ABSTRACT
Cognitive-behavioural conjoint therapy (CBCT) for PTSD has been shown to improve PTSD, relationship adjustment, and the health and well-being of partners. MDMA (3,4-methylenedioxymethamphetamine) has been used to facilitate an individual therapy for PTSD. This study was an initial test of the safety, tolerability, and efficacy of MDMA-facilitated CBCT. Six couples with varying levels of baseline relationship satisfaction in which one partner was diagnosed with PTSD participated in a condensed version of the 15-session CBCT protocol delivered over 7 weeks. There were two sessions in which both members of the couple were administered MDMA. All couples completed the treatment protocol, and there were no serious adverse events in either partner. There were significant improvements in clinician-assessed, patient-rated, and partner-rated PTSD symptoms (pre- to post-treatment/follow-up effect sizes ranged from d = 1.85–3.59), as well as patient depression, sleep, emotion regulation, and trauma-related beliefs. In addition, there were significant improvements in patient and partner-rated relationship adjustment and happiness (d =.64–2.79). These results are contextualized in relation to prior results from individual MDMA-facilitated psychotherapy and CBCT for PTSD alone. MDMA holds promise as a facilitator of CBCT to achieve more robust and broad effects on individual and relational functioning in those with PTSD and their partners.
HIGHLIGHTS
• MDMA was combined with cognitive-behavioural conjoint therapy for PTSD in six couples revealing significant improvements in PTSD, depression, sleep, emotion regulation, trauma beliefs, and relationship satisfaction.• Controlled studies are planned based on these promising results.
Se ha demostrado que la terapia conjunta cognitivo-conductual (TCCC) para el TEPT mejora TEPT, el ajuste de la relación, y la salud y el bienestar de las parejas. Se ha utilizado MDMA (3,4-metilendioximetanfetamina) para facilitar una terapia individual para el TEPT. Este estudio fue una prueba inicial acerca de la seguridad, tolerabilidad y eficacia de la TCCC facilitada por MDMA. Seis parejas con diferentes niveles de línea de base de su satisfacción en la relación de pareja, en las que uno de ellos fue diagnosticado con TEPT, participaron en una versión condensada del protocolo TCCC de 15 sesiones entregado durante 7 semanas. Hubo dos sesiones en las que a ambos miembros de la pareja se les administró MDMA. Todas las parejas completaron el protocolo de tratamiento y no hubo eventos adversos graves en ninguno de las parejas. Hubo mejorías significativas en los síntomas de TEPT evaluados por el médico, por el paciente y por la pareja (los tamaños del efecto antes y después del tratamiento/seguimiento variaron de d = 1,85 a 3,59), así como la depresión del paciente, el sueño, la regulación emocional y las creencias relacionadas con el trauma. Además, hubo mejorías significativas en la adaptación y satisfacción de la relación calificada por el paciente y la pareja (d =.64-2.79). Estos resultados se contextualizan en relación con los resultados anteriores de la psicoterapia individual facilitada por MDMA y TCCC solo para el TEPT. La MDMA se muestra prometedora como facilitadora de TCCC para lograr efectos más sólidos y amplios en el funcionamiento individual y relacional de las personas con TEPT y sus parejas.
