ABSTRACT
Posterior reversible encephalopathy syndrome (PRES) characteristically presents with rapid onset of headache, seizure, encephalopathy, and visual changes, along with evidence of parieto-occipital vasogenic edema on magnetic resonance imaging. We describe the case of a 41-year-old female with a protracted presentation of two of the four classic PRES symptoms, which were not immediately recognized as PRES due to the presence of multiple other comorbidities and reasons for encephalopathy. This case highlights the possibility of atypical presentations of PRES and the diagnostic challenges in making this clinical diagnosis when competing diagnoses are present.
1. Introduction
Posterior reversible encephalopathy syndrome (PRES) describes a group of neurological symptoms accompanied by cerebral imaging abnormalities, and is associated with a growing list of possible etiologies [Citation1]. This syndrome was first described in 1996 in a small case series of patients presenting with headaches, vomiting, confusion, seizures, cortical blindness and other visual abnormalities, and motor signs as reversible posterior leukoencephalopathy syndrome (RPLS) [Citation2]. Typical magnetic resonance imaging (MRI) findings include vasogenic edema in the subcortical white matter of the posterior parietal and occipital regions bilaterally, but additional cortical and anterior findings are possible as well [Citation3–Citation6]. Severe hypertension, abrupt increases in blood pressure, renal disease, pre-eclampsia and eclampsia, autoimmune disease, immunodeficiency states including human immunodeficiency virus infection (HIV) and use of immunosuppressants or immune modulators are the most commonly implicated causes [Citation2,Citation4,Citation7–Citation11]. The clinical and neuroradiologic findings of PRES are non-specific and may mimic other neurologic conditions, posing a diagnostic difficulty, especially in patients with multiple co-morbidities. We describe a case of PRES in a patient with atypical clinical and radiologic features and multiple risk factors.
2. Case
A 41-year-old woman with HIV/AIDS (antiretroviral naïve, CD4 count < 10 cells/μL), injection drug use, end-stage renal disease (ESRD), and poorly controlled hypertension was evaluated at an outside facility for new onset seizure. Three months later, she again presented with seizure, and evaluation revealed a single small focus of T2-weighted fluid-attenuated inversion recovery (T2/FLAIR) hyperintensity in the deep right frontal white matter on MRI and a normal electroencephalogram (EEG). She was discharged fully oriented and without cognitive dysfunction. Three days later she was brought to our emergency department for acute confusion.
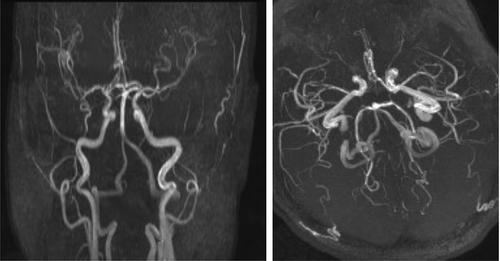
On examination, she was agitated and confused, with an initial blood pressure (BP) of 198/92 mmHg. There were no focal neurologic deficits or complaints of visual change. Cocaine metabolites were detected on urine toxicology. EEG and head computed tomography (CT) were unremarkable but brain MRI showed numerous foci of increased T2/FLAIR signals bilaterally in the cerebral and cerebellar hemispheres and brainstem (). MR angiogram (MRA) of the brain ruled out internal carotid artery occlusion, vertebrobasilar stenosis, aneurysm, and vascular malformation as a cause of her altered mental status (). Given her comorbidities and degree of immunocompromise, progressive multifocal leukoencephalopathy, HIV encephalitis, cerebral toxoplasmosis, infectious endocarditis with septic cerebral embolism, reversible cerebral vasoconstriction syndrome, and atypical PRES were all considered. Attempts at cerebral spinal fluid (CSF) collection were unsuccessful.
Figure 1. Fluid-attenuated inversion recovery (FLAIR) magnetic resonance images (MRI) at presentation showed scattered regions of hyperintense signal in the frontal, parietal, occipital, and temporal lobes bilaterally (a–d). FLAIR hyperintensities are also seen in the deep grey nuclei (b), midbrain (d–e), and cerebellum (e–f).
