ABSTRACT
Background: Dairy production in Kenya is important and dominated by small-holder farmers who market their produce through small-scale traders in the informal sector.
Method: This study aimed to determine the prevalence of aflatoxin (AFM1) in informally marketed milk in peri-urban Nairobi, Kenya, and to assess knowledge of milk traders on aflatoxins using questionnaires. A total of 96 samples were analyzed for AFM1 using enzyme-linked immunosorbent assay. In addition, boiling and fermentation experiments were carried out in the laboratory.
Results: All samples had AFM1 above the limit of detection (5 ng/kg) (mean of 290.3 ± 663.4 ng/kg). Two-thirds of the samples had AFM1 levels above 50 ng/kg and 7.5% of the samples exceeded 500 ng/kg. Most of the traders had low (69.8%) or medium (30.2%) knowledge. Educated (p = 0.01) and female traders (p= 0.04) were more knowledgeable. Experimentally, fermenting milk to lala (a traditional fermented drink) and yogurt significantly reduced AFM1 levels (p< 0.01) (71.8% reduction in lala after incubation at room temperature for 15 h, and 73.6% reduction in yogurt after incubation at 45ºC for 4h). Boiling had no effect.
Conclusion: The study concluded that the prevalence of raw milk with AFM1 was high, while knowledge was low. Fermentation reduced the AFM1 levels.
Introduction
Milk is a good source of macro- and micronutrients [Citation1]. It is affordable and can help diversify diets in developing countries [Citation2]. The dairy sector in Kenya makes up 40% of the agricultural gross domestic product (GDP) and 4% of the national GDP [Citation3]. Most of Kenya’s 3 million dairy herds are kept by small-holder farmers who contribute 70% of the total milk produced [Citation4]. On average, these farmers own between 1.2 and 2.0 hectares of land and keep about two to five cattle heads, each producing 5–10 kg milk per day [Citation4–Citation6].
Only 15% of the total produced milk is processed and marketed through the formal channel. The remainder is either consumed on-farm or marketed informally [Citation4,Citation6]. Informal distribution channels include direct sale of raw milk from the farm to consumers and raw milk sold to wholesale distributors, retailers, cooperative societies, and self-help groups. Milk is mainly used in preparation of tea or gruel but is also consumed raw, after boiling and after fermentation [Citation7].
With increased production and consumption of milk and dairy products, there is concern about the presence of hazards in milk and their effect on human health. One such hazard is aflatoxin (AF) [Citation1]. Aflatoxins are secondary metabolites of Aspergillus flavus, Aspergillus parasiticus, Aspergillus nomius, and other rarer species. The main classes of aflatoxins are B1, B2, G1, and G2. Aflatoxin M1 and M2 are hydroxylated metabolites of B1 and B2, respectively. When an animal consumes feed contaminated by AFB1, part of it is degraded in the rumen and the other part is rapidly absorbed and metabolized into AFM1 in the liver. AFM1 is absorbed into the blood and secreted in milk, urine, and bile or further metabolized [Citation8].
Both AFB1 and AFM1 are categorized as class 1 human carcinogens. However, AFM1 has been found to be less genotoxic, mutagenic, and carcinogenic compared to AFB1 [Citation9]. AF is also a significant risk factor in the occurrence of hepatocellular carcinoma (HCC) in humans. Studies have established a correlation between AF exposure through the diet and incidence of HCC in different populations [Citation10].
In children, chronic exposure to aflatoxins results in growth retardation [Citation11,Citation12]. Immune suppression is also experienced, and thus, children become more susceptible to other diseases. Growth impairment, especially stunting, has been observed, leading to increased susceptibility to infections and cognitive impairments which last beyond childhood [Citation13].
Aflatoxin contamination is also a barrier to international markets where stringent regulations are applied [Citation13,Citation14]. The European Union (EU) has set the limit of aflatoxin B1 (AFB1) in feed for dairy cattle to be 5 ng/kg, while the limit for aflatoxin M1 (AFM1) in milk is 50 ng/kg. The Codex Alimentarius limit is 500 ng/kg [Citation15], which is being adopted by Kenya.