PTSD的认知行为联合疗法 (CBCT) 已被证明可以改善PTSD, 关系调整和伴侣的身心健康。MDMA (3,4-亚甲基二氧基甲基苯丙胺) 已用于辅助PTSD的个体治疗。本研究是对MDMA辅助CBCT的安全性, 耐受性和有效性的初步测试。六对基线关系满意度不同的夫妻, 其中一名伴侣被诊断患有PTSD, 参 加了为期7周的15次精简版CBCT方案。有两次夫妇双方都被给与了MDMA。所有夫妇均完成了治疗方案, 并且任何一方均未发生严重不良事件。临床医生评估, 患者评估和伴侣评估的PTSD症状 (治疗前至治疗后/随访效果量范围为d = 1.85-3.59) 及患者抑郁, 睡眠, 情绪调节以及创伤相关信念都有了显著改善。此外, 患者和伴侣之间的关系调整和幸福感得到了显著改善 (d =.64-2.79) 。这些结果与个体MDMA辅助的心理治疗和仅针对PTSD的CBCT的先前结果相关。MDMA有望作为CBCT的辅助因素, 对PTSD患者及其伴侣的个人和关系功能产生更稳健, 更广泛的影响。
PALABRAS CLAVE:
Cognitive-behavioural conjoint therapy for PTSD (CBCT; Monson & Fredman, Citation2012) is a manualized psychotherapy shown to improve posttraumatic stress disorder (PTSD) and comorbid conditions, enhance relationship functioning, and improve intimate partner well-being (Liebman, Whitfield, Sijercic, Ennis, & Monson, Citationin press, for review). MDMA’s (3,4-methylenedioxymethamphetamine) empathogenic and neurocognitive properties make it a promising facilitator of this trauma-focused, empirically supported relational therapy (e.g. Feduccia & Mithoefer, Citation2018). Previous studies using MDMA with individual psychotherapy have yielded significant improvements in PTSD symptoms, and other outcomes such as increased openness to experience, posttraumatic growth, and improvement in comorbid conditions (Gorman et al., Citation2020; Mithoefer et al., Citation2019, Citation2018; Wagner et al., Citation2017). MDMA had not yet been tested alongside a stand-alone, empirically-supported psychotherapy for PTSD. We sought to fill this gap by using MDMA to potentially facilitate the effects of CBCT for PTSD.
CBCT for PTSD (Monson & Fredman, Citation2012) has been tested in community and military/veteran samples in uncontrolled and controlled trials. These trials have revealed significant improvements in clinician- and patient-rated PTSD severity, comorbid conditions, and relationship improvements (see Liebman et al., Citationin press for review). Compared with individual therapies for PTSD, CBCT for PTSD focuses on the relationship between the participants as the treatment target and engages participants in developing their skills as a dyad, both in terms of communication as well as reduction of avoidance and challenging of beliefs. MDMA’s empathogenic qualities are posited to be a natural fit with the purported mechanisms of CBCT for PTSD in that MDMA can support the dyadic process in psychotherapy through enhancing feelings of connection and greater ease in communicating. We therefore conducted an initial test of the safety, feasibility and efficacy of MDMA-facilitated CBCT in an uncontrolled trial of six couples with a range of baseline relationship satisfaction in which one member was diagnosed with PTSD resulting from a range of traumas. We hypothesized that MDMA-facilitated CBCT would be safe, feasible, and result in significant and sustained improvements in PTSD, its common comorbidities, and relationship adjustment and happiness.
1. Method
Relevant institutional review and research ethics boards, as well as the US Food and Drug Administration, reviewed and approved the conduct of the trial.
1.1. Participants
Inclusion in the study required that one partner has a current PTSD diagnosis according to the Clinician-Administered PTSD Scale for the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (CAPS-5; Weathers et al., Citation2013a), with a duration of at least 6 months (referred to as ‘Patient’ for simplicity). In addition, all individuals had to be at least 18 years old; generally healthy per physical examination and medical history; proficient in speaking and reading English; willing to refrain from taking any psychiatric medications during the study period; willing to follow restrictions and guidelines concerning consumption of food, beverages, and nicotine the night before and just prior to each MDMA session; practising birth control if able to bear children; willing to consent to videorecording of assessment and treatment sessions; and willing to refrain from participating in any other interventional clinical trials for the duration of this study. Exclusion criteria for all individuals were acute psychosis, acute mania, substance use disorder, pregnancy or nursing (in women), or weighing less than 48 kg.
Average age of all participants was about 47 years, all were Caucasian, and all were heterosexual couples. Regarding patient participants, four were male, five had a history of multiple traumatic events (index traumas for PTSD diagnosis included childhood physical abuse, childhood sexual abuse, and combat), and all had comorbid diagnoses and prior pharmacological and psychosocial treatment. All patients had a history of psychotherapy; 60% had previously received trauma-focused therapy and 40% had received dialectical behaviour therapy and cognitive-behavioural therapy (not trauma-focused). In terms of partners, 50% were diagnosed with at least one mental health condition (not PTSD per inclusion/exclusion criteria) according to clinician assessment. At baseline, two patients were relationally distressed and three partners were relationally distressed according to cut points on the Couples Satisfaction Index (Funk & Rogge, Citation2007; see ).
