ABSTRACT
Patients presenting with bacteremia and the presence of multiple infected emboli sites should be promptly investigated for heart valve endocarditis as the possible culprit. We present a case of Eustachian valve endocarditis secondary to multiple abdominal surgeries to highlight not only the importance of recognizing risks factors, other than intravenous drug use, in the pathogenesis of right sided endocarditis, but also to illustrate the significance of systematically interrogating all the heart valves, including the Eustachian valve during echocardiography.
1. Introduction
The Eustachian valve, also known as the valve of the inferior vena cava, is easily visualized on routine ultrasound; however, its involvement in bacterial endocarditis is rare, with only 37 cases reported since 1986 [Citation1]. The infrequent presentation of such a case makes it a diagnostic challenge and raises question of whether it is an incidental finding or if it is truly pathologic. Recent literature reviewed showed that it predominantly occurs in intravenous drug abusers (IVDA), in patients with pacemaker or central venous lines and in patients with congenital heart disease [Citation1–Citation4]; however, here we present a patient with no history of the aforementioned but with a history multiple abdominal surgeries who was found to have Eustachian valve vegetations.
2. Case presentation
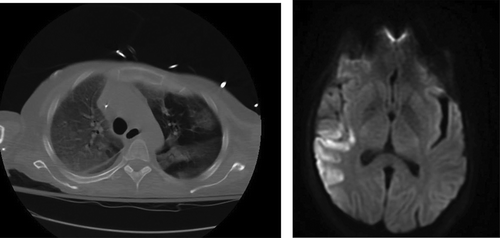
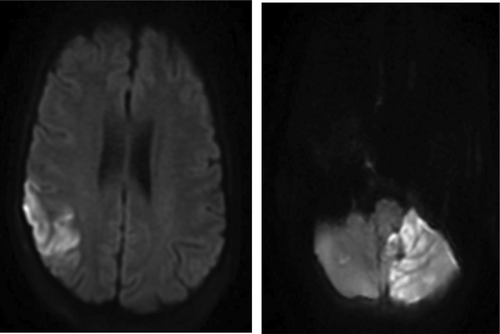
A 54 year old woman with a past medical history significant for Roux-en-Y procedure 11 years ago and a recent exploratory laparotomy for small bowel obstruction 3 months ago, with no prior history of IV drug abuse, presented to the emergency department with altered mental status described as confusion and hallucinations, and chest pain over the past 3 days. Vitals at initial assessment were: blood pressure (BP): 119/78 mmHg, pulse 85 beats per minute, temperature 97.8 F°, respiratory rate (RR): 18/minute and SpO2 of 84% at room air. On examination, patient had 13/15 on Glasgow Coma Scale (GCS), patient was alert and awake but not oriented to person, place or time, lung exam revealed bilateral coarse crackles and expiratory wheezes throughout lung fields, and cardiovascular exam revealed regular rate and rhythm, normal S1 and S2, no murmurs or gallops. Electrocardiogram (ECG) showed normal sinus rhythm with no ST or T wave changes. Abdomen examination showed present bowel sounds with mild diffuse tenderness to palpation and a 3 cm abdominal wound healing well with no surrounding erythema or exudate from recent exploratory laparotomy. Initial laboratory results showed a white blood cell count of 11.8×103/mm3 (normal value 4.5–11.0×109/L), total bilirubin of 3.2 mg/dL (normal value 0.2–1.2 mg/dL), AST 131 U/L (normal value <40 U/L), ALT 178 U/L (normal value <40 U/L), ALK phosphate of 339 IU/L (normal value 20–140IU/L). Urinalysis was negative for infections and a urine drug screen was positive for opiates and benzodiazepines. Arterial blood gas showed pH of 7.37, PCO2 of 40.7, PO2 of 56, and a HCO3 of 23.3. Chest X-ray revealed that the patient had multi-lobar pneumonia () and further workup of her AMS, which included a brain MRI, revealed a right fronto-parietal and a left cerebellar Infarct (, ).
Figure 2. MRI brain showing acute ischemic edema involving the right posterior frontal parietal distribution.