The carryover of aflatoxins from feeds to milk produced by animals can be reduced by good agricultural practices and different control and mitigation methods [Citation16,Citation17]. Physical and chemical methods have been used in control of aflatoxins in food and feed. However, uptake of these technologies has been restricted due to cost, practicability, and implications for safety and nutritional quality of the product [Citation18]. Biological strategies are, therefore, considered as an alternative to these methods. Lactic acid bacteria (LAB) bind aflatoxins, reducing their bioavailability in food products. LAB are also nutritionally beneficial as probiotics [Citation19,Citation20].
A recent study in Kenya has estimated dietary exposure to AFM1 in milk to be 0.2 ng/kg Body Weight (BW) per day for an average adult [Citation21]. Higher levels of exposure to AFM1 (of up to 6.5 and 8.8 ng/kg BW) have been reported in infants and young children because of their increased milk consumption relative to adults [Citation22]. Individuals involved in milk trading, including retailers, are more likely to consume milk due to ready availability and thus may be at a greater risk of exposure to AFM1 [Citation23].
The informal marketing channel is growing and reaches a wide population, especially due to convenience and cost-effectiveness [Citation4,Citation5]. Informal traders are, therefore, important stakeholders and play a pivotal role in the dairy value chain. This study was done to determine the occurrence of AFM1 in informally marketed milk in a peri-urban area of Nairobi, assessed knowledge of aflatoxin among the traders involved, and evaluated the effect of fermentation and boiling on the concentration of AFM1.
Methodology
Study site
The study had two parts: field survey and an experimental (fermentation and boiling trials). The field study was conducted in Kasarani sub-county, Nairobi County, Kenya, in June 2018. Kasarani is a peri-urban sub-county administratively divided into five wards, with an estimated human population of 525,624 [Citation24]. Kasarani was chosen because of the intensive small-scale dairy farming activities that take place in the area, as reported by officials from the Ministry of Agriculture Livestock and Fisheries. In addition, an intervention involving the use of aflatoxin binders and training on milk safety had been conducted in the area.
Sampling and sample size determination
A cross-sectional study design approach was used for the survey. The sample size (n = 96) was calculated using Fischer’s formula. The expected (p) prevalence of samples exceeding 50 ng/kg AFM1 was assumed to be 50%, with a desired normal deviation of 1.96 which corresponds to 95% and a 10% degree of precision [Citation25]. A list of informal milk traders operating in Kasarani ward was established through the help of the sub-county administration, and 96 traders were randomly selected from the list, including on-farm milk kiosks (shops selling milk produced within the same place), dairy shops (shops either selling milk exclusively or other goods together with milk), milk ATMs, and street vendors (milk stands along streets). Milk ATM is a Kenyan term for automated milk dispensing machines which allows consumers to purchase milk in quantities they can afford. Milk is dispensed through a nozzle in units as small as 100 ml. The machine should have an in-built refrigeration system maintained between 5°C and 10°C, although this is not always the case.
Data collection
Traders were interviewed face-to-face either in English or Swahili depending on the preferred language, using pretested semi-structured questionnaires (Supplementary material). Informed consent was obtained prior to the interview. Questions were related to business characteristics of the trader (level of education, role in the business, sources of marketed milk, volume sold, price of buying and selling, etc.), knowledge of aflatoxins and willingness to pay for milk with reduced aflatoxins, and milk consumption practices (consumption of milk in the trader’s household and consumption of milk by children between 6 months and 3 years in the household). A set of 10 questions was used in assessing knowledge of aflatoxins. Milk samples were collected from the interviewed traders, but where milk was not present at the time of the interview, the team recorded details of the outlet and requested that a sample be put aside from the next batch to be received and the sample was collected the following day. Samples were collected in sterile 50 ml tubes and transported in cool boxes to Biosciences for east and central Africa, International Livestock Research Institute (BecA-ILRI) laboratories within 8 h, where they were stored at −20ºC before analysis.
Laboratory experiment
The milk used in the experiments was obtained from the University of Nairobi veterinary farm in Kanyariri, Lower Kabete, Nairobi. Two different raw-bulk samples were obtained, one for fermentation and one for boiling. The samples were drawn in sterilized 50 ml tubes and analyzed for AFM1 before the start of the experiments.
Boiling
Boiling of milk was conducted according to the description given by the traders as follows. Briefly, 2 l of milk was heated until it started to boil, and the heat was then turned down so that the milk was boiling at low heat until steady bubbles and foam formed. A stirrer was used to break up the foam and allow the steam to escape. After this, the milk was allowed to boil for 2 min with constant stirring. After cooling to 23°C, the milk was stored at 4°C for 7 days. Samples were taken immediately after boiling, after cooling to 23°C, and after storage at 4°C on days 1, 2, and 7.
