ABSTRACT
Background: Diabetes is a very common cause of cardiovascular disease, and metformin remains the first-line treatment of diabetes. Many trials were conducted to prove the efficacy and safety of other antidiabetic medication as the best add-on medication. Objectives: We aimed to evaluate the atherosclerotic effect of incretin mimetics in patients with diabetes.Methods: We searched in PubMed, clinicaltrials.gov and Cochrane Library for randomized controlled trials (RCTs) comparing incretin mimetic with conventional treatment. The primary outcome was the change in carotid intima-media thickness (CIMT) at the end of the trials.Results: Five RCTs (n = 1241), the mean age of patients included in the trials is 64.3 ± 11.4. The primary outcome was statistically significant for CIMT improvement in terms of long-term follow-up analysis between the incretin mimetic group and conventional group (mean difference [MD] −0.031; 95% Confidence interval [CI] −0.049 to 0.012; P = 0.001), whereas at short-term follow-up it wasn’t (MD −0.004; 95% CI −0.024 to 0.016; P = 0.7) in the overall group of study participants. Additionally, the mean change in body mass index (BMI) (MD 0.064; 95% CI −0.54 to 0.67; P = 0.8), and mean change in systolic blood pressure (MD −0.42; 95% CI −3.2 to 2.3; P = 0.8) or diastolic blood pressure (MD 0.25; 95% CI −1.18 to 1.68; P = 0.7) were not significant.Conclusion: Long-term use of incretin mimetic medication results in significant improvement of atherosclerosis, which leads to fewer vascular events, with no apparent effect on blood pressure or BMI. Further dedicated trials are required to show the superiority of adding these medications to conventional treatment versus placebo.
1. Introduction
Diabetes mellitus prevalence is trending up worldwide, part of which is related to increased prevalence of obesity and dietary changes due to assimilation of western countries [Citation1,Citation2]. Cardiovascular disease remains the leading cause of death among diabetic patients [Citation3–Citation6].Trends to lower glycosylated hemoglobin have reduced the microvascular complications but still there is controversy regarding the impact of tight glycemic control on the macrovascular complications [Citation7,Citation8].
Although most guidelines recommend metformin as the first line to be used in drug-naïve patients, other drugs may play a role as an add-on treatment [Citation9,Citation10]. Cardiovascular safety and efficacy of anti-diabetic medications have been reported in many randomized controlled trials [Citation11]. The cardiovascular impact of antidiabetic agents is variable, but many agents did show a positive impact on the reduction of cardiovascular events [Citation12,Citation13].
Incretin mimetics’ efficacy in controlling blood glucose has been proven by many trials [Citation14], and many trials were done to show their efficacy in improving cardiovascular outcomes [Citation12,Citation15,Citation16]. Incretin receptors on vascular smooth muscle cells have a role in causing atherosclerosis, and this was studied in experimental studies [Citation17,Citation18]. Furthermore, incretin mimetics showed a favorable effect in reducing endothelial dysfunction [Citation19].
Importantly, several randomized control trials have investigated the effect of incretin mimetics on atherosclerosis risk. However, these trials did show variable effect. So, we conducted this meta-analysis on randomized control trials that investigated the effect of incretin mimetics on carotid intima-media thickness (CIMT) as a strong foreteller of future cardiac and cerebrovascular events [Citation20].
2. Methodology
2.1. Literature search and data source
An electronic literature search was performed independently by two investigators (A.A. and Y.Z.) in accordance with the recommendations of the Cochrane Collaboration, using PubMed, clinicaltrials.gov and Cochrane Library from inception through 8 March 2018. Any disagreement was resolved by a discussion of the two reviewers and a third investigator (M.B.). Neither language nor demographic restrictions were applied. All references from papers obtained through the databases were reviewed manually. The search terms were: ‘sitagliptin’, ‘saxagliptin’, ‘vildagliptin’, ‘alogliptin’, ‘linagliptin’, ‘dutogliptin’, ‘gemigliptin’, ‘DPP’, ‘incretin’, ‘dipeptidyl peptidase-4’, ‘GLP-1’, ‘exenatide’, ‘liraglutide’, ‘albiglutide’, ‘dulaglutide’, ‘lixisenatide’, ‘intima media thickness’, ‘carotid’, ‘atherosclerosis’, ‘arteriosclerosis’, ‘plaque’. The electronic search was archived through Mendeley and is available on request.