Table 1. Demographic characteristics for patients and partners
1.2. Treatment
Participants received all of the session content comprising CBCT; MDMA sessions were timed to synergize with the CBCT interventions (e.g. following the development of communication skills, and in the midst of cognitive processing; see MAPS, Citation2016; Wagner, Mithoefer, Mithoefer, & Monson, Citation2019 for protocol specifics). CBCT for PTSD includes content related to psychoeducation about trauma and relationships, increasing relational safety, communication skills, tools for behavioural approach, and dyadic cognitive intervention related to problematic trauma-related and relationship cognitions. The first three sessions of CBCT were delivered in person the day before the first MDMA session (4 hours), and sessions 4 and 5 focused on feelings and thoughts (1 hour) were delivered in person the morning before MDMA administration (6–8 hours). An integration session (1.5 hours) took place the morning after the MDMA session. Following this intensive weekend of treatment, sessions 6 through 9 of CBCT were delivered biweekly via secure video over the next 2.5 weeks (1.25 hours each). The second intensive weekend consisted of sessions 10 and 11 focused on appraising blame and trust (2 hours) delivered in person the day before the second MDMA session, the second MDMA session (6–8 hours), and an integration session (1.5 hours) the following day. The final four sessions of CBCT (sessions 12 to 15) were conducted weekly over video (1.25 hours each). The entire duration of treatment was 7 weeks.
Each partner was given 75 mg MDMA in the first MDMA session, and 100 mg in the second MDMA session, with an optional supplemental half-dose 1.5 hours later in both sessions (participants were informed that the supplemental half-doses could prolong the therapeutic window of MDMA effects). Co-therapists with expertise in MDMA and CBCT, respectively, delivered the therapy sessions. Adverse events were monitored from study enrolment to treatment completion, and at 3-month and six-month follow-up. Spontaneously reported experiences and specific adverse effects were monitored on the day of each experimental session and subsequent contact days with a standardized list used in MDMA studies (MAPS, Citation2016).
1.3. Assessment
Each member of the couple was scheduled for assessment at pre-treatment, mid-treatment, post-treatment, and 3- and 6-month follow-ups. In addition, participants completed assessments of self- and partner-reported PTSD symptoms and overall relationship happiness at those assessment points, as well as at each treatment occasion. Well-validated measures of the outcomes were used, and independent assessors trained to reliability completed the clinician interviews. There was no missing clinician interview for PTSD data. Across all the other self-report outcome measures and occasions, the overall assessment completion rate was 86%.
Participants were assessed using the Structured Clinical Interview for DSM-5 (SCID-5; First, Williams, Karg, & Spitzer, Citation2015) at baseline to determine eligibility and characterize the sample. The Clinician-Administered PTSD Scale-5 (CAPS-5; Weathers et al., Citation2013a) was used as the clinician-rated primary outcome measure of PTSD symptoms, and the PTSD Checklist (PCL-5, patient and partner versions; Weathers et al., Citation2013b) and Couples Satisfaction Index (CSI; Funk & Rogge, Citation2007) were used as self-report primary outcomes. The overall relationship happiness item from the CSI was also completed at each visit.
Secondary outcome measures included self-report measures of the Beck Depression Inventory (BDI-II; Beck, Steer, & Brown, Citation1996) for depression, the Pittsburgh Sleep Quality Questionnaire for sleep disturbances (Buysse, Reynolds, Monk, Berman, & Kupfer, Citation1989), the Emotion Regulation Questionnaire (Gross & John, Citation2003) for emotion regulation strategies related to reappraisal and suppression, and the Traumatic and Attachment Beliefs Scale for trauma-related beliefs (Pearlman, Citation2003).
1.4. Analyses
Analyses were conducted in SPSS Version 26 (Citation2018). Growth curve models were used to analyse outcomes at each assessment, with time transformed to be the natural log function of the number of days since baseline. Due to the small sample size, slopes were fixed and intercepts were allowed to vary to account for different starting values in each outcome. In accordance with recommended guidelines (Feingold, Citation2009), within-group effect sizes (Cohen’s d) from pre-treatment to each major assessment time point were calculated on estimated means from the models for each outcome and raw pooled standard deviations for the relevant assessment period.
2. Results
All couples completed the protocol, and there were no serious adverse events. As documented in the supplementary tables, the most common reactions following MDMA sessions in patients and partners were diminished appetite, anxiety, headache, and jaw tightness.