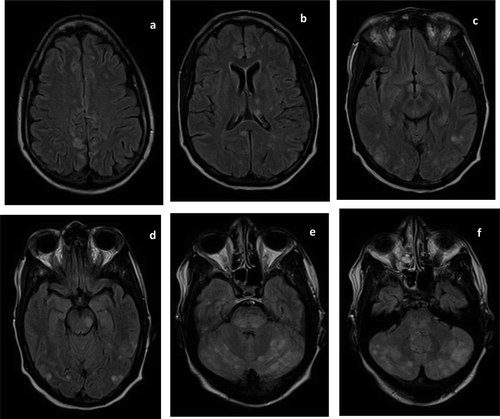
Figure 2. At presentation, magnetic resonance angiogram (MRA) of the circle of Willis showed no abnormality.
She was treated with empiric clindamycin, pyrimethanine and folinic acid for toxoplasmosis, combination antiretroviral therapy (cART) for HIV/AIDS, hemodialysis for ESRD, and intravenous hydralazine for hypertension. BP was labile, ranging from 70/38 to 215/165. Over the course of five days, her confusion and agitation slowly resolved and her blood pressure was brought down to a goal of 120/80 with the addition of oral amlodipine.
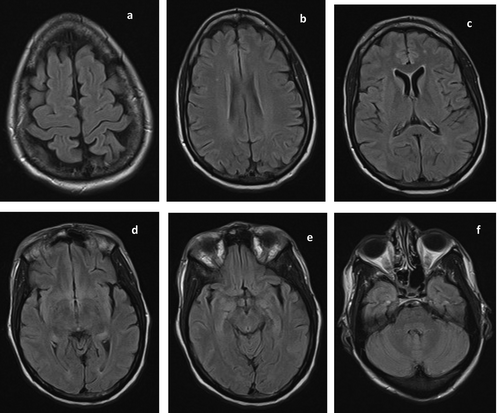
A repeat MRI done on hospital day 7 showed resolution of the previously noted signal abnormality, except for two punctate foci of subcortical white matter signal abnormalities (). Thus, a retrospective diagnosis of PRES was made and treatment for toxoplasmosis was withdrawn.
Figure 3. Repeat MRI done on hospital day 7 showed resolution of the previously noted extensive signal abnormalities. However, two punctate foci of FLAIR hyperintensities persisted in the right corona radiata (b) and left frontal lobe (c). These may represent age-related white matter changes or white matter hyperintensities of presumed vascular origin.
3. Discussion
PRES is a clinico-radiologic entity characterized by distinct clinical signs and neuroradiologic features. Clinically, PRES is typified by headache, altered mental status, visual abnormalities, and seizure activity, which can occur in various combinations. In our patient, the only clinical feature present on admission to our hospital was altered mental status, which has been reported in 13–90% of cases [Citation12]. Seizure is the most common feature of PRES, occurring in up to 92% of cases [Citation12]. Although she had seizure episodes three months prior and three days prior to presentation, there was no seizure demonstrated clinically or by EEG during the third presentation, when MRI findings consistent with PRES were first noted. Visual abnormality and headaches were absent during all three presentations, though they are noted to be present in up to 67% and 53% of cases, respectively [Citation12].
The characteristic neuro-radiologic feature of PRES is white matter vasogenic edema notable on brain MRI as hyper-intense lesions on T2/FLAIR sequences [Citation3,Citation6]. While the patterns of distribution of such signal hyper intensities are now known to vary, historically PRES was thought to produce bilateral and symmetric lesions in the posterior temporal, parietal, and occipital regions of the brain [Citation2,Citation6]. The posterior circulation was believed to be preferentially affected due to its relatively poorer sympathetic innervation [Citation13]. Cohort studies [Citation6,Citation12] have described four different radiological patterns of PRES and reported that this archetypal pattern, referred to as ‘the dominant parietal-occipital pattern’, occurred in only 22% in their studied cohort of 136 cases. The topographic distribution seen in our case with signal hyperintensities present in the frontal, parietal, and occipital lobes bilaterally as well as the brainstem and pons best fits the description of a ‘holohemispheric watershed pattern’. This pattern of distribution was present in 23% of their cohort.
