure of PTSD symptom severity, in addition to CAPS total scores. It was shown to have high test–retest reliability over a three-day period (r = 0.96) and very high internal consistency (α = 0.97) as well as a high correlation with CAPS (Blanchard, Citation1996). This measure was cross-translated to Hebrew and used in previous studies (Ben-Zion et al., Citation2018; Fine, Achituv, Etkin, Merin, & Shalev, Citation2018).
Beck Depression Inventory (BDI-II) (Beck, Steer, & Brown, Citation1996). 21-item self-report questionnaire, which will assess the severity of current depressive symptoms. The BDI was shown to have high internal consistency (α = 0.97) and high test–retest reliability (r = 0.93). It’s Hebrew version was used in prospective studies of PTSD, where it was found to be a good predictor of chronic PTSD at 1-week and 1-month after trauma (Freedman et al., Citation1999a).
Beck Anxiety Inventory (BAI) (Beck, Epstein, Brown, & Steer, Citation1988). 21-item self-report questionnaire measuring concurrent anxiety symptoms. The BAI was shown to have high internal consistency (α = 0.88) and good test–retest reliability over 1-week (r = 0.75). Previously, BAI discriminated anxious diagnostic groups (panic disorder, generalized anxiety disorder, etc.) from non-anxious diagnostic groups (major depression, dysthymic disorder, etc.), and was moderately correlated with other anxiety rating scales (Fydrich, Dowdall, & Chambless, Citation1992).
Participants’ Clinical Global Impression scale (CGI-P) (Guy, Citation1976). Self-report, easily understood practical measurement tool (Busner & Targum, Citation2007), which will be used to evaluate patients’ subjective impression, on a scale between 1 (‘normal feeling’) to 7 (‘the worst feeling there is’).
2.4. Cognitive functioning measures
Cognitive tests will be administered via WebNeuro, a validated world-wide-web-based comprehensive battery of cognitive functioning (Silverstein et al., Citation2007). To standardize testing conditions, all tests will be taken in the Hebrew language at our laboratory, rather than in participants’ homes. Performance in the different tasks included in WebNeuro battery will be calculated using an automated software program, standardizing performance for age and years of education. For each participant, standardized z-scores will be derived from all the relevant outcome measured, at each of the following 11 cognitive functions: motor coordination, processing speed, sustained attention, controlled attention, cognitive flexibility, response inhibition, working memory, recall memory, executive function, emotion identification, and emotional bias (see the specific tests, measured constructs, instructions and outcome measures in ).
Table 1. Cognitive measures. Specific cognitive tests, measured constructs, instructions for participants, and outcome measures of the cognitive measures which will be used in the study.
2.5. Imaging data acquisition
Structural and functional scans will be performed in a 3.0 Tesla Siemens MRI system (MAGNETOM Prisma, Germany), using a twenty-channel head coil, located at the Sagol Brain Institute at Tel-Aviv Sourasky Medical Centre. To allow high-resolution whole-brain structural images, both T1-weighted magnetization prepared rapid gradient echo (MPRAGE) (TR/TE = 2400/2.29 ms, flip angle = 8°, voxel size 0.7 × 0.7 × 0.7 mm, FOV = 224 × 224 mm) and Diffusion Kurtosis Imaging (DKI) sequences (30 diffusion directions, b = 0/1000/2000 s/mm2, TR/TE = 8200/69.0 ms, voxel size 2.2 × 2.2 × 2.2 mm, FOV = 220 × 220 mm) will be acquired. Functional whole-brain scans will be performed in an interleaved order, using a T2*-weighted gradient echo-planar imaging pulse sequence (TR/TE = 2000/28 ms, flip angle = 90°, voxel size 2.2 × 2.2 × 2.2 mm, FOV = 220 × 220 mm, slice thickness = 3 mm, 36 slices per volume).
2.6. Imaging data analysis
Imaging data will be analyzed using validated softwares, including FMRIB Software Library (FSL) (Jenkinson, Beckmann, Behrens, Woolrich, & Smith, Citation2012), Statistical Parametric Mapping (SPM) (Friston et al., Citation1994), and FreeSurfer (Fischl, Citation2012).
2.7. fMRI tasks
2.7.1. Resting-state scan
A functional resting-state scan (duration = 10 min, TR = 2000 ms, TE = 28 ms, 3 × 3 × 3mm voxel size), in which participants will be instructed to keep their eyes open and fixed on a fixation cross, will be used to examine functional activity and connectivity of different neural regions (e.g. amygdala, hippocampus, prefrontal cortex) and networks at rest (e.g. salience network, default-mode network).
2.7.2. Emotional reactivity task
Subjects will perform the emotional faces matching task (Hariri, Bookheimer, & Mazziotta, Citation2003), in which they will be instructed to select the shape (located at the bottom right or bottom left of the screen) that matches the target face/shape (located at the top of the screen), as accurate and as quickly as possible. The task will include four blocks of shapes, and four blocks of emotional faces (angry, fearful, surprised and neutral faces). The order of the emotional faces blocks will be counterbalanced between subjects, as well as within-subjects across different time-points, using four different versions for this task. We will use this task mainly to evaluate individuals’ emotional reactivity and regulation.