Fermentation of yogurt
Milk was pasteurized at 90ºC for 30 min and then cooled to 43ºC. Yogurt starter culture (Lactobacillus delbrueckii ssp. bulgaricus and Streptococcus salivarius ssp. thermophiles) was added (YF-L903, Thermophilic Yoghurt Culture -Yo Flex®, Batch No. 2402687, CHR Hansen). The sample was incubated at 45ºC for 4 h. The milk was then cooled overnight to 3–4°C and then stored at 4ºC for 7 days. The pH was measured after incubation and during sampling on days 1, 2, and 7 at 4ºC using a pH-meter (MetroHM 632-pH Meter, Type 1.632.00, Number 6HI/265 made in Switzerland). Samples were drawn after pasteurization, after cooling to 43°C, after incubation, after overnight cooling, and after storage on days 1, 2, and 7. The samples were stored at −20°C until the day of AFM1 analysis. Samples were analyzed in triplicate.
Fermentation of lala
Lala is sour milk, made either traditionally by natural fermentation process or commercially made by adding mesophilic culture. In this experiment, mesophilic culture was used. Milk was pasteurized at 90ºC for 30 min and then cooled to 23ºC. Lala starter culture (Streptococcus lactis and Leuconostoc mesenteroide ssp. mesenteroides) was added (CHN-22, Mesophilic Aromatic Culture -Yo Flex®, Batch No. 3399963). This was incubated at room temperature for 15 h. The milk was then cooled overnight to 3–4ºC and stored at 4ºC for 7 days. The pH was measured after incubation and after storage on days 1, 2, and 7 at 4ºC. Samples were drawn after pasteurization, after cooling to 23ºC, after incubation, and after storage on days 1, 2, and 7 at 4ºC. Samples were stored at −20ºC until the day of analysis. Samples were analyzed in triplicate.
Aflatoxin M1 assays
Quantitative detection of AFM1 for all raw milk samples and fermented samples was done using competitive enzyme-linked immunosorbent assay kit (Helica Biosystems Inc., Santa Ana, CA, USA, catalog no. 961AFLM01M-96) following the manufacturer’s instructions.
The kit had a limit of detection of 5 ng/kg, and the highest standard was 100 ng/kg. Samples that exceeded this limit were diluted with skim milk provided with the kit and retested. Optical density was read at 450 nm using a microplate reader. AFM1 levels for the samples were quantified using a logarithmic standard curve generated using optical densities of standards with R2 readings above 97%.
Statistical analysis
Data obtained were entered in Microsoft Excel and analyzed using Genstat (version 15.1) and IBM SPSS (version 20) statistical tools. A knowledge score was computed as a percentage of the sum of correct description and positive responses to the questions. Respondents with absolutely no information on aflatoxins got a score of zero which was upscaled to 1 to avoid corner point solution. Knowledge of aflatoxins was categorized into three: high knowledge, medium knowledge, and low knowledge. Based on the percentage scores, low knowledge was computed to be between 10% and 37.5%, while high knowledge scores were above 75% [Citation26,Citation27]. Descriptive statistics including arithmetic means (±standard error), minimum and maximum values, and frequencies were used. Chi-square tests were used to assess the associations between categorical variables. One-way analysis of variance was used in multiple mean comparisons. p-Value of <0.05 was considered significant in all statistical comparisons.
Results
Milk distribution and consumption
A total of 96 respondents were interviewed, women comprised 52% while 48% were men. Businesses identified in our study included on-farm shops or kiosks (41.1%), dairy shops (38.9%), milk dispensing machines (ATMs) (15.6%), and street vendors (4.4%).
Most traders (64.8%) sourced milk from distributors located in distant areas of the country including Embu, Murang’a, Mount Kenya, Limuru, Kinangop, Kerugoya, and Nyeri. Other traders obtained milk directly from nearby farms alone (27.5%), farms and/or distributors (3.3%), dairy shops (2.2%), and aggregation centers (2.2%).
