ABSTRACT
Discovered by Louis Pasteur in 1880, Pasteurella multocida is the most common cause of zoonotic infection in humans which is transmitted via pet bites and/or scratches. However, animal contact may be absent or not identified in up to 40% of cases which usually occur in individuals with comorbidities. Despite having a low virulence, PM can cause serious and life threatening infections in rare instances. In such cases, prompt diagnosis and appropriate treatment can lead to miraculous recovery.
1. Introduction
‘Zoonoses’ are a group of infections in humans caused by various micro-organisms, primarily transmitted via some form of animal exposure. Pasteurella multocida is one such organism which is characterized as a gram-negative coccobacillus and is part of the normal flora in domestic animals (cats and dogs). It causes infections in humans through pet bites/scratches. However, severe infections may occur in the absence of animal bites or scratches especially with immune suppression. Although, commonly associated with soft tissue infections, rare cases of Pasteurella multocida as the causative agent of prosthetic joint infection have been documented in medical literature.
Here we present an interesting case of Pasteurella multocida infection highlighting the unusual presentation and lack of animal exposure.
2. Case description
A 71-year-old gentleman with history of alcohol abuse, chronic obstructive pulmonary disease (COPD) and left hip replacement presented to the emergency department for evaluation of acute-onset left lower extremity pain. Of note, he had been admitted 2 months prior for treatment of a right lung abscess attributed to aspiration. A complete infectious workup including blood and sputum cultures was unremarkable at that time and due to his comorbidities, it was decided not to pursue any further invasive investigations. He was given IV ampicillin-sulbactam for 4 weeks and a repeat CT chest afterwards showed resolution of the abscess.
During this presentation, he described the pain in his posterior thigh region which usually worsened on walking but improved on rest. He denied having any trauma or recent surgery or fall. He had undergone a total hip replacement 25 years back with revision surgery performed 10 years later. A cobalt-chromium prosthesis had been inserted at that time.
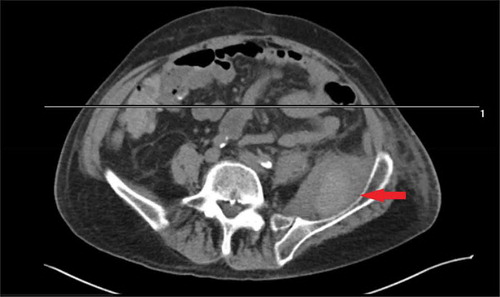
On examination, he was normotensive, afebrile and not in any respiratory distress. Pulses were palpable in lower extremities. Right leg examination was unremarkable. Left leg showed no warmth, skin changes, wound or discharge. Tenderness on deep palpation was noted in the medial and posterior aspect of the left thigh. Range of motion in left hip was limited both on active and passive motion. Sensations were intact. The left knee was unremarkable. Laboratory investigations revealed an leukocytosis of 27,000 with 40% bands. Metabolic profile, lactic acid and urinalysis were unremarkable. Blood cultures were drawn and patient was started on intravenous vancomycin and piperacillin-tazobactam. Chest x-ray showed no new infiltrates or cavitatory changes. Venous doppler of the concerned extremity ruled out a venous thrombus. However, CT scan of the left leg showed ‘5x5.5x8 cm peripherally calcified mass in the medial left thigh anterior to the left hip’ ().
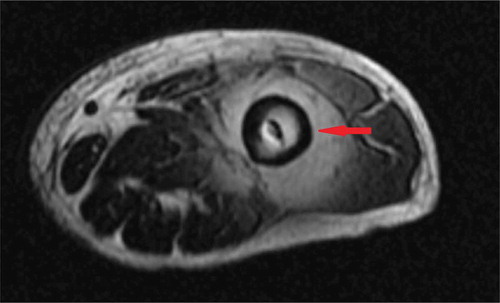
Orthopedic surgery was consulted and ultrasound-guided drainage of the mass was performed during which 100 ml of thick viscous black colored fluid was aspirated. As it was not clear whether there was involvement of the left hip, an MRI hip was done which demonstrated ‘5x6.5x8 cm circumscribed fluid collection around and anterior to the left hip joint ().
Figure 2. MRI without contrast of the hip showing the fluid collection around left hip joint (red arrow).
Meanwhile, blood and fluid cultures showed growth of gram-negative rods; it was determined to be Pasteurella multocida on final report. On close questioning, the patient and the family denied having any pet or animal contact. No bites or scratch marks were noted on examination.
The patient underwent irrigation, debridement of the left hip joint followed by Explant and antibiotic spacer placement. The infectious disease consultant recommended IV ampicillin-sulbactam for 6 weeks after susceptibility testing while orthopedic surgery decided to perform definitive revision hip arthroplasty after completion of the course.
Despite all these interventions, the patient failed to improve and opted for palliative care; therefore, no further aggressive management was pursued and he was transferred to a hospice facility.
3. Discussion
Pasteurella multocida (PM) is a gram-negative, non-spore forming coccobacillus belonging to the Pasteurella species; named after the ‘father of microbiology’ Loius Pasteur who isolated it in 1880 [Citation1].
Classically, PM infections in humans are categorized under ‘Zoonoses’ as the mode of transmission is through bites or scratches by animals such as dog and cats; in these animals, PM is part of the normal gastrointestinal and upper respiratory tract flora [Citation2].