2.1. PTSD and patient comorbid outcomes
Growth curve modelling revealed significant and sustained improvements in clinician-assessed PTSD (B = −4.64, p < .001), with d = 1.88–2.25 at post-treatment and follow-ups (see ). All but one patient showed a sustained remission in their clinician-assessed PTSD diagnosis. Self- (B = −8.14, p < .001) and partner-rated (B = −5.90, p < .001) PTSD symptoms significantly improved, with d = 2.72–3.59 for patients, and 1.85–2.72 for partners at post-treatment and follow-ups, respectively. includes actual means across visits and follow-ups, and in relation to the assessments done after each MDMA session.
Figure 1. Patient- and partner-rated actual mean PTSD symptoms and relationship happiness across treatment and follow-ups
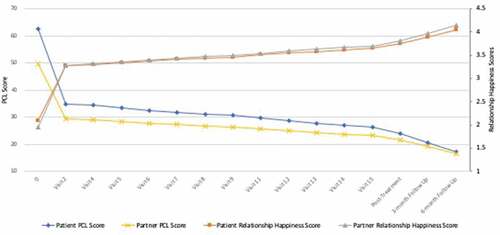
Growth curve models also revealed overall improvements in patients’ depression (B = −4.24, d = 1.50–2.53), sleep (B = −.81, p < .01, d = .88–1.18), emotion regulation (reappraisal B = 1.38, p < .01, d = 1.09–1.15; suppression B = −1.12, p < .01, d = 1.12–1.20), and overall trauma-related beliefs (B = −12.01, p < .01, d = .98–1.17) at post-treatment and follow-ups (see ).
Table 2. Estimated means and standard deviations at each assessment point
Table 3. Effect size changes for outcomes (Cohen’s d)
2.2. Intimate relationship outcomes
Growth curves revealed significant improvements in overall patient and partner relationship satisfaction (B = 4.55, p < .05, d = .82–1.22 for patients and B = 5.73, p < .05, d = .64-.80 for partners). Both patients who were relationally distressed at baseline were in the satisfied range at posttreatment and follow-ups. One patient who was relationally satisfied at baseline was relationally dissatisfied at posttreatment and follow-ups (this patient also retained their PTSD diagnosis). Of the three partners who were relationally distressed at baseline, only 1 was relationally distressed at posttreatment and follow-ups (partner of the patient with retained PTSD). Growth curve modelling of the relationship happiness item across treatment and follow-ups also revealed significant improvements for both patients (B = .35, p < .001) and partners (B = .33, p < .001), with d = 1.42–2.79 for patients and 1.30–1.78 for partners at post-treatment and follow-ups (see ).
3. Discussion
This is the first study to investigate the use of MDMA to facilitate a therapy established to be efficacious outside of MDMA treatment studies. Our initial data indicates that MDMA delivered in combination with CBCT for PTSD appears to be safe, does not appear to be treatment-interfering, and may potentiate the treatment effects for PTSD and the larger relationship context in which it exists. In the 1970s and 1980s, before MDMA was placed on schedule 1, it was used in clinical practice to facilitate couple therapy, although no controlled trials were conducted. MDMA has more recently been studied to facilitate an individual therapy for PTSD (Mithoefer et al., Citation2019). This initial study suggests that MDMA-facilitated CBCT holds promise in facilitating trauma recovery and achieving broader relational outcomes not fully realized with individual evidence-based treatment for PTSD.
The effect sizes for improvements in PTSD and common co-occurring conditions in this pilot study were greater than those found with individually delivered MDMA-facilitated psychotherapy for PTSD (see Mithoefer et al., Citation2019 for pooled pre- to post-treatment effect in six phase 2 RCTs of approximately d = 1.20). In addition, the effects on PTSD, other symptoms, and relationship outcomes in this study were on par with, or greater than, those previously achieved with CBCT alone. More specifically, the largest pre- to post-treatment PTSD effect sizes found with CBCT come from Monson et al.’s (Citation2012) waitlist controlled trial with a mixed trauma sample. In that study, the effects for clinician-rated PTSD from pre- to post-treatment were g = 1.82, and g = .64 for patient-rated and g = .15 for partner-rated relationship adjustment. In comparison, the effects in the current trial were d = 2.10 for clinician-rated PTSD and d = .82 for patient-rated and g = .64 for partner-rated overall relationship adjustment. Interestingly, in the current study, across all outcomes, the effects were generally largest at 6-month follow-up, suggesting that MDMA facilitation may confer ongoing benefits. It is important to note that comparison of these effect sizes is tentative because of the small sample and lack of a control condition in the current study.