The pathogenesis of PRES remains unclear. However, Legriel et al describe two hypotheses [Citation12]. Although both theories oppose each other, they both propose that blood–brain barrier (BBB) dysfunction with resultant vasogenic edema is the final end point. The BBB is a major physical and physiological barrier that regulates the passage of solute, macromolecules, and cells from the circulation into the brain interstitial fluid. It is formed mainly by the intercellular tight junctions of the brain microvascular endothelial cells [Citation14,Citation15]. The commonly accepted theory proposes that an increase in blood pressure above the autoregulatory capacity of the cerebral circulation successively results in cerebral hyperperfusion via arteriolar dilation, leading to cerebral endothelial damage, loss of BBB integrity, and fluid and protein extravasation. This cascade ultimately results in vasogenic edema. This theory is supported by the high incidence of hypertension (up to 80%) in patients with PRES [Citation12]. The alternate contradictory theory proposes an immune mediated mechanism in which endothelial cell activation leads to the release of pro-inflammatory cytokines, leukocyte chemoattractants, and vasoconstrictors. This results in leukocyte trafficking, endothelial damage, vasoconstriction, and hypoperfusion, ultimately culminating in BBB dysfunction and vasogenic edema. This mechanism is believed to be predominant in normotensive patients with PRES.
PRES can be caused by diverse medical conditions. Depending on the clinical setting, either of the proposed pathophysiologies may contribute to the development of PRES in different disease conditions. Acute severe hypertension is the most classic precipitant of PRES [Citation2]. Other well-documented causes include HIV infection, renal failure, pre-eclampsia, injection drug use, sepsis, electrolyte imbalance, organ transplantation, autoimmune diseases and immunosuppressive drugs [Citation2,Citation4,Citation7–Citation11]. Possible causes in our patient include severe variation in BP, HIV/AIDS, ESRD, and cocaine use.
In this patient, mean arterial pressure ranged between 49 and 182 mmHg immediately upon presentation, which falls outside of the cerebral auto regulatory range of 60–120 mmHg [Citation12]. Either hypoperfusion at lower BP or hyperperfusion at higher BP could have precipitated PRES.
Our patient also had advanced HIV infection with a very low CD4 cell count. HIV infection has been implicated as the etiological factor in many published cases of PRES [Citation8,Citation9,Citation11,Citation13]. HIV virus has been shown to induce endothelial cell apoptosis, tight junction disruption, oxidative stress, and the expression of pro-inflammatory cytokines and adhesion molecules which stimulate endothelial cell inflammation, all of which culminates in BBB dysfunction [Citation11,Citation16,Citation17]. Most reported cases of PRES occurred in patients with low CD4 counts (range 16–284 cells/μl) [Citation9,Citation13,Citation18–Citation21], however, HIV-induced pathology has not been shown to be related to viral load [Citation22].
Substance abuse is believed to accelerate disease progression and aggravate the neuropathologic effect of HIV in infected patients [Citation16]. Cocaine has been shown to synergistically enhance the pathologic processes induced by HIV infection [Citation23–Citation25]. Cocaine is also known to induce oxidative stress, tight junction disruption, endothelial cell inflammation dysfunction, and loss of BBB integrity [Citation26–Citation28]. Hence cocaine use in this patient may have precipitated PRES by itself or in conjunction with HIV infection.
Another possible inciting factor in this patient was ESRD; she had not been completely adherent to her hemodialysis schedule, and received hemodialysis infrequently. Both volume overload resulting from irregular hemodialysis and the fluid shifts that occur during hemodialysis can lead to endothelial dysfunction in patients with ESRD and have been implicated as precipitants of PRES in patients with ESRD [Citation1,Citation9,Citation13].
Any of the aforementioned conditions could have provoked PRES individually or synergistically, as this patient had multiple risk factors.