2.7.3. Risk and reward sensitivity task
Participants will play an interactive-modified domino game (Admon et al., Citation2013), in which they will be forced to take risks in order to win (Kahn et al., Citation2002). This paradigm involves four intervals: decision making (goal-conflict behaviour), execution (risky vs. safe choice), anticipation (emotional regulation), and outcome (punishment, non-punishment, reward, non-reward). We will use this task mainly to evaluate sensitivity to signals of risk and reward, emotional regulation motivation processing.
2.7.4. Emotion regulation task
Attention- and appraisal-based emotion regulation will be probed using the Shifted-Attention Emotion Appraisal Task (SEAT) (Duval, Joshi, Russman Block, Abelson, & Liberzon, Citation2018; Klumpp et al., Citation2011; Sripada et al., Citation2013). Stimuli in this task include composite pictures of superimposed faces (foreground) and buildings (background), as well as 20 pictures of faces or buildings only. The face pictures depict neutral, angry, or fearful expressions, and the building pictures depict indoor or outdoor scenes. In three different conditions, participants will be asked to respond to three different questions: (1) Gender (Male/Female): whether the face in the foreground is male or female; (2) Inside/Outside: whether the scene in the background is indoors or outdoors; and (3) Like/Dislike: whether the face in the foreground is liked or disliked. This task will probe multiple components of emotion regulation, including (1) implicit emotional processing, (2) attentional modulation of emotion, and (3) modulation of emotion by appraisal.
2.8. Procedure
2.8.1. Source of participants
Potential trauma survivors will be identified via Tel Aviv Sourasky Medical Centre’s ER computerized medical records, following potential traumatic events.
2.8.2. Screening and initial telephone assessment
Within 10–14 days after trauma exposure, and after being discharged from the hospital, identified individuals will be contacted by telephone for an initial screening. After explaining the purpose of the call and obtaining verbal consent, a modified dichotomous version of the PCL will be administered. Potential participants which will report symptoms that will meet DSM-IV or DSM-5 PTSD diagnostic criteria (except for the 1-month duration), and will not meet any of the exclusion criteria, will receive further verbal information about the research and will be invited for a face-to-face interview. The telephone screening will be conducted by B.A. and M.A. level students which will receive specialized training and will be certified to use these specific assessment tools.
2.8.3. Clinical assessment
After signing an informed consent, the participants will be evaluated by CAPS and SCID (described above), will complete four self-report questionnaires (PCL, BDI-II, BAI, and CGI) and a battery of cognitive tests (WebNeuro). Participants who will meet CAPS-based DSM-IV or DSM-5 Acute PTSD diagnostic criteria, and will not meet any of the exclusion criteria, will be invited for a structural and functional MRI scan. Although the study will preferentially enrol survivors who meet PTSD diagnosis (and are thus at risk of chronic PTSD), we will also enrol individuals with sub-threshold PTSD.
It is important to note that the selection of individuals with initial high PTSD symptom severity was intended to generate a large enough sample of PTSD individuals at the end of the study. This is based on previous literature showing that survivors with early PTSD symptoms are at high risk of developing chronic PTSD, and that an absence of early symptoms reliably predicts a good prognosis (Freedman, Brandes, Peri, & Shalev, Citation1999b; Shalev et al., Citation2012b; Shalev, Freedman, Peri, & Brandes, Citation1997; Shalev et al., Citation2019).
The first clinical meeting will take approximately 3 h, including clinical interview, self-report questionnaires and cognitive assessment (Meeting #1). The second meeting will take approximately 2 h, including high-resolution structural and functional MRI scan (Meeting #2). Both meetings will take place at our laboratory, within 1 month after trauma (Time Point 1, TP1). Identical follow-up meetings (similar to both Meeting #1 and Meeting #2) will be conducted at 6 and 14 months after trauma (Time Point 2 and Time Point 3, TP2 and TP3 accordingly). Participants will receive financial remuneration at the end of each meeting of each time-point (TP1, TP2 and TP3), according to the ethics committee regulations and approval.
2.9. Sample size
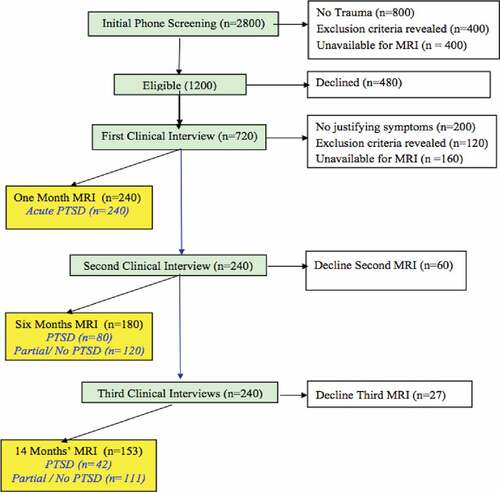
We estimated sample size based on previous ER prospective studies of recent survivors, including attrition in an MRI study (Bonne et al., Citation2001) and in a prevention study using similar recruitment and follow-up approach (Shalev et al., Citation2012b, Citation2011). The number of subjects was calculated with the goal of reaching n = 42 individuals diagnosed with PTSD at 14 months (TP3), considering expected subject loss (including MRI-related dropouts), as well as estimated spontaneous recovery (see ).