Interestingly, cases have been reported on ‘non-zoonotic’ PM infections where patients had no identifiable animal exposure [Citation3]. A similar scenario was observed in our patient who denied any animal contact; in addition, no scratch, bite mark or wound could be identified on a detailed examination.
PM usually causes soft-tissue infections in the form of cellulitis, lymphangitis, abscesses and necrotizing fascitis. However, other systems are not that commonly involved ().
Table 1. Unusual manifestations of Pasteurella multocida infection in humans [Citation6].
PM is known to cause respiratory tract infections and lung abscesses in patients with underlying lung disease. This, along with the fact that PM is susceptible to beta-lactams, suggests that our patient may have had lung abscess secondary to PM which resolved after IV beta-lactam therapy. Furthermore, PM bacteremia and prosthetic joint infection especially in the absence of animal exposure is a rare medical occurrence. A review of medical literature shows that so far only 33 such cases have been reported [Citation4,Citation5].
A study found that PM infections without a bite history usually occur in older patients with serious comorbidities, malignancy, chronic lung disease, cirrhosis, prolonged steroid use, diabetes and autoimmunity [Citation6]. In addition, PM can colonize the respiratory tract of immunocompetent individuals who are directly exposed to animals without causing an infection. Any close contact with such colonized individuals can lead to infections in immunocompromised patients. Rarely, transmission via contaminated body fluids/tissue, blood products and intra-partum infection have also been reported [Citation3,Citation7]. We speculate that since our patient was an elderly gentleman with history of COPD, an upper respiratory colonization with PM could have resulted in transmucosal transmission of the pathogen.
In our patient, I&D of the joint demonstrated a thick black aspirate which is not characteristically seen in septic arthritis. This was likely due to infection-induced metallic synovitis and breakdown of the joint prosthesis; with resultant metallic debris imparting the dark color to the pus.
It is worth mentioning here that Gram stains are not useful for diagnosing PM infection as the pathogen can be misidentified initially. This is because on gram-stain they can appear either as single bacillus, in pairs or short chains [Citation8]. Therefore, the diagnosis of PM infection is made by isolation of the organism in culture. In our patient, the initial GS of blood and joint aspirate demonstrated gram-negative rods but the final cultures confirmed PM.
PM is susceptible to a number of antibiotics including penicillins. However, the pathogen can have beta-lactamase activity resulting in treatment failures or resistance [Citation5,Citation9]. Therefore, antibiotic susceptibility testing is recommended in severe infections. In presence of beta-lactamase activity, treatment should be pursued with one of the following: ampicillin-sulbactam, piperacillin-tazobactam, quinolones, broad-spectrum cephalosporins and carbapenams. This is important as mortality rate can be as high as 31% among bacteremic patients and early initiation of appropriate therapy can help improve outcomes [Citation10].
4. Conclusion
Our case highlights an unusual presentation of PM infection in addition to highlighting several learning points. First, contrary to the common belief, Pasteurella multocida can cause ‘non-zoonotic’ infections in humans. This is especially true for individuals who are immunocompromised. Second, the pathogen can be misidentified on gram-staining of fluid/tissue. Last, once identified on cultures, prompt antibiotic therapy with susceptibility testing should be pursued to improve outcomes on these potentially fatal infections.
Disclosure statement
No potential conflict of interest was reported by the author.
References
- Henderson A. Pasteurella multocida infection in man; A review of the literature. Antonie Van Leeuwenhoek. 1963;29:359–367.
- Owen CR, Buker EO, Bell JF, et al. Pasteurella multocida in animals’ mouths. Rocky Mt Med J. 1968;65(11):45–46.
- Orsini J, Perez R, Llosa A, et al. Non-zoonotic Pasteurella multocida infection as a cause of septic shock in a patient with liver cirrhosis: a case report and review of the literature. J Glob Infect Dis. 2013;5(4):176–178.
- Honnorat E, Seng P, Savini H, et al. Prosthetic joint infection caused by Pasteurella multocida: a case series and review of literature. BMC Infect Dis. 2016;16:435.
- Runnstrom M, Hyde R, Shahb K. Pasteurella multocida prosthetic joint infection. IDCases. 2018;13:e00429.
- Weber DJ, Wolfson JS, Swartz MN, et al. Pasteurella multocida infections. Report of 34 cases and review of the literature. Medicine (Baltimore). 1984;63(3):133.
- Siahanidou T, Gika G, Skiathitou AV, et al. Pasteurella multocida infection in a neonate: evidence for a human-to-human horizontal transmission. Pediatr Infect Dis J. 2012;31(5):536.
- Ashley BD, Noone M, Dwarakanath AD, et al. Fatal Pasteurella dagmatis peritonitis and septicaemia in a patient with cirrhosis: a case report and review of the literature. J Clin Pathol. 2004;57(2):210.
- Oehler RL, Velez AP, Mizrachi M, et al. Bite-related and septic syndromes caused by cats and dogs. Lancet Infect Dis. 2009;9(7):439–447.
- Raffi F, Jacques B, Baron D, et al. Pasteurella multocida bacteremia: report of thirteen cases over twelve years and review of the literature. Scand J Infect Dis. 1987;19(4):385–393.