There was no significant difference in buying price of milk from the different sources (farms, distributors, dairy shops, aggregation centers, and farm and/or distributors) (p = 0.75). Prices are given in Kenyan Shilling (KES), where 1KES = 0.01 USD in 2018. The highest mean buying price was KES 52.18 ± 0.43 for milk sourced from distributors, and the lowest was at KES 47.5 ± 7.50 obtained directly from dairy farms. On average, the traders sold 78.25 ± 10.61 (median = 50, range = 797) liters of milk in a day, at KES 62.71 (±0.53) per liter (median = 65, range = 30).
An average household size had four members (median = 4, range = 9) and consumed an average of 1.57 ± 0.14 (median = 1.00, range = 9.75) liters of milk in a day. shows the different forms of milk consumed in the traders’ households and by children between the age of 6 months and 3 years within the household.
Milk safety and knowledge of aflatoxins
According to the traders, milk safety can be judged by senses, mainly by sight and taste (52.6%). A number of traders (44.3%) thought that senses alone cannot be used to judge the safety of milk, while some traders (3.1%) said quick tests such as lactometer test could show whether the milk is safe or not. Most of the traders (76.9%) stored their milk at refrigeration temperatures between 5 and 10ºC prior to selling, in milk dispensers (which have in-built refrigeration system), or in normal refrigerators. Some traders (16.5%) stored milk in clear plastic buckets where they could be seen by potential customers while others used aluminum cans (5.5%) or stored their milk in cold places (1.1%) which included cold areas within the room or immersing containers with milk in cold water. Some traders (43.8%) reported having milk spoilt from time to time. Of these, 45.5% discarded the milk, 15.9% gave the milk to animals such as calves, pigs, and dogs, and 18.2% made lala out of the milk. Traders, especially those with a direct connection with the suppliers (20.5%), were able to alert their suppliers whenever there were cases of spoilage and where possible the product was returned to the supplier.
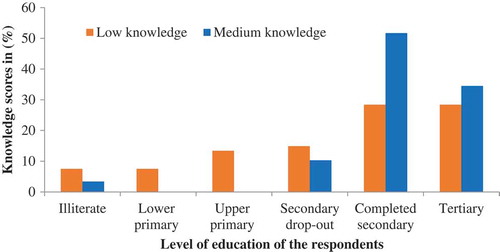
Though 68.4% of the traders had heard about aflatoxins, of these, only 26.8% could correctly describe aflatoxins, 57.7% gave incorrect description, while 15.5% had no idea completely what aflatoxins are. The highest knowledge score was 65% so no trader was categorized as having high knowledge, while 69.8% and 30.2% of the traders demonstrated low and medium knowledge levels, respectively. There was no association (p = 0.64) between the wards where the respondents came from and their awareness on aflatoxins. Individuals that had attained secondary and college level education were significantly more aware of aflatoxins than those with primary level education and those with no education at all (p = 0.015) (). Women traders (46 out of 96) demonstrated significantly more knowledge than men (p = 0.04).
Aflatoxin M1 in milk
Samples from the trader shops
A total of 96 raw milk samples were collected and analyzed for AFM1. All samples collected were contaminated with AFM1. The mean level of AFM1 in milk was 290.3 (±663.4) ng/kg. The minimum level detected was 15.4 and the maximum was 4563 ng/kg. Of the samples analyzed, 66.4% were above 50 ng/kg which is the legal limit allowed by the EU, while 7.5% of the samples exceeded 500 ng/kg, the legal limit allowed by the Food and Drug Administration of the USA. The means are summarized in .
Table 1. The different forms of milk consumed by the household and children between 6 months and 3 years of age.
Boiling
There was no significant change in AFM1 levels after boiling and during storage in days 1 and 2 (p = 0.42). However, there was significant change after 7 days of storage (p-value = 0.016) () .
Fermentation of yogurt and lala
The pH of yogurt was 4.4 after incubation and reduced to 4.0 after day 7 at 4ºC. The pH of lala was 4.6 after incubation and reduced to 4.2 after day 7 at 4ºC. There was a significant reduction in AFM1 during lala and yogurt processing (p < 0.01). After incubation, 71.8% reduction in AFM1 level was recorded for lala as compared to 73.6% reduction for yogurt. There was no significant difference in the reduction of AFM1 in both lala and yogurt during storage. The mean levels of AFM1 during the processes of production are presented in .
Table 2. Mean levels of AFM1 in milk (ng/kg) sourced from farms, dairy shops, and distributors and for different business types, Kasarani, June 2018.