We intend to screen 2800 adult civilians admitted to the hospital’s ER, to confirm the occurrence of a psychologically traumatic event, and identify and assess those expressing initial PTSD symptoms via telephone-based structured interviews within 10–14 days of ER admission (target n = 1200). We then plan to conduct face-to-face clinical interviews of 720 consenting participants within 30 days of ER admission (TP1), and enrol 240 consenting participants, performing the first MRI scans within 30 days of ER admission (TP1). Assuming 36% loss of subjects to MRI sessions, we will perform 180 MRI sessions at 6 months (TP2) and 153 at 14 months after trauma (TP3). Clinical interviews will proceed regardless of MRI participation, with expected 240 participants at 6 and 14 months after trauma (TP2 and TP3). Structured telephone interviews could replace clinical assessments in participants who might decline repeated clinical contacts. Taken into account spontaneous recovery, we expect n = 42 individuals with PTSD diagnosed at 14-months post-trauma (TP3), and the remaining n = 111 individuals with partial or no PTSD diagnosis (see ).
2.10. Statistical analysis
PTSD latent symptom trajectories will be calculated using Latent Group Mixture Modelling (LGMM) analytic approach. Cognitive domains, brain structure and function will be examined in relation to these symptom trajectories. A ‘trajectory’ group x time factorial analysis will be conducted to identify progressive changes in cognitive domains, brain structure and function, associated with the different trajectories. Our statistical analyses will include key-covariates such as PTSD comorbidities, trauma type, age and gender. Corrections for multiple testing will be addressed through false discovery rate (FDR) corrected p values.
3. Conclusions
This work presents a study design aiming to uncover neurobehavioral moderators of PTSD trajectories in recent trauma survivors at substantial risk for PTSD (i.e., individuals with high initial symptoms). The study protocol is outlined in order to offer researchers an opportunity to evaluate and implement the methodology carefully chosen for this research.
Longitudinal studies of the early development of post-traumatic psychopathology present significant technical and conceptual challenges (Fine et al., Citation2018; Qi et al., Citation2018; Shalev et al., Citation2011). Technical challenges include reaching out to, enrolling, evaluating and retaining participants in sufficient numbers and proportions to ensure power and reduce retention bias. It is also critical to choose adequate tools and probes which are pertinent to constructs of interest (i.e. disease trajectories, hypothetical pathogenic and pathophysiological mechanisms). Conceptually, and more importantly, studies’ design and implementation must address sample representativeness, for purposes of generalizability and specification of the population sample.
This study is designed to address some of these challenges in the following matter. First and foremost, PTSD definition of post-traumatic psychopathology is a symptom configuration that varies upon DSM taxonomic classification. Although DSM-based PTSD criteria is the most extensively studied outcome of trauma exposure, the specific diagnostic criteria has been recently modified and is an object of ongoing discussion (Do, Citation2011; Galatzer-Levy & Bryant, Citation2013; Zoellner, Bedard-Gilligan, Jun, Marks, & Garcia, Citation2013). In order not to limit ourselves to one of the frequently changing definitions of PTSD, we will use an assessment instrument that combines the two most recent symptom templates (i.e. DSM-IV and DSM-5), as well as self-report measures including indicators of anxiety and depression, that will portray a full picture of diagnostic features that are associated to PTSD manifestation.
Second, determining the timing of assessments is crucial for deriving adequate conclusions (Shalev et al., Citation2011). Overall, this study will include four repeated measurements of clinical data in the first year after trauma, chosen to assess the development and persistence of PTSD during this year. This design will allow us to capture: (1) early emerging symptoms (at 10–14 days post-trauma); (2) the key time point to discriminate early PTSD symptoms from fully developed syndrome (at 1-month post-trauma) (Shalev et al., Citation2012b); (3) an intermediary point enabling us to capture the evolving pathogenesis and pathophysiological PTSD mechanisms (at 6 months post-trauma); (4) endpoint hallmark encompassing the probability of sustaining chronic PTSD (at 14 months post-trauma), by which over 90% of recovery from PTSD is expected (Kessler et al., Citation1995; Shalev & Freedman, Citation2005). This endpoint was chosen to avoid the 1-year ‘anniversary’ exacerbation of traumatic memories and symptoms.
Third, minimizing the subject loss in longitudinal designs is a well-known, often unresolved challenge. Here we address this a-priori by choosing to conduct the study on the Israeli population, known for its relatively high compliance, as well as situating it in a large metropolitan, easily accessible to the large population. Furthermore, the study will enable considerable flexibility and special attention to each one of the participants’ needs (e.g. arranging transportation, flexible times of assessments, modified MRI scans, etc.). In addition, special emphasis will be given on strengthening the rapport between the participants and the research team (e.g. frequent contact with participants after and between different time points, issuing a study newsletter, etc.).