There are several limitations to the current study. First and foremost, there was no randomization or control condition, which severely limits the conclusions we can draw. We are currently preparing a Phase 2 randomized controlled trial to more rigorously investigate the safety and efficacy of MDMA-facilitated CBCT. Moreover, the therapy was delivered by expert therapists in each of the modalities, which is expected at this stage of treatment development, but limits generalizability. The size of the trial precluded examination of potential moderators of treatment outcomes (e.g. gender of patient and partner, pre-treatment relationship distress, type of index trauma), as well as mechanisms of treatment action. These are important questions to consider in future studies examining the use of MDMA to facilitate existing evidence-based treatments.
Although there are frontline, recommended therapies for PTSD that produce excellent outcomes for many individuals with PTSD (Forbes, Bisson, Monson, & Berliner, Citationin press), there is still a need to innovate treatment approaches for those individuals who do not tolerate these therapies or respond adequately to them. In addition, there is room to expand the breadth of outcomes that might be achieved, including improvements in intimate relationship functioning. MDMA-facilitated CBCT may be one avenue of facilitating psychotherapy to improve the lives of those who suffer from PTSD, as well as the lives of their loved ones.
COI Statements
Dr Monson receives book royalties from Guilford Press related to the sale of the treatment manual of interest to this study. Drs. Monson and Wagner received payment from MAPS for the provision of treatment for the study. Dr and Ms Mithoefer receive payments from MAPS Public Benefit Corporation related to their work in Medical Affairs, Training and Supervision, and from Awakn Life Sciences as members of their Scientific Advisory Board. Drs. Feduccia and Jerome received payment from MAPS Public Benefit Corporation as employees for work related to this study. Dr Yazar-Klosinski is a salaried employee of MAPS and received payment for work related to this study. Dr Doblin receives payment from MAPS for his work as Executive Director, which included work related to this study. Dr Liebman has no conflict of interests to disclose.
Supplemental Material
Download MS Word (23.6 KB)Supplemental Material
Download MS Word (28.3 KB)Acknowledgments
ClinicalTrials.gov identifier (NCT number): NCT02876172. This study was funded by the Multidisciplinary Association for Psychedelic Studies. The authors would like to extend their gratitude to the couples who graciously agreed to allow us to work with them toward trauma recovery. In addition, the authors would like to acknowledge Drs. Matthew Friedman, Paula Schnurr, and John Krystal for originally recognizing the promise of the combination of CBCT and MDMA. This paper is dedicated to the memory of Sarah Sadler - a remarkable person and beloved colleague. Without her, this study would not have been possible.
Data sharing statement
The data that support the findings of this study are openly available in the Open Science Framework Registry at https://osf.io/7fuwd, DOI: 10.17605/OSF.IO/7FUWD.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Beck, A. T., Steer, R. A., & Brown, G. K. (1996). Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation.
- Buysse, D. J., Reynolds, C. F., III, Monk, T. H., Berman, S. R., & Kupfer, D. J. (1989). The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Journal of Psychiatric Research, 28(2), 193–7.
- Feduccia, A. A., & Mithoefer, M. C. (2018). MDMA-assisted psychotherapy for PTSD: Are memory reconsolidation and fear extinction underlying mechanisms? Progress in Neuropsychopharmacology & Biological Psychiatry, 84, 221–228.
- Feingold, A. (2009). Effect sizes for growth-modeling analysis for controlled clinical trials in the same metric as for classical analysis. Psychological Methods, 14(1), 43–53.
- First, M. B., Williams, J. B. W., Karg, R. S., & Spitzer, R. L. (2015). Structured clinical interview for DSM-5-Research version (SCID-5 for DSM-5, research version; SCID-5 RV). Arlington, VA: American Psychiatric Association.
- Forbes, D., Bisson, J. I., Monson, C. M., & Berliner, L. A. (in press). Effective treatments for PTSD: Practice guidelines from the International Society for Traumatic Stress Studies (3rd ed.). New York: Guilford.