Table 3. Mean AFM1 levels (ng/kg) during fermentation and boiling processes.
Discussion
This study was conducted to evaluate the presence of AFM1 in informally marketed milk and the effects of processing through fermentation and boiling on the concentration of AFM1. Dairy actors in Kenya include dairy cooperatives, wholesale and retail traders in dairy shops, and itinerant traders such as hawkers. Cooperative societies are an integral part of the milk marketing system in Kenya. Small-holder farmers organize themselves in groups where their milk is collected, bulked, and distributed to the bigger processors and high potential markets such as Nairobi [Citation28]. This explains why distributors have become a chief source of milk in peri-urban centers such as Kasarani.
Farm shops or kiosks are popular in informal settings because they allow personal interaction between the producer and consumer, thereby establishing trust [Citation29]. A number of street vendors declined to participate in the study in fear of any possible legal implications since they are unlicensed; they are, therefore, underrepresented in this study.
Milk dispensing machines (ATMs) have become popular in peri-urban centers in Kenya. This is because of convenience, as consumers are able to access milk in economic packages depending on their purchasing power [Citation30]. Moreover, the Kenya Bureau of Standards discourages the sale of raw milk and promotes the sale of pasteurized milk either packaged or sold through ATMs [Citation31]. While it is recommended that ATMs sell pasteurized milk and are maintained at 5–7°C, some sell raw milk, as seen in this study.
Generally, there was low knowledge of aflatoxins among milk traders. Similar results were found among dairy farmers by Kiama and others [Citation32]. The education level of traders was significantly associated with knowledge of aflatoxins. This confirms a previous study by Limbikani and others [Citation27]. More educated individuals have been seen to be more aware of food safety risks brought about by chemical hazards such as pesticide residues [Citation33]. Poor knowledge of aflatoxins affects the adoption of technologies for the management of aflatoxins [Citation34]. Higher knowledge of aflatoxins among women as compared to men is possibly because of their key role in food production and household food security [Citation35].
The mean level of AFM1 in the milk was higher than those reported in some previous studies in Nairobi [Citation11,Citation23,Citation36]. It, however, was lower than found in one study in Kenya in 2016 [Citation37]. Variation in AFM1 could be linked to milk originating from areas of different agroecological conditions and husbandry practices. Conditions of high humidity provide a good environment for the growth of fungi and production of mycotoxin in feed and feed ingredients [Citation37,Citation38]. Accessibility to feed in different regions and/or seasons may also be part of the variation of aflatoxin levels observed in milk [Citation36], which results in some farmers using feed concentrate and stored feed other than green forage which is a factor in AFM1 contamination in milk [Citation39].
While boiling has been little studied before, pasteurization has been studied and seen to bring about no significant change in AFM1 level. In our study, boiling and storage of milk at 4°C for 1 and 2 days did not reduce the level of AFM1. This was expected as aflatoxins are known to be heat stable [Citation40,Citation41]. However, after 7 days, there was a significant change in AFM1 concentration which could potentially be explained by initial stages of natural fermentation of the milk during storage.
LAB used in milk fermentation have been proven for their ability in binding aflatoxins [Citation41,Citation42]. Thermophilic (Lactobacillus bulgaricus and Streptococcus thermophiles) and mesophilic (S. lactis and Leuconostoc mesenteroides) culture for yogurt and lala, respectively, demonstrated the ability to bind AFM1. El Khoury recorded 87.6% binding in Phosphate Buffer Saline (PBS) medium after incubating for 14 h at 37°C and 46.7% binding in milk after incubating for 6 h at 42°C [Citation42]. Shigute and Washe [Citation43] noted 54% decrease during fermentation using a stock of LAB culture, including L. bulgaricus, S. thermophiles, and L. mesenteroides after incubation for 5 days at 20–30°C. A gradual decrease in pH was also noted. In their study, Adibpour and others [Citation44] noted that with an increase in storage time at 4°C, AFM1 binding increased, which was not the case in our study.
There are various factors that affect the process of fermentation, among them are incubation period, temperature of incubation, and pH of the medium [Citation45]. At 4ºC, though the bacterial cells remain viable, their activity is reduced since the temperature is below the minimum required for growth; fermentation rate is, therefore, reduced [Citation46,Citation47]. This possibly reduces the ability of the cells to efficiently bind the toxin. The mechanism for decontamination of milk by LAB has not been fully understood. However, some studies have suggested the ability of the toxin to bind to the cell wall of the bacteria during fermentation. Polysaccharide and peptidoglycan components of the cell wall play a major role in this [Citation19].