2.2. Study selection
We included randomized controlled trials (RCTs) comparing dipeptidyl peptidase-4 (DPP-4) inhibitors or Glucagon-like peptide-1 (GLP-1) analogues versus placebo or conventional treatment in patients with diabetes mellitus that studied the effect of treatment on CIMT, which was our primary outcome. Retrospective studies were excluded to decrease bias and confounding variables. We excluded all case reports, case series, letters and replies.
2.3. Outcomes
The primary outcome was change of intima-media thickness (IMT) of the carotid artery on short- and long-term treatment from baseline. Short term was defined as treatment for 1 year or less, while long term was treatment for more than 1 year. Secondary outcomes were systolic and diastolic blood pressure change, and body mass index (BMI) change. All outcomes were measured as the mean difference between the two groups.
2.4. Statistical analysis
Effect estimates in the form of mean difference were extracted from each study. These were directly extracted from the article (when available) or calculated indirectly based on the available data presented in the text of the article. We calculated the weighted mean difference and 95% Confidence interval [CI] using the inverse variance test. The random effect model was used to account for between-study variation and heterogeneity between studies was explored by I-squared (I2) statistic. In addition, we performed a subgroup analysis for duration of outcomes (short vs long) as well as sensitivity analysis in the group with the short duration, excluding the study by Dejgaard et al [Citation21]. since it had the shortest follow-up. We did not perform an assessment for publication bias given the small number of studies included in the analysis. For each endpoint, all statistical tests were two-sided and p values less than 0.05 were considered to be statistically significant. Analyses were conducted with Comprehensive Meta-analysis software version 3.3.070.
3. Results
3.1. Summary of the studies
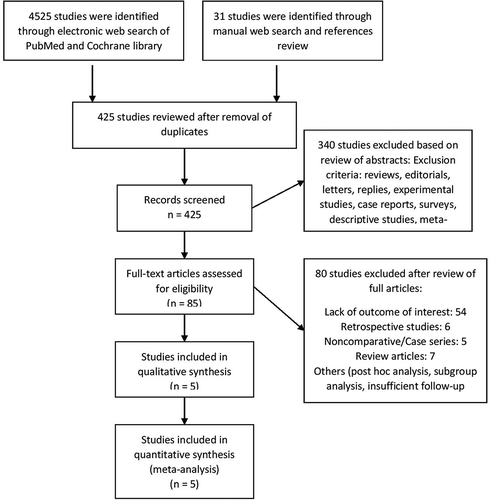
A thorough literature search resulted in 401 articles from electronic searches and 24 articles from other sources, including a manual search and references review. We included 5 prospective studies that compared DPP-4 inhibitors or GLP-1 analogues versus placebo or conventional treatment of diabetes mellitus [Citation21–Citation25]. Included Studies are summarized in . shows the information relevant to the search process. The effect on CIMT after short-term (6 months to 1 year) treatment was reported in the 5 included studies, while the effect after long-term (2 years) treatment was reported in 3 out of the 5 included studies [Citation22–Citation24]. All secondary outcomes were analyzed at 2 years and were reported in 3 studies. The mean age of patients included in the trials is 64.3 ± 11.4. A total of 1241 patients were included in the analysis of the short-term effect of treatment on CIMT, while analyses of the long-term effect on CIMT and all other secondary outcomes included 1057 patients. Demographic features of the included studies described in .
Table 1. Summary of included studies.
Table 2. Demographic features.