Nevertheless, this study design has also several limitations. First, as in most PTSD studies, there is a lack of baseline measurement before trauma exposure. However, as structural brain changes typically take time to occur, it is likely to assume that structural abnormalities detect pre-disposition factors, rather than consequence of trauma exposure. Second, for ethical reasons, we will not prevent individuals from seeking and participating in different treatments (pharmacological or psychotherapy), even though it might affect the natural course of the pathophysiologic development. Nevertheless, information regarding treatment timing and type will be recorded, thus could be accounted for. In addition, dissociation was not evaluated in depth (i.e. CAPS de-realization and de-personalization items were the only dissociative features captured in this work). Although dissociative PTSD subtype was previously associated with altered neural mechanisms (Lanius et al., Citation2010), it was most common among PTSD stemming from childhood trauma (De Bellis, Woolley, & Hooper, Citation2013; Rivera-Vélez, González-Viruet, Martínez-Taboas, & Pérez-Mojica, Citation2014).
Despite many breakthroughs in technology, medicine and neuroscience research, there are still many gaps in our understanding and ability to predict PTSD trajectories. Being among one of the only mental disorders that has a defined onset, PTSD is an excellent target for secondary prevention and for mapping pathogenic processes. This study proposes the next necessary step, a large functional and structural brain imaging study of the evolving psychopathological consequences of traumatic events, enhancing our understanding of the nature of traumatic stress responses and its aftermath. Linking PTSD symptom trajectories with pertinent brain alterations (present initially, or developing simultaneously) and cognitive functioning, will contribute to classification and prediction of PTSD. While non-remitting symptom trajectory will inform the pathogenesis of chronic PTSD, a rapid remission trajectory may inform the science of resilience and recovery from trauma. Finally, our integrative, mechanism-oriented exploratory approach, may lead to improved early treatment and prevention of PTSD, thus improving the life of trauma survivors and increasing the cost-effectiveness of personalized intervention.
Authors’ contributions
ZB and NK carried out the procedural aspects of the study, the research assistants training, guidance and monitoring, management of participants and QA of data. PH managed the hospital interface, specifically with the ER at TLV. ZB and NF drafted the manuscript and finalized it, with the guidance of AS. RA, TH, IL, and AS initiated and supervised all procedures at TLV site. TH, IL, and AS designed, obtained funding and oversaw the implementation of the study. All authors have read and approved the final manuscript.
Declarations
Ethics: The research study meets all ethical regulations as required by the ethics committee at Tel-Aviv Sourasky Medical Center (Reference number 0207/14). All subjects will give written informed consent in accordance with the Declaration of Helsinki. The study ClinicalTrials.gov registration ID is NCT03756545; It was ‘retrospectively registered’ 28th of November 2018.
Acknowledgments
The authors would like to thank the research team at Tel-Aviv Sourasky Medical Center - including Nili Green, Mor Halevi, Sheli Luvton, Yael Shavit, Olga Nevenchannaya, Iris Rashap, Efrat Routledge and Ophir Leshets - for their major contribution in carrying out this research, including subjects’ recruitment and screening, and performing clinical, cognitive and neural assessments. We also extend our gratitude to all the participants of this study, which completed all the assessments at three different time-points after experiencing a traumatic event.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The datasets which will be used and/or analyzed during the current study will be available after QA and once brought to maturity, by contacting the study PI on reasonable request.
Additional information
Funding
References
- Abe, O., Yamasue, H., Kasai, K., Yamada, H., Aoki, S., Iwanami, A., … Ohtomo, K. (2006). Voxel-based diffusion tensor analysis reveals aberrant anterior cingulum integrity in posttraumatic stress disorder due to terrorism. Psychiatry Res - Neuroimaging, 146(3), 231–13.
- Admon, R., Leykin, D., Lubin, G., Engert, V., Andrews, J., Pruessner, J., & Hendler, T. (2013). Stress-induced reduction in hippocampal volume and connectivity with the ventromedial prefrontal cortex are related to maladaptive responses to stressful military service. Human Brain Mapping, 34(11), 2808–2816.
- Admon, R., Lubin, G., Rosenblatt, J. D., Stern, O., Kahn, I., Assaf, M., & Hendler, T. (2013). Imbalanced neural responsivity to risk and reward indicates stress vulnerability in humans. Cerebral Cortex (New York, N.Y.: 1991), 23(1), 28–35.
- Aupperle, R. L., Melrose, A. J., Stein, M. B., & Paulus, M. P. (2012). Executive function and PTSD: Disengaging from trauma. Neuropharmacology, 62(2), 686–694.
- Ayling, E., Aghajani, M., Fouche, J. P., & van der Wee, N. (2012). Diffusion tensor imaging in anxiety disorders. Current Psychiatry Reports, 14(3), 197–202.