Conclusion
The informal milk sector is dominant in Kenya, mostly made up of small-holder farmers who sell their milk through cooperative societies who then distribute the milk. Milk traders are important agents in the milk value chain, yet most of them have low knowledge of aflatoxins. In the survey, we found levels of AFM1 above the recommended limit of 50 ng/kg which may bring about health implications over a long period, even though these are not fully understood [Citation48].
Thermophilic and mesophilic cultures used in milk fermentation have demonstrated the ability to bind AFM1 and reduce milk contamination. Incubation temperature and time are important in the reduction of AFM1 level. Boiling of milk, which is a common practice among households, only destroys bacterial cells and not aflatoxins in the milk.
It is important to raise awareness and education on aflatoxins across the general public and identify appropriate technologies to reduce AFM1 contamination in milk.
Ethical approval
Written informed consent was obtained from the participants prior to the interview, and their anonymity was maintained. The study design and tool underwent ethical review and approval by the ILRI Institutional Research and Ethics Committee (IREC) under the reference number ILRI IREC 2017-10.
Supplemental Material
Download MS Word (35.2 KB)Acknowledgments
The authors wish to acknowledge all the milk traders that participated in the survey and the research assistants, the Livestock Department of Kasarani subcounty, Department of Food Science, Nutrition and Technology, and BecA-ILRI Hub for technical assistance.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental material
Supplementary data for this article can be accessed here.
Additional information
Funding
Notes on contributors
Maureen M. Kuboka
Maureen Kuboka is a Graduate Student in the Department of Food Science, Nutrition and Technology at the University of Nairobi and a Graduate Fellow at ILRI. Her background is in Food Science and her research interests are in Food Safety and Nutrition.
Jasper K. Imungi
Jasper Imungi is a Professor at the Department of Food Science, Nutrition and Technology at the University of Nairobi. His research interests are in Food Science and Technology. His administrative appointments include being Chairman of the Department and Dean of the Faculty of Agriculture. He also works as a Consultant Food Scientist with UNIDO.
Lucy Njue
Lucy Njue is a Lecturer at the Department of Food Science, Nutrition and Technology at the University of Nairobi with a background in Microbiology. Her research interests are in Food safety and microbiology and she supervises and PhD students. .
Florence Mutua
Florence Mutua is a Lecturer at the Department of Public Health Pharmacology and Toxicology, University of Nairobi and currently on a year’s sabbatical with ILRI. Her research interests are in Zoonoses and Epidemiology.
Delia Grace
Delia Grace is an Epidemiologist and leads the Health Program at ILRI and the Flagship on Food Safety in the CGIAR research program on Agriculture for Nutrition and Health. She has led or contributed to evidence syntheses and investment advice for World Bank, DFID, USAID, ACIAR, BMGF, FAO, OIE, WHO, AU-IBAR, OECD, and others.
Johanna F. Lindahl
Johanna Lindahl is a joint appointee of ILRI, Swedish University of Agriculture and Uppsala University. She has a background in veterinary medicine and 10 years’ experience in academia and research. She leads research work on aflatoxins under ILRI Health Program and recently edited a widely cited special edition on aflatoxins in maize and dairy in East Africa.
References
- Mcmahon D. Dairy products in human nutrition dairy products. Rome, Italy: Food and Agriculture Organization; 2013.
- Finnell KJ, John R. Research to understand milk consumption behaviors in a food-insecure low-income SNAP population in the US. Beverages. 2017;3(3):1.
- Ministry of Livestock Development. Kenya national dairy master plan. Nairobi, Kenya; 2010.
- Muriuki HG. Dairy development in Kenya. Rome, Italy: Food and Agriculture Organization; 2011; p. 1–8.
- Muriuki HG. A review of small-scale dairy sector-Kenya. Rome, Italy: Food and Agriculture Organization; 2003.
- Thorpe W, Muriuki H, Omore AO, et al. Development of smallholder dairying in Eastern Africa with particular reference to Kenya. In: Paper prepared for the UZ/RVAU/DIAS/DANIDA-ENRECA PROJECT REVIEW WORKSHOP; 2000 Jan 10–13; Bronte Hotel, Harare, Zimbabwe. 2000.