When PRES occurs in patients with multiple co-morbidities not only is it difficult to pinpoint the inciting factor, the diagnosis itself may be difficult, as was the case in our patient. The non-specific and varied clinical and radiologic presentation of PRES also contributes to the diagnostic dilemma. The alternate diagnoses that we entertained in this case were HIV related encephalitis, septic or aseptic emboli, progressive multifocal leukoencephalopathy, reversible cerebral vasoconstriction syndrome, and toxoplasmosis. HIV related encephalitis, progressive multifocal leukoencephalopathy, and toxoplasmosis were considered because our patient had advanced HIV infection and immunodeficiency. Combination antiretroviral therapy (cART) was commenced on the second day of admission. Empiric treatment of toxoplasmosis was started on admission and discontinued after PRES was diagnosed retrospectively. Embolism was a diagnostic consideration because the lesions were concentrated around the gray-white junction. PRES has been found to be present in about 9% of cases of reversible cerebral vasoconstriction syndrome which is also associated with cocaine use. The absence of cerebral vasoconstriction or stenosis on MRA ruled out this condition. In such challenging cases it is imperative to direct therapy at correcting possible inciting factors. Resolution of clinical and radiologic features following management of the provoking condition confirms the diagnosis of PRES retrospectively, as occurred in our case.
The subcortical white matter hyperintensities that persisted after treatment are nonspecific and may represent non-pathological age-related white matter changes or white matter hyperintensities of presumed vascular origin (WMHPV) [Citation29,Citation30], chronic changes unrelated to the acute event. WMHPV are not uncommon in the general population and have hypertension and chronic kidney disease among their significant risk factors [Citation29].
PRES is usually reversible with most patients showing clinical and radiologic resolution within 5 days to 17 months [Citation12]. However, permanent neurological deficit and severe complications have been associated with this condition. Recognized complications include cerebral ischemia, cerebral hemorrhage, cerebral herniation and death which has been reported in as much as 15% of cases [Citation12]. Hence, early diagnosis of PRES, identification of its inciting factor(s), and prompt management is necessary to avoid possible compilations including death.
4. Conclusion
The archetypal PRES symptoms and radiologic features do not occur in every patient. In patients with multiple risk factors for PRES, the syndrome may be triggered by one or more such causes. Because of the nonspecific nature of PRES, diagnosis may be difficult, especially in patients with many comorbidities, and may only be confirmed retrospectively, such as in this patient. The prognosis of PRES is excellent with clinical and radiological resolution when the underlying cause is recognized and corrected.
Clinicians should be aware that PRES may present atypically. It is important to consider PRES in the differential diagnosis of patients with multiple co-morbidities presenting with any one of the characteristic symptoms: mental status change, headache, seizure, or visual disturbance. A high index of suspicion is necessary for early diagnosis and initiation of therapeutic measures in order to avoid the possible detrimental complications of PRES.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Fugate JE, Rabinstein AA. Posterior reversible encephalopathy syndrome: clinical and radiological manifestations, pathophysiology, and outstanding questions. The Lancet Neurology;14(9):914–925.
- Hinchey J, Chaves C, Appignani B, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334(8):494–500.
- Bartynski WS. Posterior reversible encephalopathy syndrome, part 1: fundamental imaging and clinical features. AJNR Am J Neuroradiol. 2008;29(6):1036–1042.
- Schaefer PW, Buonanno FS, Gonzalez RG, et al. Diffusion-weighted imaging discriminates between cytotoxic and vasogenic edema in a patient with eclampsia. Stroke. 1997;28(5):1082.
- Schwartz RB, Mulkern RV, Gudbjartsson H, et al. Diffusion-weighted MR imaging in hypertensive encephalopathy: clues to pathogenesis. AJNR Am J Neuroradiol. 1998;19(5):859–862.
- Bartynski WS, Boardman JF. Distinct imaging patterns and lesion distribution in posterior reversible encephalopathy syndrome. AJNR Am J Neuroradiol. 2007;28(7):1320–1327.
- Baizabal-Carvallo JF, Barragán-Campos HM, Padilla-Aranda HJ, et al. Posterior reversible encephalopathy syndrome as a complication of acute lupus activity. Clin Neurol Neurosurg. 2009;111(4):359–363.
- Ribeiro S, Monteiro M, Moreira B, et al. Rare posterior reversible encephalopathy syndrome in a patient with HIV. BMJ Case Rep. 2013;2013:bcr2013201495.
- Kurukumbi M, Castellanos MI, Crawford AK, et al. Posterior reversible encephalopathy syndrome in a patient with newly diagnosed HIV infection and end stage renal disease. Case Rep Neurol Med. 2013;2013:1–5.
