ABSTRACT
Focal segmental glomerulosclerosis (FSGS) is a common cause of nephrotic syndrome, accounting for 40% of nephrotic syndrome in adults. FSGS has diverse clinical and morphological features and underlying pathogenesis. We present a case of a 33-year-old male presenting with acute systolic heart failure complicated with left ventricular thrombus with embolism to coronary circulation and bilateral deep vein thrombosis. He was found to have nephrotic range proteinuria with kidney biopsy showing FSGS. Association of FSGS with cardiomyopathy has been reported in children. However, in adults, according to our best knowledge, there have not been any report of FSGS and non-ischemic cardiomyopathy or it is at least underreported.
Abbreviations
FSGS: Focal segmental glomerulosclerosis; ESRD: End-stage renal disease; NOS: Not otherwise specified; LV: Left ventricle
1. Introduction
FSGS has an estimated prevalence of 4% and is the most common primary glomerular disease resulting in end-stage renal disease in the USA. FSGS accounts for 7–20% of idiopathic nephrotic syndrome in children and 40% in adults [Citation1]. The clinical course and prognosis is heterogeneous. Proteinuria ranging from nephrotic to sub-nephrotic range is usually the presenting feature of FSGS; other associated features are hypertension, microscopic hematuria, renal insufficiency and hypercoagulability-related complications like arterial and venous thrombosis [Citation2]. The role of FSGS in cardiomyopathy has not been established. Although, in pediatric literature, there have been reports of cardiac complications related to FSGS, the association in adults has not been recognized and reported [Citation3]. Our patient presented with heart failure secondary to cardiomyopathy along with left ventricular clot, bilateral deep vein thrombosis and nephrotic range proteinuria. In ESRD, cardiac morbidity and mortality related to microvascular disease, accelerated atherosclerosis and arteriosclerosis is well established [Citation4]. However, the association of nephrotic syndrome, specifically FSGS, with cardiomyopathy needs further investigation.
2. Case report
A 33-year-old male presented to the emergency department for shortness of breath and bilateral leg swelling for 2 weeks. He endorsed orthopnea and paroxysmal nocturnal dyspnea but denied any history of chest pain, palpitations, recent viral illness, drug use, or alcohol use. On examination, he had increased jugular venous distension, bibasilar crackles and bilateral 2+ pedal edema. The rest of the physical exam was unremarkable.
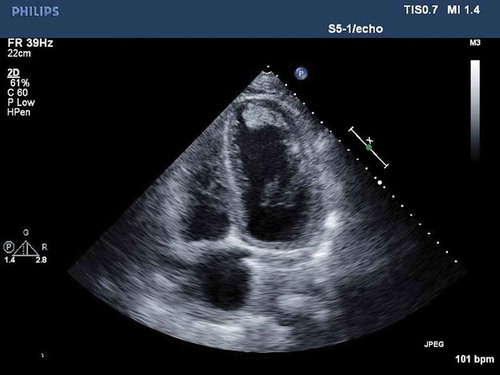
Labs on presentation showed blood urea nitrogen of 90 mg/dL, creatinine of 6.43 mg/dL, glomerular filtration rate of 10 mL/min, troponin of 52.94 ng/mL and total creatine kinase over 4000 U/L. The complete blood count and lipid profile were within normal limits. Electrocardiogram showed sinus tachycardia at 114 beats per minute, right axis deviation, normal PR interval, incomplete right bundle branch block, poor R wave progression, no ST-segment elevations or depressions, normal QTc interval, no T wave inversions. The patient was admitted to the cardiac care unit and further workup for non-ST segment elevation myocardial infarction and renal failure was done. Transthoracic echocardiogram showed ejection fraction of 15–20%, severely dilated LV with mild concentric hypertrophy with severe global diffuse LV hypokinesis, grade II diastolic dysfunction and layered echogenic material extending from the mid to distal anterior lateral wall, anterior wall, inferior wall and apex which represented a layered thrombus ().
Figure 1. Transthoracic echocardiogram in the apical four-chamber view showing layered echogenic material extending from the mid to distal anterior lateral wall, anterior wall, descending inferior wall and apex which represented a layered thrombus.
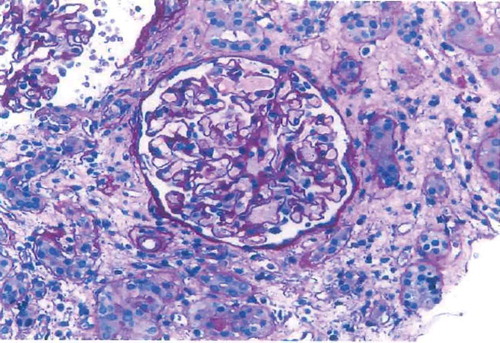
Twenty-four-hour total urine protein was 4325 mg, C3 complement was 78 mg/dL, C4 complement was 37 mg/dL, and both serum and urine electrophoresis were within normal limits. Further immunological and infectious serologies were found to be negative. Renal ultrasound showed echogenic kidneys compatible with parenchymal renal disease. Cardiac catheterization showed that the left main, left anterior descending, left circumflex, right coronary arteries were normal; however, the obtuse marginal was 100% occluded which was likely from an embolism from the LV thrombus. Given the high suspicion of deep venous thrombosis in the setting of nephrotic syndrome, a bilateral venous ultrasound of legs was done which showed chronic non-occlusive thrombus in the bilateral tibialis posterior veins. A kidney biopsy showed FSGS, NOS type with widespread foot process effacement, global glomerulosclerosis, severe arteriosclerosis moderate interstitial fibrosis, moderate tubular atrophy and no evidence of immune complex-related glomerular disease ().