- Baltenweck I, Staal SJ, Owango M, et al. Intensification of dairying in the greater Nairobi milk-shed: spatial and household analysis. MoA/KARI/ILRI-Collaborative Research Project Report; 1998.
- Prandini A, Tansini G, Sigolo S, et al. On the occurrence of aflatoxin M1 in milk and dairy products. Food Chem Toxicol. 2009;47(5):984–991.
- International Agency for Research on Cancer. IARC monographs on the evaluation of carcinogenic risks to humans. Some traditional herbal medicines, some mycotoxins, naphthalene and styrene. World Health Organisation; 2002.
- Wang J, Tang L. Epidemiology of aflatoxin exposure and human liver cancer. J Toxicol Toxin Rev. 2004;23(2–3):249–271.
- Kiarie GM, Dominguez-Salas P, Kang’ethe SK, et al. Aflatoxin exposure among young children in urban low-income areas of Nairobi and association with child growth. Afr J Food Agric Nutr Dev. 2016;16:10967–10990.
- Khlangwiset P, Shephard GS, Wu F. Aflatoxins and growth impairment: a review. Crit Rev Toxicol. 2011;41(9):740–755.
- Unnevehr L, Grace D. Aflatoxins finding solutions for improved food safety. Washington, DC: International Food Policy Research Institute; 2013.
- Udomkun P, Wiredu AN, Nagle M, et al. Innovative technologies to manage aflatoxins in foods and feeds and the profitability of application – a review. Food Control. 2017;76:127–138.
- European Commission. Directive 2002/32/EC of the European Parliament and of the Council of 7th May 2002 on undesirable substances in Animal Feed. Vol. L 269; 2000 Sep. p. 1–15.
- Pettersson H. Controlling mycotoxins in animal feed in Mycotoxins in Food: Detection and Control. In: Magan N, Olsen M. Cambridge, UK: Woodhead Publishing Ltd.; 2004, ch 12, p. 262–304.
- Battacone G, Nudda A, Cannas A, et al. Excretion of aflatoxin M1 in milk of dairy ewes treated with different doses of aflatoxin B1. J Dairy Sci. 2003;86(8):2667–2675.
- Adibpour N, Soleimanian-Zad S. Effect of storage time and concentration of aflatoxin M 1 on toxin binding capacity of L. acidophilus in fermented milk product. J Agr Sci Tech. 2016;18:1209–1220.
- Bulent K, Dibson A. Biological strategies to counteract the effects of mycotoxins. J Food Prot. 2009;72(9):2006–2016.
- Ahlberg SH, Joutsjoki V, Korhonen HJ. Potential of lactic acid bacteria in aflatoxin risk mitigation. Int J Food Microbiol. 2015;207:87–102.
- Sirma A, Lindahl JF, Makita K, et al. The impacts of aflatoxin standards on health and nutrition in sub-Saharan Africa: the case of Kenya. Glob Food Sec. 2018 August;18:57–61.
- Food and Agriculture Organisation. Aflatoxins. Rome, Italy: Food and Agriculture Organisation; 2018.
- Kirino Y, Makita K, Grace D, et al. Survey of informal milk retailers in Nairobi, Kenya and prevalence of aflatoxin M1 in marketed milk. Afr J Food, Agric Nutr Dev. 2016;16(3):11022–11038.
- Kenya National Bureau of Statistics (KNBS). Kenya – demographic and health survey 2008–2009. Nairobi, Kenya: Kenya National Bureau of Statistics; 2010.
- Naing L, Winn T, Rusli B. Sample size calculator for prevalence studies. 2006. p. 9–14. Available from: www.kck.usm.my/ppsg/stats_resources.htm
- Ilesanmi FF, Ilesanmi OS. Knowledge of aflatoxin contamination in groundnut and the risk of its ingestion among health workers in Ibadan, Nigeria. Asian Pac J Trop Biomed. 2011;1(6):493–495.
- Matumba L, Monjerezi M, Kankwamba H, et al. Knowledge, attitude, and practices concerning presence of molds in foods among members of the general public in Malawi. Mycotoxin Res. 2016;32(1):27–36.