- Beck, A., Epstein, N., Brown, G., & Steer, R. A. (1988). An inventory for measuring clinical anxiety: Psychometric properties. Journal of Consulting and Clinical Psychology, 56(6), 893.
- Beck, A. T., Steer, R. A., & Brown, G. K. (1996). Beck depression inventory-II. San Antonio, 78(2), 490–498.
- Ben-Zion, Z., Fine, N. B., Keynan, N. J., Admon, R., Green, N., Halevi, M., & Shalev, A. Y. (2018). Cognitive flexibility predicts PTSD symptoms: Observational and interventional studies. Frontiers in Psychiatry, 9, 477.
- Blake, D. D., Weathers, F. W., Nagy, L. M., Kaloupek, D. G., Gusman, F. D., Charney, D. S., & Keane, T. M. (1995). The development of a clinician-administered PTSD scale. Journal of Traumatic Stress, 8(1), 75–90.
- Blanchard, E. (1996). Psychometric properties of the PTSD checklist (PCL). Behaviour Research and Therapy, 34(8), 669–673.
- Bonne, O., Brandes, D., Gilboa, A., Gomori, J. M., Shenton, M. E., Pitman, R. K., & Shalev, A. Y. (2001). Longitudinal MRI study of Hippocampal volume in trauma survivors with PTSD. The American Journal of Psychiatry, 158(8), 1248–1251.
- Bremner, J. D., Elzinga, B., Schmahl, C., & Vermetten, E. (2007). Structural and functional plasticity of the human brain in posttraumatic stress disorder. Progress in Brain Research, 167, 171–186.
- Busner, J., & Targum, S. D. (2007). The clinical global impressions scale: Applying a research tool in clinical practice. Psychiatry (Edgmont), 4(7), 28–37.
- Casada, J. H., & Roache, J. D. (2005). Behavioral inhibition and activation in Posttraumatic stress disorder. The Journal of Nervous and Mental Disease, 193(2), 102–109.
- Davidson, J. R. T., Stein, D. J., Shalev, A. Y., & Yehuda, R. (2004). Posttraumatic stress disorder: Acquisition, recognition, course, and treatment. The Journal of Neuropsychiatry and Clinical Neurosciences, 16(2), 135–147.
- De Bellis, M. D., Woolley, D. P., & Hooper, S. R. (2013). Neuropsychological findings in pediatric maltreatment. Child Maltreatment, 18(3), 171–183.
- Do, L. L. T. N. (2011). American Psychiatric Association diagnostic and statistical manual of mental disorders (DSM-IV). In GoldsteinS., Naglieri J.A. (eds.). Encyclopedia of child behavior and development (pp. 84–85). Boston, MA: Springer US. doi:10.1007/978-0-387-79061-9_113.
- Duval, E. R., Joshi, S. A., Russman Block, S., Abelson, J. L., & Liberzon, I. (2018). Insula activation is modulated by attention shifting in social anxiety disorder. Journal of Anxiety Disorders, 56, 56–62.
- Fani, N., King, T. Z., Jovanovic, T., Glover, E. M., Bradley, B., Choi, K., & Ressler, K. J. (2012). White matter integrity in highly traumatized adults with and without post-traumatic stress disorder. Neuropsychopharmacology, 37(12), 2740–2746.
- Ferrari, M. C. F., Busatto, G. F., McGuire, P. K., & Crippa, J. A. S. (2008). Structural magnetic resonance imaging in anxiety disorders: An update of research findings. Revista Brasileira De Psiquiatria (Sao Paulo, Brazil: 1999), 30(3), 251–264.
- Fine, N. B., Achituv, M., Etkin, A., Merin, O., & Shalev, A. Y. (2018). Evaluating web-based cognitive-affective remediation in recent trauma survivors: Study rationale and protocol. European Journal of Psychotraumatology, 9(1), 1442602.
- First, M. B., Spitzer, R. L., Gibbon, M., & Williams, J. B. W. (1995). Biometrics research. New York State Psychiatric Institute; New York: 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. Clinical Trials Version (SCID-CT), 9(2), 92–104.
- Fischl, B. (2012). FreeSurfer. Neuroimage, 62(2), 774–781.
- Freedman, S. A., Brandes, D., Peri, T., & Shalev, A. (1999a). Predictors of chronic post-traumatic stress disorder. A prospective study. The British Journal of Psychiatry: the Journal of Mental Science, 174(4). Retrieved from http://bjp.rcpsych.org/content/174/4/353.short
- Freedman, S. A., Brandes, D., Peri, T., & Shalev, A. Y. (1999b). Predictors of chronic post-traumatic stress disorder: A prospective study. The British Journal of Psychiatry: the Journal of Mental Science, 174(4), 353–359.
- Friston, K. J., Holmes, A. P., Worsley, K. J., Poline, J.-P., Frith, C. D., & Frackowiak, R. S. J. (1994). Statistical parametric maps in functional imaging: A general linear approach. Human Brain Mapping, 2(4), 189–210.