In terms of the short-term follow-up there was no statistically significant improvement in the CIMT for the treatment group in comparison to the conventional group (mean difference −0.004; 95% CI −0.024 to 0.016; P = 0.7) . A sensitivity analysis was done by excluding the study by Dejgaard et al. (as this trial had an inordinately short follow-up period (6 months) and included only patients with type 1 diabetes and a low atherosclerotic risk profile) and again there was no statistically significant difference between the 2 groups (mean difference −0.012; 95% CI −0.034 to 0.010; P = 0.28) (Supplementary material Figure 1).
Figure 2. Forest plots summarizing the short term (6–12 months) changes of the carotid intima-media thickness.
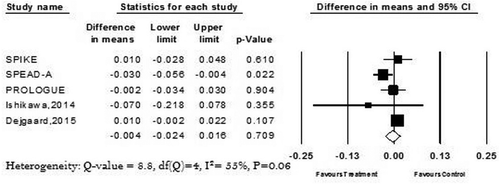
In the long-term follow-up group, which included three studies, the results showed statistically significant improvement in the CIMT for the treatment group in comparison to the conventional group (mean difference −0.031; 95% CI −0.049 to 0.012; P = 0.001) .
Figure 3. Forest plots summarizing the Long term (24 months) changes of the carotid intima-media thickness.
For secondary outcomes, our analysis did not show any statistically significant difference between the two groups in systolic blood pressure (mean difference −0.42; 95% CI −3.2 to 2.3; P = 0.8) diastolic blood pressure (mean difference 0.25; 95% CI −1.18 to 1.68; P = 0.7), or BMI (mean difference 0.064; 95% CI −0.54 to 0.67; P = 0.8) (supplementary material Figures 2–4, respectively).
4. Discussion
In this meta-analysis we included five RCTs that evaluated the effect of incretin mimetics on CIMT. The novel finding of our meta-analysis is the long-term improvement of CIMT in patients receiving GLP-1 mimetics. CIMT has been validated as a good predictor for the incidence of vascular events, especially stroke [Citation20]. This can be translated into low vascular events in patients who are receiving the GLP-1 mimetics in the long term.
Data on long-term outcomes were available in 3 trials: SPIKE, SPEAD-A, and PROLOGUE. The authors of SPIKE and SPEAD-A found an improvement in CIMT in comparison to the baseline at 2 years’ follow-up; this effect was not statistically significant in the PROLOGUE trial. Many inflammatory markers, like IL-6, are suppressed by GLP-1 mimetics in human macrophages through inhibition of protein kinase C [Citation26], and this discordance between PROLOGUE and the other two trials could be explained by the lower level of HbA1c in the study participants; as well-controlled diabetes has a lesser expression of inflammatory markers [Citation27], this could attenuate the anti-inflammatory effect of GLP-1 mimetics in these patients[Citation28]. Also, other medications like pioglitazone and metformin can attenuate the progression of IMT [Citation29,Citation30], and these medications were used more in the conventional group of PROLOGUE participants than the treatment group, which attenuates the effect of sitagliptin [Citation24].
Cardiovascular safety was studied by two large non-inferior randomized control trials: the TECOS and EXAMINE trials, which studied the clinical efficacy and safety of Sitagliptin and Alogliptin, respectively [Citation31,Citation32]. Together with our result that used the CIMT as a proxy for future vascular events, there is a greater impetus for doing superiority trials regarding the vascular events reduction effect of these medications in the future.
Interestingly, the short-term (less than one year) analysis failed to show improvement in the CIMT for the GLP-1 memetics group, and this highlights the time-dependent beneficial effect of these medications. Anti-atherosclerotic effect of anti-diabetic agents usually needs time to be seen and this has been highlighted in the United Kingdom Prospective Diabetes Study (UKPDS), which proposed 10 years of follow-up for the treatment to show a beneficial effect [Citation4].
Four trials evaluated the anti-atherosclerotic effect of DPP4 inhibitors (Sitagliptin in 3 trials and Alogliptin in one trial) at one year. The SPIKE trial was the only one which didn’t show any improvement at this point of time, however, the minimal improvement the other trials showed at one year was not statistically significant [Citation22–Citation25]. The proposed latent effect of DPP4 may be the main reason for this trivial benefit.