Figure 2. Kidney biopsy showed FSGS, NOS type with widespread foot process effacement, global glomerulosclerosis, severe arteriosclerosis, moderate interstitial fibrosis, and moderate tubular atrophy.
The patient was started on aspirin, clopidogrel, heparin drip and atorvastatin. During the hospital course, a tunneled dialysis catheter was placed, and he was started on dialysis. He was started on anticoagulation with a heparin drip for the LV thrombus which was later transitioned to warfarin. The patient was discharged on warfarin, carvedilol, lisinopril, aspirin, atorvastatin, furosemide and omeprazole and had outpatient follow with cardiology and nephrology.
On follow up with cardiology, he had improvement in his ejection fraction to 35–40% with guideline-directed medical therapy. He was continued on hemodialysis without complications and had placement of an arteriovenous fistula. He had repeat ultrasound of lower extremities which were negative for deep venous thrombosis.
3. Discussion
FSGS is a histologic lesion, rather than a clinical disease, characterized by focal and segmental obliteration of glomerular capillary tufts with increased matrix. The Columbia classification of FSGS based on the location and character of the sclerotic lesion is as follows: collapsing, tip, cellular, perihilar and NOS. NOS variant is the most common form both in adults and children [Citation5]. Hypercoagulability is an established feature in nephrotic syndrome. The possible mechanisms of the hypercoagulable state include increased plasma level of procoagulant factors (fibrinogen, Factor V, Factor VIII and Factor X) secondary to increased liver synthesis, low plasma concentration of antithrombin III due to renal losses, and depression of fibrinolysis in nephrotic syndrome [Citation6]. In our patient, there was a clot in the LV with a likely embolism to the coronary circulation. In addition to hypercoagulability from nephrotic syndrome, low ejection fraction secondary to cardiomyopathy results in low regional intracardiac blood flow velocity which may also have contributed to the clot formation.
The American Heart Association defines cardiomyopathies as a heterogeneous group of diseases of the myocardium associated with mechanical and/or electrical dysfunction that usually exhibit inappropriate ventricular hypertrophy or dilation and are due to a variety of causes that frequently are genetic. Cardiomyopathies are categorized as primary cardiomyopathies which predominantly involve the heart and secondary cardiomyopathies which are accompanied by other organ system involvement [Citation7]. The cardiovascular and renal system interact with each other to maintain hemodynamic stability and vascular tone. Cardio-renal syndrome ensues when dysfunction in one system leads to progressive decline in both systems. ESRD factors, such as activation of the renin-angiotensin-aldosterone system, chronic damage by uremic toxins and dysfunction of calcium and phosphate metabolism are involved in the progressive decline of the cardiovascular system. These factors chronically lead to LV hypertrophy, interstitial fibrosis, microvascular disease and vasculopathy in the form of atherosclerosis and arteriosclerosis [Citation4]. Atherosclerosis is a primarily intimal disorder of medium-sized arteries characterized by plaque formation and subsequent narrowing and occlusion of the vessels resulting in impaired conduit function. These changes lead to chronic myocardial ischemia, myocardial fibrosis, heart failure and sudden cardiac death [Citation8]. Arteriosclerosis – hallmark of arterial remodeling in ESRD – leads to calcification and increased wall thickness of the medial layer of the aorta and its branches [Citation9]. Cardiovascular mortality accounts for 40% of all-cause mortality in these patients. With ESRD, these changes occur chronically [Citation10].
Adedoyin et al compared the occurrence of cardiac disease in children with glomerular causes of primary nephrotic syndrome. Cardiac diseases such as cardiomyopathy, congestive heart failure, and LV hypertrophy occurred in six cases – all with FSGS. It was noted that black ethnicity and female sex are more susceptible to develop cardiac complications among patients with FSGS [Citation3].
Primary FSGS is considered to be related to podocyte injury, and the pathogenesis of podocyte injury has been actively investigated. Several circulating factors such as Cardiotrophin-like cytokine-1 (CLC-1), anti-CD40 antibodies and soluble urokinase-type plasminogen activator receptor affecting podocyte permeability barrier have been proposed [Citation11]. It is possible that the circulating factors associated with FSGS may also account for the initiation of cardiomyopathy in patients with this glomerulopathy. The role of a permeability factor in the pathogenesis of FSGS is suggested by the high recurrence rate of 20–50% in FSGS after kidney transplantation [Citation12], reports of substantial reduction in proteinuria after plasmapheresis [Citation13], plasma or plasma fraction from patients with FSGS injected into rats causing proteinuria [Citation14] and possible maternal transplacental transmission of FSGS causing proteinuria in the infant [Citation15]. It is possible that the permeability factor involved in the pathogenesis of FSGS might also be involved in causing cardiomyopathy in these patients.