- Fydrich, T., Dowdall, D., & Chambless, D. L. (1992). Reliability and validity of the beck anxiety inventory. Journal of Anxiety Disorders, 6(1), 55–61.
- Galatzer-Levy, I. R., Ankri, Y., Freedman, S., Israeli-Shalev, Y., Roitman, P., Gilad, M., … Felmingham, K. (2013). Early PTSD symptom trajectories: Persistence, recovery, and response to treatment: Results from the Jerusalem Trauma Outreach and Prevention Study (J-TOPS). PloS One, 8(8), e70084.
- Galatzer-Levy, I. R., & Bryant, R. A. (2013). 636,120 ways to have posttraumatic stress disorder. Perspectives on Psychological Science: A Journal of the Association for Psychological Science, 8(6), 651–662.
- Gilbertson, M. W., Shenton, M. E., Ciszewski, A., Kasai, K., Lasko, N. B., Orr, S. P., & Pitman, R. K. (2002). Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nature Neuroscience, 5(11), 1242–1247.
- Guy, W. (1976). CGI. Clinical global impressions. ECDEU, Assessment Manual for Psychopharmacology, 217–222.
- Hariri, A. R., Bookheimer, S. Y., & Mazziotta, J. C. (2003). Modulating emotional responses. Neuroreport, 11(1), 43–48.
- Hart, R. P., Bagrodia, R., Rahman, N., Bryant, R. A., Titcombe-Parekh, R., Marmar, C. R., & Brown, A. D. (2017). Neuropsychological predictors of trauma centrality in OIF/OEF veterans. Frontiers in Psychology, 8, 1120.
- Hirschfeld, R. M. A., Williams, J. B. W., Spitzer, R. L., Calabrese, J. R., Flynn, L., Keck, P. E., … Zajecka, J. (2000). Development and validation of a screening instrument for bipolar spectrum disorder: The mood disorder questionnaire. The American Journal of Psychiatry, 157(11), 1873–1875.
- Jenkinson, M., Beckmann, C. F., Behrens, T. E. J., Woolrich, M. W., & Smith, S. M. (2012). FSL. Neuroimage, 62(2), 782–790.
- Johnsen, G. E., & Asbjørnsen, A. E. (2008). Consistent impaired verbal memory in PTSD: A meta-analysis. Journal of Affective Disorders, 111(1), 74–82.
- Kahn, I., Yeshurun, Y., Rotshtein, P., Fried, I., Ben-Bashat, D., & Hendler, T. (2002). The role of the amygdala in signaling prospective outcome of choice. Neuron, 33(6), 983–994.
- Kasai, K., Yamasue, H., Gilbertson, M. W., Shenton, M. E., Rauch, S. L., & Pitman, R. K. (2008). Evidence for acquired pregenual anterior cingulate gray matter loss from a twin study of combat-related posttraumatic stress disorder. Biological Psychiatry, 63(6), 550–556.
- Kessler, R. C. (2000). Posttraumatic stress disorder: The burden to the individual and to society. The Journal of Clinical Psychiatry, 61, 4–12.
- Kessler, R. C., Sonnega, A., Bromet, E., Hughes, M., & Nelson, C. B. (1995). Posttraumatic stress disorder in the national comorbidity survey. Archives of General Psychiatry, 52(12), 1048.
- Kim, S. J., Jeong, D. U., Sim, M. E., Bae, S. C., Chung, A., Kim, M. J., & Lyoo, I. K. (2007). Asymmetrically altered integrity of cingulum bundle in posttraumatic stress disorder. Neuropsychobiology, 54(2), 120–125.
- Klumpp, H., Ho, S. S., Taylor, S. F., Phan, K. L., Abelson, J. L., & Liberzon, I. (2011). Trait anxiety modulates anterior cingulate activation to threat interference. Depression and Anxiety, 28(3), 194–201.
- Koch, S. B. J., van Zuiden, M., Nawijn, L., Frijling, J. L., Veltman, D. J., & Olff, M. (2016). Aberrant resting-state brain activity in posttraumatic stress disorder: A meta-analysis and systematic review. Depression and Anxiety. doi:10.1002/da.22478
- Koenen, K. C., Driver, K. L., Oscar-Berman, M., Wolfe, J., Folsom, S., Huang, M. T., & Schlesinger, L. (2001). Measures of prefrontal system dysfunction in posttraumatic stress disorder. Brain and Cognition, 45(1), 64–78.
- Koren, D., Arnon, I., & Klein, E. (2001). Long term course of chronic posttraumatic stress disorder in traffic accident victims: A three-year prospective follow-up study. Behaviour Research and Therapy, 39(12), 1449–1458.
- Lanius, R. A., Vermetten, E., Loewenstein, R. J., Brand, B., Schmahl, C., Bremner, J. D., & Spiegel, D. (2010). Emotion modulation in PTSD: Clinical and neurobiological evidence for a dissociative subtype. The American Journal of Psychiatry, 167(6), 640–647.