Many previous trials studied the blood pressure effect of incretin mimetics and showed variable results, but none of them was a clinically significant reduction [Citation33,Citation34], as vildagliptin improved blood pressure by less than 3 mmHg [Citation35]. In this meta-analysis the included trials failed to show a significant reduction of both systolic and diastolic blood pressure. Although more than 50% of included patients were hypertensive, their blood pressure was controlled and, interestingly, the dosage of sitagliptin used in the SPIKE and PROLOGUE trials was lower than the experimented dose required in a patient who has diabetes and hypertension [Citation36].
Incretin mimetics in general have a favorable effect on weight reduction, although the data support GLP-1 agonist more than DPP4 drugs [Citation37,Citation38]. At 2 years’ follow-up, our data didn’t show significant reduction in BMI, even though it was significant in SPEAD-A, in which Alogliptin was used in the treatment group [Citation23], this may show the variable effect within the DPP4 inhibitor group, as sitagliptin was the treatment drug in the other 2 trials [Citation22,Citation24]. Studies were heterogenous regarding this outcome. This could be explained by the clinical diversity of study populations between the PROLOGUE trial and the other two trials, as the demographic feature of participants in the PROLOGUE trial is quite different, represented by lower HbA1c and higher usage of medications such as metformin and thiazolidinedione [Citation24]. So, a random-effects model was used to overcome this heterogeneity.
The lipid-lowering effect of anti-diabetic medication is variable, but incretin mimetics did show a favorable effect on the lipid profile of diabetic patients in observational and small randomized trials [Citation39,Citation40]. In our analysis we tried to analyze low density lipoprotein (LDL) and high density lipoprotein (HDL) levels, but it was impossible as the numbers were given in different formats; in the PROLOGUE trial the numbers were given in crude value, while in SPIKE and SPEAD-A the numbers were given in percent of change from baseline. Study heterogeneity was variable in the studied outcomes and it was statistically significant in BMI.
5. Limitations
There are some limitations in this meta-analysis that should be acknowledged. First, the trials included in this pooled analysis are not powered enough to detect the vascular events and metabolic effect of these medications. Second, all the included trials in long-term analysis are only on Japanese patients, thus the generalizability of the data is limited. Third, PROBE design was used to conduct the design of included trials; and this may bias the outcome assessment. Fourth, the SPIKE and SPEAD-A trials are multicenter-trials and intersonographer difference in measurement of CIMT is possible. Fifth, the PROLOGUE trials’ outcomes were adjusted to the baseline characteristic, while the other trials didn’t adjust their results.
6. Conclusion
Long-term (2 years) use of incretin mimetics did show a significant improvement of CIMT, which has a strong correlation with vascular events, but these medications failed to show this effect at 1 year. Cardiovascular mortality and morbidity benefits of these medications were studied in non-inferiority trials, and our data support the idea of doing a superiority trial regarding the use of these medications and the conventional treatment. On the other hand, this analysis failed to show a significant effect of these medication on BMI, blood pressure, and lipid profile.
Supplemental Material
Download MS Word (419.5 KB)Acknowledgments
We would like to thank Katherine Negele, editorial assistant, research department, Hurley Medical Center, for assistance with manuscript editing.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this can be accessed here.
References
- Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414(6865):782–787.
- Stamatouli A, Inzucchi S. Implications of the EMPA-REG trial for clinical care and research. Curr Diab Rep. 2016;16(12). DOI:10.1007/s11892-016-0822-7
- Khan SS, Butler J, Gheorghiade M. Management of comorbid diabetes mellitus and worsening heart failure. JAMA. 2014;311(23):2379–2380.
- Holman RR, Paul SK, Bethel MA, et al. 10-Year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–1589.
- Fox CS, Coady S, Sorlie PD, et al. Trends in cardiovascular complications of diabetes. JAMA. 2004;292(20):2495–2499.
- Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017;135(10):e146–e603.