- Funk, J. L., & Rogge, R. D. (2007). Testing the ruler with item response theory: Increasing precision of measurement for relationship satisfaction with the Couples Satisfaction Index. Journal of Family Psychology, 21(4), 572–583.
- Gorman, I., Belser, A. B., Jermone, L., Hennigan, C., Shechet, B., Hamilton, S., & Feduccia, A. A. (2020). Posttraumatic growth after MDMA-Assisted psychotherapy for posttraumatic stress disorder. Journal of Traumatic Stress, 33(2), 161–170.
- Gross, J. J., & John, O. P. (2003). Individual differences in two emotion regulation processes: Implications for affect, relationships, and well-being. Journal of Personality and Social Psychology, 85(2), 348–362.
- IBM Corp. Released 2018. IBM SPSS statistics for windows, version 26.0. Armonk, NY: Author.
- Liebman, R. E., Whitfield, K., Sijercic, I., Ennis, N., & Monson, C. M. (in press). Harnessing the healing power of relationships in trauma recovery: A review of cognitive-behavioral conjoint therapy for PTSD. Current Treatment Options in Psychiatry.
- MAPS. (2016, March). A phase 1/2 open-label treatment development study of MDMA-assisted Cognitive-Behavioral Conjoint Therapy (CBCT) in dyads in which one member has chronic posttraumatic stress disorder (PTSD). Retrieved from https://s3-us-west-1.amazonaws.com/mapscontent/research-archive/MPVA-1+Protocol+Amend+2+V1_Final_02Mar2016_WEB.pdf
- Mithoefer, M. C., Feduccia, A. A., Jerome, L., Mithoefer, A., Wagner, M., Walsh, Z., … Doblin, R. (2019). MDMA-assisted psychotherapy for treatment of PTSD: Study design and rationale for phase 3 trials based on pooled analysis of six phase 2 randomized controlled trials. Psychopharmacology, 236(9), 2735–2745.
- Mithoefer, M. C., Mithoefer, A. T., Feduccia, A. A., Jerome, L., Wagner, M. T., Wymer, J., … Doblin, R. (2018). 3,4-methylenedioxymethamphetamine (MDMA)-assisted psychotherapy for post-traumatic stress disorder in military veterans, firefighters, and police officers: A randomised, double-blind, dose-response, phase 2 clinical trial. The Lancet Psychiatry, 5(6), 486–497.
- Monson, C. M., & Fredman, S. J. (2012). Cognitive-behavioral conjoint therapy for PTSD. New York: Guilford Press.
- Monson, C. M., Fredman, S. J., Macdonald, A., Pukay-Martin, N. D., Resick, P. A., & Schnurr, P. P. (2012). Effect of cognitive-behavioral couple therapy for PTSD: A randomized controlled trial. Journal of the American Medical Association, 308(7), 700–709.
- Pearlman, L. A. (2003). Trauma and attachment belief scale. Los Angeles, CA: Western Psychological Services.
- Wagner, A. C., Mithoefer, M. C., Mithoefer, A. T., & Monson, C. M. (2019). Combining cognitive-behavioral conjoint therapy for PTSD with 3,4-methylenedioxymethamphetamine (MDMA): A case example. Journal of Psychoactive Drugs, 51(2), 166–173.
- Wagner, M. T., Mithoefer, M. C., Mithoefer, A. T., MacAulay, R. K., Jerome, L., Yazar-Klosinski, B., & Doblin, R. (2017). Therapeutic effect of increased openness: Investigating mechanism of action in MDMA-assisted psychotherapy. Journal of Psychopharmacology, 31(8), 967–974.
- Weathers, F. W., Blake, D. D., Schnurr, P. P., Kaloupek, D. G., Marx, B. P., & Keane, T. M. (2013a). The Clinician-Administered PTSD Scale for DSM-5 (CAPS-5). Washington, DC: U.S. Department of Veterans Affairs. Interview available from the National Center for PTSD at www.ptsd.va.gov.
- Weathers, F. W., Litz, B. T., Keane, T. M., Palmieri, P. A., Marx, B. P., & Schnurr, P. P. (2013b). The PTSD checklist for DSM-5 (PCL-5). Washington, DC: U.S. Department of Veterans Affairs. Scale available from the National Center for PTSD at www.ptsd.va.gov.