- Leskin, L. P., & White, P. M. (2007). Attentional networks reveal executive function deficits in posttraumatic stress disorder. Neuropsychology, 21(3), 275–284.
- Liberzon, I., & Sripada, C. S. (2007). The functional neuroanatomy of PTSD: A critical review. Progress in Brain Research, 167, 151–169.
- Marmar, C. R., Weiss, D. S., Schlenger, W. E., Fairbank, J. A., Jordan, B. K., Kulka, R. A., & Hough, R. L. (1994). Peritraumatic dissociation and posttraumatic stress in male Vietnam theater veterans. The American Journal of Psychiatry, 151(6), 902–907.
- Mayou, R., Tyndel, S., & Bryant, B. (1997). Long-term outcome of motor vehicle accident injury. Psychosomatic Medicine, 59(6), 578–584.
- O’Donnell, M. L., Creamer, M., Bryant, R. A., Schnyder, U., & Shalev, A. (2003). Posttraumatic disorders following injury: An empirical and methodological review. Clinical Psychology Review, 23(4), 587–603.
- Peleg, T., & Shalev, A. Y. (2006). Longitudinal studies of PTSD: Overview of findings and methods. CNS Spectrums, 11(8), 589–602.
- Perkonigg, A., Pfister, H., Stein, M. B., Höfler, M., Lieb, R., Maercker, A., & Wittchen, H.-U. (2005). Longitudinal course of posttraumatic stress disorder and posttraumatic stress disorder symptoms in a community sample of adolescents and young adults. The American Journal of Psychiatry, 162(7), 1320–1327.
- Phan, K. L., Orlichenko, A., Boyd, E., Angstadt, M., Coccaro, E. F., Liberzon, I., & Arfanakis, K. (2009). Preliminary evidence of white matter abnormality in the uncinate fasciculus in generalized social anxiety disorder. Biological Psychiatry, 66(7), 691–694.
- Pineles, S. L., Shipherd, J. C., Mostoufi, S. M., Abramovitz, S. M., & Yovel, I. (2009). Attentional biases in PTSD: More evidence for interference. Behaviour Research and Therapy, 47(12), 1050–1057.
- Pitman, R. K., Rasmusson, A. M., Koenen, K. C., Shin, L. M., Orr, S. P., Gilbertson, M. W., … Liberzon, I. (2012). Biological studies of post-traumatic stress disorder. Nature Reviews. Neuroscience, 13(11), 769–787.
- Polak, A. R., Witteveen, A. B., Reitsma, J. B., & Olff, M. (2012). The role of executive function in posttraumatic stress disorder: A systematic review. Journal of Affective Disorders, 141(1), 11–21.
- Qi, W., Ratanatharathorn, A., Gevonden, M., Bryant, R., Delahanty, D., Matsuoka, Y., … Shalev, A. (2018). Application of data pooling to longitudinal studies of early post-traumatic stress disorder (PTSD): The international consortium to predict PTSD (ICPP) project. European Journal of Psychotraumatology, 9, 1.
- Reitan, R. (1958). Validity of the trail making test as an indicator of organic brain damage. Perceptual and Motor Skills, 8, 271–276.
- Rivera-Vélez, G. M., González-Viruet, M., Martínez-Taboas, A., & Pérez-Mojica, D. (2014). Post-traumatic stress disorder, dissociation, and neuropsychological performance in latina victims of childhood sexual abuse. Journal of Child Sexual Abuse. doi:10.1080/10538712.2014.864746
- Rohde, P., Lewinsohn, P. M., & Seeley, J. R. (1997). Comparability of telephone and face-to-face interviews in assessing axis I and II disorders. The American Journal of Psychiatry, 154(11), 1593–1598.
- Samuelson, K. W. (2011). Post-traumatic stress disorder and declarative memory functioning: A review. Dialogues in Clinical Neuroscience, 13(3), 346–351. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/22033732
- Schuff, N., Zhang, Y., Zhan, W., Lenoci, M., Ching, C., Boreta, L., & Neylan, T. C. (2011). Patterns of altered cortical perfusion and diminished subcortical integrity in posttraumatic stress disorder: An MRI study. Neuroimage, 54, S62–S68.
- Scott, J. C., Matt, G. E., Wrocklage, K. M., Crnich, C., Jordan, J., Southwick, S. M., & Schweinsburg, B. C. (2015). A quantitative meta-analysis of neurocognitive functioning in posttraumatic stress disorder. Psychological Bulletin, 141(1), 105–140.
- Shalev, A. Y., Freedman,S., Peri, T., Brandes, D., & Sahar, T. (1997). Predicting PTSD intrauma survivors: prospective evaluation of self-report and clinicianadministeredinstruments. The British Journal of Psychiatry, 170(6), 558–564.
- Shalev, A., Liberzon, I., & Marmar, C. (2017). Post-traumatic stress disorder. The New England Journal of Medicine, 376(25), 2459–2469.
- Shalev, A. Y. (1996). Prospective follow up studies of PTSD: Psychological and biological predictors. European Neuropsychopharmacology: The Journal of the European College of Neuropsychopharmacology, 8, S112–S113.