- Dei Cas A, Khan SS, Butler J, et al. Impact of diabetes on epidemiology, treatment, and outcomes of patients with heart failure. JACC Heart Fail. 2015;3(2):136–145.
- Ray KK, Seshasai SRK, Wijesuriya S, et al. Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: a meta-analysis of randomised controlled trials. Lancet. 2009;373(9677):1765–1772.
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35(6):1364–1379.
- Harper W, Clement M, Goldenberg R, et al. Pharmacologic management of type 2 diabetes. Can J Diabetes. 2013;37(Suppl 1):S61–8.
- Goyat R, Rai P, Chang J, et al. Cardiovascular mortality of oral antidiabetic drugs approved before and after the 2008 US FDA guidance for industry: a systemic review and meta-analysis. Clin Drug Invest. March 2018. DOI:10.1007/s40261-018-0639-z.
- Wu S, Cipriani A, Yang Z, et al. The cardiovascular effect of incretin-based therapies among type 2 diabetes: a systematic review and network meta-analysis. Expert Opin Drug Saf. 2018;17(3):243–249.
- Kosiborod M, Birkeland KI, Cavender MA, et al. Rates of myocardial infarction and stroke in patients initiated on SGLT2-inhibitors versus other glucose-lowering agents in real-world clinical practice: results from the CVD-REAL study. Diabetes Obes Metab. 2018 March. DOI:10.1111/dom.13299.
- Waldrop G, Zhong J, Peters M, et al. Incretin-based therapy in type 2 diabetes: an evidence based systematic review and meta-analysis. J Diabetes Complications. 2018;32(1):113–122.
- Marfella R, Sardu C, Balestrieri ML, et al. Effects of incretin treatment on cardiovascular outcomes in diabetic STEMI-patients with culprit obstructive and multivessel non obstructive-coronary-stenosis. Diabetol Metab Syndr. 2018;10:1.
- Li Y-R, Tsai -S-S, Chen D-Y, et al. Linagliptin and cardiovascular outcomes in type 2 diabetes after acute coronary syndrome or acute ischemic stroke. Cardiovasc Diabetol. 2018;17(1):2.
- Jojima T, Uchida K, Akimoto K, et al. Liraglutide, a GLP-1 receptor agonist, inhibits vascular smooth muscle cell proliferation by enhancing AMP-activated protein kinase and cell cycle regulation, and delays atherosclerosis in ApoE deficient mice. Atherosclerosis. 2017;261:44–51.
- Shi L, Ji Y, Jiang X, et al. Liraglutide attenuates high glucose-induced abnormal cell migration, proliferation, and apoptosis of vascular smooth muscle cells by activating the GLP-1 receptor, and inhibiting ERK1/2 and PI3K/Akt signaling pathways. Cardiovasc Diabetol. 2015;14:18.
- Nakamura K, Oe H, Kihara H, et al. DPP-4 inhibitor and alpha-glucosidase inhibitor equally improve endothelial function in patients with type 2 diabetes: EDGE study. Cardiovasc Diabetol. 2014;13:110.
- Lorenz MW, Markus HS, Bots ML, et al. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation. 2007;115(4):459–467.
- Dejgaard TF, Johansen NB, Frandsen CS, et al. Effects of liraglutide on cardiovascular risk factors in patients with type 1 diabetes. Diabetes Obes Metab. 2017;19(5):734–738.
- Mita T, Katakami N, Shiraiwa T, et al. Sitagliptin attenuates the progression of carotid intima-media thickening in insulin-treated patients with type 2 diabetes: the Sitagliptin Preventive Study of Intima-Media Thickness Evaluation (SPIKE): a randomized controlled trial. Diabetes Care. 2016;39(3):455–464.
- Mita T, Katakami N, Yoshii H, et al. Alogliptin, a dipeptidyl peptidase 4 inhibitor, prevents the progression of carotid atherosclerosis in patients with type 2 diabetes: the Study of Preventive Effects of Alogliptin on Diabetic Atherosclerosis (SPEAD-A). Diabetes Care. 2016;39(1):139–148.