- Mbogoh S, Okoth M. Technical and economic considerations in the establishment of a milk collection and cooling centre. FAO sponsored Workshop on Regional Exchange Network for Market-Oriented Dairy Development, December 4–8, 1995, Harare, Zimbabwe.
- Mbogoh S. Comparative evaluation of dairy marketing systems. In: Strategies for market orientation of small scale milk producers and their organisations. Workshop proceedings Mogororo, Tanzania, 20–24 March; 1995.
- Galičič A, Kranjec N, Kirbiš A, et al. Consumer ’ s attitude and manipulation of raw milk from milk vending machines. Sanitary Engineering Research. 2015;9(1):21–34.
- Kenya Bureau of Standards KEBS. Code of hygienic practice for milk and milk products. Nairobi, Kenya: Kenya Bureau of Standards; 2015. p. 1–27.
- Kiama TN, Lindahl JF, Sirma AJ, et al. Kenya dairy farmers perceptions of moulds and mycotoxins and implications for exposure to aflatoxins: a gendered analysis. Afr J Food Agric Nutr Dev. 2016;16(3):11106–11125.
- Dosman DM, Adamowicz WL, Hrudey SE. Socioeconomic determinants of health- and food safety-related risk perceptions. Risk. Anal. 2001;21(2).
- Kumar GDS, Popat MN. Knowledge and adoption of aflatoxin management practices in groundnut farming in Junagadh, Gujarat, India. Journal of SAT Agricultural Research. 2007;3(1):2–3.
- Quisumbing AR, Brown LR, Feldstein HS, et al. Women: the key to food security. Food Policy Rep. 1995 December;2:26.
- Lindahl J, Kagera I, Grace D. Aflatoxin M1 levels in different marketed milk products in Nairobi, Kenya Mycotoxin Research. Mycotoxin Res. 2018;34:289–295.
- Senerwa D, Sirma AJ, Mtimet N, et al. Prevalence of aflatoxin in feeds and cow milk from five counties in Kenya. Afr J Food, Agric Nutr Dev. 2016;16(03):11004–11021.
- Koteswara Rao V, Aruna B, Rafiyuddin M, et al. Effect of relative humidity on biodeterioration of poultry feed and ochratoxin A production by Penicillium species PUBLIC INTEREST STATEMENT. Cogent Food Agric. 2016;2(2):1–9.
- Kang’ethe EK, Lang’a KA. Aflatoxin B1 and M1 contamination of animal feeds and milk from urban centers in Kenya. Afr Health Sci. 2009 Dec;9(4):218–226.
- Galvano F, Galofaro V, Galvano G. Occurrence and stability of aflatoxin M1 in milk and milk products. A worldwide review. J Food Prot. 1996;59(10):1079–1090.
- Giovati L, Magliani W, Ciociola T, et al. AFM1 in milk: physical, biological, and prophylactic methods to mitigate contamination. Toxins (Basel). 2015;7(10):4330–4349.
- El Khoury A, Atoui A, Yaghi J. Analysis of aflatoxin M1 in milk and yogurt and AFM1 reduction by lactic acid bacteria used in Lebanese industry. Food Control. 2011;22(10):1695–1699.
- Shigute T, Washe AP. Reduction of aflatoxin M1 levels during ethiopian traditional fermented milk (Ergo) production. J Food Quality. 2018;2018(2016).
- Adibpour N, Soleimanian-Zad S, Sarabi-Jamab M, et al. Effect of storage time and concentration of aflatoxin m1 on toxin binding capacity of L. acidophilus in fermented milk product. J Agric Sci Technol. 2016;18(5):1209–1220.
- Dalié DKD, Deschamps AM, Richard-Forget F. Lactic acid bacteria – potential for control of mould growth and mycotoxins: a review. Food Control. 2010 Apr;21(4):370–380.
- Vaningelgem F, Zamfir M, Adriany T, et al. Fermentation conditions affecting the bacterial growth and exopolysaccharide production by Streptococcus thermophilus ST 111 in milk-based medium. J Applied Microb. 2004. p. 1257–1273.
- Hamann WT, Marth EH. Survival of Streptococcus thermophilus and Lactobacillus bulgaricus in commercial and experimental yogurts. J Food Prot. 1984;47(10):781–786.
- Ahlberg S, Vesa J, Hannu JK. A risk assessment of aflatoxin M1 exposure in low and mid-income dairy consumers in Kenya. Toxins 2018. 2018 Aug;10(9):348.