Our patient presented with heart failure secondary to cardiomyopathy along with a LV clot, bilateral deep vein thrombosis, and nephrotic range proteinuria. Certain factors involved in the pathogenesis of FSGS might also be involved in causing cardiomyopathy in these patients; larger studies are needed to understand the factors involved in concomitant cardiac and renal failure in these patients. Unique to our patient was the acute presentation; ischemic cardiomyopathy was ruled out by the nonischemic cardiac catheterization findings. The complete total occlusion of obtuse marginal branch would not explain the low ejection fraction and the wall motion abnormalities noted on the initial echocardiogram. We attribute the obtuse marginal occlusion as being secondary to embolism from the intracardiac thrombus.
4. Conclusion
In conclusion, our case and review of literature highlights an important correlation between FSGS and cardiomyopathy as its management differs from other forms of cardiomyopathy. Health-care providers should be aware of the cardiac complications of FSGS, and high index of suspicion should be kept in mind for the appropriate clinical scenario. Though in pediatrics, bilateral iliac and popliteal arterial thrombosis [Citation16] as well as pulmonary embolism [Citation17] have been documented, to our knowledge, this is the only case of FSGS in adult population that presented with left ventricular thrombus.
Disclosure statement
There are no conflicts of interest.
References
- Braden GL, Mulhern JG, O’Shea MH, et al. Changing incidence of glomerular diseases in adults. Am J Kidney Dis. 2000;35:878–883.
- Swaminathan S, Leung N, Lager DJ, et al. Changing incidence of glomerular disease in Olmsted County, Minnesota: a 30-year renal biopsy study. Clin J Am Soc Nephrol. 2006;1:483–487.
- Adedoyi O, Frank R, Vento S, et al. Cardiac disease in children with primary glomerular disorders— role of focal segmental glomerulosclerosis. Pediatr Nephrol. 2004;19:408–412.
- Chirakarnjanakorn S, Navaneethan SD, Francis GS, et al. Cardiovascular impact in patients undergoing maintenance hemodialysis: clinical management considerations. Int J Cardiol. 2017;232:12–23.
- Lim BJ, Yang JW, Do WS, et al. Pathogenesis of focal segmental glomerulosclerosis. J Pathol Transl Med. 2016;50:405–410.
- Chen TV, Hung CC, Tsao CJ. Hemostatic molecular markers in nephrotic syndrome. Am J Hematol. 1993;44:276–279.
- Maron BJ, Towbin JA, Thiene G, et al. American heart association; council on clinical cardiology, heart failure and transplantation committee; quality of care and outcomes research and functional genomics and translational biology interdisciplinary working groups; and council on epidemiology and prevention. Circulation. 2006;113:1807–1816.
- London GM, Drueke TB. Atherosclerosis and arteriosclerosis in chronic renal failure. Kidney Int. 2002;73:678–1695.
- London GM, Marchais SJ, Guerin AP. Arterial stiffness and function in end-stage renal disease. Adv Chron Kidney Dis. 2004;11: 202–209.
- de Jager DJ, Grootendorst DC, Jager KJ, et al. Cardiovascular and noncardiovascular mortality among patients starting dialysis. JAMA. 2009;302:1782–1789.
- Wada T, Nangaku M. A circulating permeability factor in focal segmental glomerulosclerosis: the hunt continues. Clin Kidney J. 2015;8:708–715.
- Vincenti F, Ghiggeri GM. New insights into the pathogenesis and the therapy of recurrent focal glomerulosclerosis. Am J Transplant. 2005;5:1179–1185.
- Davenport RD. Apheresis treatment of recurrent focal segmental glomerulosclerosis after kidney transplantation: re-analysis of published case-reports and case-series. J Clin Apher. 2001;16:175–178.
- Sharma M, Sharma R, Reddy SR, et al. Proteinuria after injection of human focal segmental glomerulosclerosis factor. Transplantation. 2002;73:366–372.
- Kemper MJ, Wolf G, Muller-Wiefel DE. Transmission of glomerular permeability factor from a mother to her child. N Engl J Med. 2001;344:386–387.
- Han KH, Park JY, Min SK, et al. Bilateral iliac and popliteal arterial thrombosis in a child with focal segmental glomerulosclerosis. Korean J Pediatr. 2016;59(5):242–245.
- Guenther RA, Kemp WL. Delayed death due to saddle pulmonary thromboembolus in child with nephrotic syndrome induced by focal segmental glomerulosclerosis. Am J Forensic Med Pathol. 2018;39(4):370.