- Oyama J-I, Murohara T, Kitakaze M, et al. The effect of sitagliptin on carotid artery atherosclerosis in type 2 diabetes: the PROLOGUE randomized controlled trial. PLoS Med. 2016;13(6):e1002051.
- Ishikawa S, Shimano M, Watarai M, et al. Impact of sitagliptin on carotid intima-media thickness in patients with coronary artery disease and impaired glucose tolerance or mild diabetes mellitus. Am J Cardiol. 2014;114(3):384–388.
- Rizzo MR, Barbieri M, Marfella R, et al. Reduction of oxidative stress and inflammation by blunting daily acute glucose fluctuations in patients with type 2 diabetes: role of dipeptidyl peptidase-IV inhibition. Diabetes Care. 2012;35(10):2076–2082.
- Chacon MR, Vendrell J, Miranda M, et al. Different TNFalpha expression elicited by glucose in monocytes from type 2 diabetes mellitus patients. Atherosclerosis. 2007;194(2):e18–25.
- Lee Y-S, Jun H-S. Anti-inflammatory effects of GLP-1-based therapies beyond glucose control. Mediators Inflammation. 2016;2016:3094642.
- Matsumoto K, Sera Y, Abe Y, et al. Metformin attenuates progression of carotid arterial wall thickness in patients with type 2 diabetes. Diabetes Res Clin Pract. 2004;64(3):225–228.
- Mazzone T, Meyer PM, Feinstein SB, et al. Effect of pioglitazone compared with glimepiride on carotid intima-media thickness in type 2 diabetes: a randomized trial. JAMA. 2006;296(21):2572–2581.
- Green JB, Bethel MA, Armstrong PW, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373(3):232–242.
- White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369(14):1327–1335.
- Kumarathurai P, Anholm C, Fabricius-Bjerre A, et al. Effects of the glucagon-like peptide-1 receptor agonist liraglutide on 24-h ambulatory blood pressure in patients with type 2 diabetes and stable coronary artery disease: a randomized, double-blind, placebo-controlled, crossover study. J Hypertens. 2017;35(5):1070–1078.
- Leung M, Leung DY, Wong VW. Effects of dipeptidyl peptidase-4 inhibitors on cardiac and endothelial function in type 2 diabetes mellitus: a pilot study. Diab Vasc Dis Res. 2016;13(3):236–243.
- Evans M, Schweizer A, Foley JE. Blood pressure and fasting lipid changes after 24 weeks’ treatment with vildagliptin: a pooled analysis in >2,000 previously drug-naive patients with type 2 diabetes mellitus. Vasc Health Risk Manag. 2016;12:337–340.
- Wilson JR, Shuey MM, Brown NJ, et al. Hypertension and type 2 diabetes are associated with decreased inhibition of dipeptidyl peptidase-4 by sitagliptin. J Endocr Soc. 2017;1(9):1168–1178.
- Aschner P, Kipnes MS, Lunceford JK, et al. Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care. 2006;29(12):2632–2637.
- Buse JB, Klonoff DC, Nielsen LL, et al. Metabolic effects of two years of exenatide treatment on diabetes, obesity, and hepatic biomarkers in patients with type 2 diabetes: an interim analysis of data from the open-label, uncontrolled extension of three double-blind, placebo-controlled trials. Clin Ther. 2007;29(1):139–153.
- Masuda D, Kobayashi T, Sairyou M, et al. Effects of a dipeptidyl peptidase 4 inhibitor sitagliptin on glycemic control and lipoprotein metabolism in patients with type 2 diabetes mellitus (GLORIA trial). J Atheroscler Thromb. 2017 December. DOI:10.5551/jat.41343.
- Hasegawa Y, Hori M, Nakagami T, et al. Glucagon-like peptide-1 receptor agonists reduced the low-density lipoprotein cholesterol in Japanese patients with type 2 diabetes mellitus treated with statins. J Clin Lipidol. 2018;12(1):62–69.e1.