- Shalev, A. Y., Ankri, Y., Israeli-Shalev, Y., Peleg, T., Adessky, R., & Freedman, S. (2012a). Prevention of posttraumatic stress disorder by early treatment. Archives of General Psychiatry, 69(2), 166.
- Shalev, A. Y., Ankri, Y., Israeli-Shalev, Y., Peleg, T., Adessky, R., & Freedman, S. (2012b). Prevention of posttraumatic stress disorder by early treatment. Archives of General Psychiatry, 69(2), 166–176.
- Shalev, A. Y., Ankri, Y. L. E., Peleg, T., Israeli-Shalev, Y., & Freedman, S. (2011). Barriers to receiving early care for PTSD: Results from the Jerusalem trauma outreach and prevention study. Psychiatric Services (Washington, D.C.), 62(7), 765–773.
- Shalev, A. Y., & Freedman, S. (2005). PTSD following terrorist attacks: A prospective evaluation. The American Journal of Psychiatry, 162(6), 1188–1191.
- Shalev, A. Y., Gevonden, M., Ratanatharathorn, A., Laska, E., van der Mei, W. F., Qi, W., & Koenen, K. C. (2019). Estimating the risk of PTSD in recent trauma survivors: Results of the international consortium to predict PTSD (ICPP). World Psychiatry: Official Journal of the World Psychiatric Association (WPA), 18(1), 77–87.
- Shalev, A. Y., Peri, T., Canetti, L., & Schreiber, S. (1996). Predictors of PTSD in injured trauma survivors. The American Journal of Psychiatry, 153, 219–225.
- Shin, L. M., Rauch, S. L., & Pitman, R. K. (2006). Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Annals of the New York Academy of Sciences, 1071(1), 67–79.
- Silverstein, S., Berten, S., Olson, P., Paul, R., Williams, L. M., Cooper, N., & Gordon, E. (2007). Development and validation of a World-Wide-Web-based neurocognitive assessment battery: WebNeuro. Behavior Research Methods, 39(4), 940–949.
- Sripada, R. K., King, A. P., Welsh, R. C., Garfinkel, S. N., Wang, X., Sripada, C. S., & Liberzon, I. (2012). Neural dysregulation in posttraumatic stress disorder: Evidence for disrupted equilibrium between salience and default mode brain networks. Psychosomatic Medicine, 74(9), 904–911.
- Sripada, R. K., Marx, C. E., King, A. P., Rampton, J. C., Ho, S. S., & Liberzon, I. (2013). Allopregnanolone elevations following pregnenolone administration are associated with enhanced activation of emotion regulation neurocircuits. Biological Psychiatry, 73(11), 1045–1053.
- Stein, M. B., Walker, J. R., Hazen, A. L., & Forde, D. R. (1997). Full and partial posttraumatic stress disorder: Findings from a community survey. The American Journal of Psychiatry, 154(8), 1114–1119.
- Stroop, J. R. (1935). Stroop color word test. Journal of Experimental Pathology, (18), 643–662. doi:10.1007/978-0-387-79948-3
- Vasterling, J. J., Brailey, K., Constans, J. I., & Sutker, P. B. (1998). Attention and memory dysfunction in posttraumatic stress disorder. Neuropsychology, 12(1), 125–133.
- Weathers, F. W., Bovin, M. J., Lee, D. J., Sloan,D. M., Schnurr, P. P., Kaloupek, D. G., ... & Marx, B. P. (2018). The Clinician-Administered PTSD Scale for DSM–5 (CAPS-5): Development and initial psychometric evaluation in military veterans. Psychological Assessment, 30(3), 383.
- Weathers, F. W., Litz, B. T., Herman, D. S., Huska, J. A., & Keane, T. M. (1993). The PTSD checklist: Reliability, validity, and diagnostic utility. In Annual convention of the international society for traumatic stress studies, San Antonio, TX (Vol. 462).
- Yehuda, R., McFarlane, A. C., & Shalev, A. Y. (1998a). Predicting the development of posttraumatic stress disorder from the acute response to a traumatic event. Biological Psychiatry, 44(12), 1305–1313.
- Yehuda, R., McFarlane, A. C., & Shalev, A. Y. (1998b). Predicting the development of posttraumatic stress disorder from the acute response to a traumatic event. Biological Psychiatry, 44(12), 1305–1313.
- Zhang, Z.-J., Tang, L.-H., Wang, -Z.-Z., Yin, H., Chen, Y.-C., Wang, H.-N., & Li, L.-J. (2010). Psychopathological, biological, and neuroimaging characterization of posttraumatic stress disorder in survivors of a severe coalmining disaster in China. Journal of Psychiatric Research, 44(6), 385–392.
- Zoellner, L. A., Bedard-Gilligan, M. A., Jun, J. J., Marks, L. H., & Garcia, N. M. (2013). The evolving construct of posttraumatic stress disorder (PTSD): DSM-5 criteria changes and legal implications. Psychological Injury and Law, 6(4), 277–289.