- Rajasekhar A, George TJJr. Gemcitabine-induced reversible posterior leukoencephalopathy syndrome: a case report and review of the literature. Oncologist. 2007;12(11):1332–1335.
- Nightingale S, Wood C, Ainsworth J. The posterior reversible encephalopathy syndrome in HIV infection. BMJ Case Rep. 2012;2012: bcr0120125647. doi:10.1136/bcr.01.2012.5647.
- Legriel S, Pico F, Azoulay E. Understanding posterior reversible encephalopathy syndrome. In: Vincent J-L, editor. Annual update in intensive care and emergency medicine 2011. Berlin, Heidelberg: Springer; 2011. p. 631–653.
- Chang OH-C, Stanculescu A, Dola C, et al. Recurrent posterior reversible encephalopathy syndrome potentially related to AIDS and end-stage renal disease: a case report and review of the literature. Case Rep Med. 2012;2012:1–3.
- Abbott NJ, Patabendige AAK, Dolman DEM, et al. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37(1):13–25.
- Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16(1):1–13.
- Tai H, Lai H, Jani J, et al. HIV infection and cocaine use induce endothelial damage and dysfunction in African Americans. Int J Cardiol. 2012;161(2):83–87.
- Andras IE, Pu H, Deli MA, et al. HIV-1 Tat protein alters tight junction protein expression and distribution in cultured brain endothelial cells. J Neurosci Res. 2003;74(2):255–265.
- Tanioka R, Yamamoto Y, Sakai M, et al. Convalescence of atypical reversible posterior leukoencephalopathy syndrome in human immunodeficiency virus infection. J Med Invest. 2007;54(1–2):191–194.
- Sasson SC, Oon A, Chagantri J, et al. Posterior reversible encephalopathy syndrome (PRES) in an HIV-1 infected patient with disseminated varicella zoster virus: a case report. BMC Infect Dis. 2013;13:396.
- Guerriero S, Ciracì L, Centoducati T, et al. Bilateral visual loss as presenting symptom of posterior reversible encephalopathy syndrome in a patient with HIV/tuberculosis coinfection: a case report. Case Rep Ophthalmol Med. 2012;2012:1–4.
- Saeed MU, Dacuycuy MAC, Kennedy DJ. Posterior reversible encephalopathy syndrome in HIV patients: case report and review of the literature. AIDS. 2007;21(6):781–782.
- Paiardini M, Müller-Trutwin M. HIV-associated chronic immune activation. Immunol Rev. 2013;254(1):78–101.
- Prasad A, Kulkarni R, Jiang S, et al. Cocaine enhances DC to T-cell HIV-1 transmission by activating DC-SIGN/LARG/LSP1 complex and facilitating infectious synapse formation. Sci Rep. 2017;7:40648.
- Dahal S, Chitti SVP, Nair MPN, et al. Interactive effects of cocaine on HIV infection: implication in HIV-associated neurocognitive disorder and neuroAIDS. Front Microbiol. 2015;6:931.
- Dalvi P, Wang K, Mermis J, et al. HIV-1/cocaine induced oxidative stress disrupts tight junction protein-1 in human pulmonary microvascular endothelial cells: role of Ras/ERK1/2 pathway. PLoS One. 2014;9(1):e85246.
- Bazuaye-Ekwuyasi EA, Ogunbileje JO, Kaphalia BS, et al. Comparative effects of cocaine and cocaethylene on alveolar epithelial type II cells. Toxicology Mechanisms and Methods 2015;25(8):604–613.
- Cerretani D, Fineschi V, Bello S, et al. Role of oxidative stress in cocaine-induced cardiotoxicity and cocaine-related death. Curr Med Chem. 2012;19(33):5619–5623.
- Tacker DH, Okorodudu AO. Evidence for injurious effect of cocaethylene in human microvascular endothelial cells. Clin Chim Acta. 2004;345(1–2):69–77.
- Shi Y, Wardlaw JM. Update on cerebral small vessel disease: a dynamic whole-brain disease. BMJ. 2016;1:83–92.
- Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12(8):822–838.