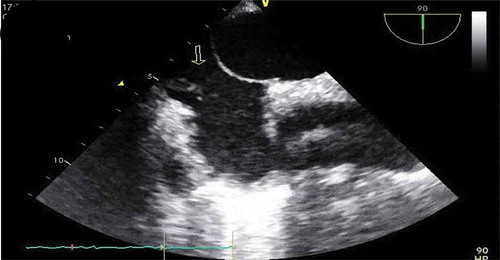
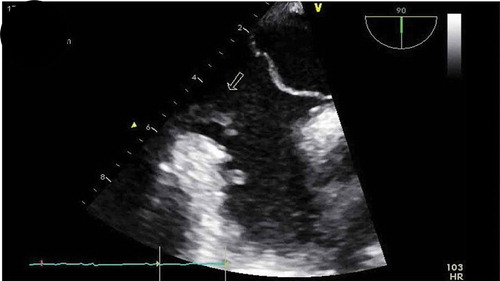
Patient was placed on BiPAP due to her hypoxemia, blood cultures were drawn, IV antibiotics were started, including Vancomycin, Cefepime, and Metronidazole, and patient transferred to the critical care unit for further management. During the course of her hospitalization, patient developed severe acute hypoxic respiratory failure requiring intubated. As part of the work up for the multiple-lobar brain infarct, a trans-esophageal echocardiogram (TEE) was done within 24 h of diagnosis of multi-lobar infarct to evaluate for cardiogenic source of ischemic cerebrovascular accident. The TEE revealed a prominent Eustachian valve with multiple vegetations, diffuse hypokinesia, and a left ventricle ejection fraction of 30% with a patent foramen ovale as seen in . Blood cultures revealed Staphylococcus capitis, which was thought to be a contaminant, but a bronchoalveolar lavage (BAL) culture revealed Methicillin Resistant Staphylococcus Aureus. Patient continued to improve on medications and was successfully extubated a few days later and mental status gradually improved, although patient was noted to have some residual deficits that included a left facial droop and left upper extremity weakness. One week after initiation of antibiotics, repeat transthoracic echocardiogram showed an ejection fraction of 45–50% and a normal Eustachian valve. Additionally, repeat blood cultures 4 days after initiation of antibiotic therapy were negative. Patients symptoms continued to improve and were discharged to a skilled nursing facility and were set to continue IV Vancomycin and Cefepime for a total duration of 6 weeks from first negative blood culture. Although a trans-esophageal echocardiogram could not be repeated due to patient non-compliance repeat transthoracic echocardiogram was performed at a follow up exam after completion of antibiotics, which again showed a prominent Eustachian valve but no vegetation, and an ejection fraction that improved to 55%.
3. Discussion
Infective endocarditis refers to a bacterial or, rarely, a fungal infection of the cardiac valves, and involves almost exclusively the left side of the heart, except in patients who are intravenous drug users or with other known risks factors like valve injury from pulmonary artery catheters, in whom infection of the right side of the heart may occur, most commonly at the site of the tricuspid valve.
The Eustachian valve, a remnant of the right sinus venosus valve, is seen at the junction of the Inferior Vena Cava (IVC) and Right Atrium (RA) in approximately 25% of individuals and appears as an elongated, membranous structure that can extend from the IVC to the border of the fossa ovalis [Citation4,Citation5], and, in rare cases, it can become a focus of infection.
Eustachian valve endocarditis was first described in 1986 by Edwards et al. [Citation6], and from 1986 to 2018, only 37 cases have been reported, 46% of which was caused by intravenous drug use and 24% by indwelling intravenous lines [Citation1]. Other usual predisposing risk factors for Eustachian valve endocarditis include rheumatic heart disease, pacemaker wires, and immunological compromise [Citation1–Citation9]. This case illustrates the importance of recognizing risk factors, other than IV drug use, in the pathogenesis of Eustachian valve endocarditis;
This case also demonstrates the significance of systematically interrogating the Eustachian valve during an echocardiography in all patients suspected of having endocarditis, including a careful assessment of atrial septal patency in right-sided endocarditis due to cerebral and/or systemic embolism are possible complications. This systematic approach is necessary, as studies have shown that it might be more frequent than previously thought but easily missed during routine studies [Citation1,Citation4]. In this particular case, the presence of a patent foramen ovale explains the septic emboli affecting both lungs as well as the multi-lobar infarcts on the brain.
Previous studies have shown that both TTE and TEE offer similar information in the diagnosis of Eustachian valve endocarditis, and it is usually recommended to start with TTE, but more recent studies have determined that TEE is a more sensitive diagnostic tool for right-sided and Eustachian valve endocarditis than TTE [Citation1,Citation4,Citation7–Citation9]. In this particular case, several TTE were done that did not show the valve vegetations due to poor acoustic window, consequently a TEE was performed that revealed the Eustachian valve vegetations. This demonstrates the value of using TEE for the diagnosis of suspected right-sided endocarditis, as its superiority at detecting smallest size vegetations on the right side and to help define complications if present, as valve perforations.
A repeat TEE could not be completed to evaluate the response to antibiotics due patient noncompliance, but a transthoracic echocardiogram with a subcostal view showed marked improvement in the ejection fraction 1 week after initiation of antibiotics from 30% to 45–50%, and patient symptoms gradually improved as well.
4. Conclusion
This case highlights the importance of recognizing all the risk factors, not just IV drug use, in the pathogenesis of right sided endocarditis, including Eustachian valve endocarditis, and the importance of interrogating all the valves during an echocardiography in all patients where there is a high suspicion for having endocarditis, as it can be easily missed if not done in a systematic way. Furthermore, it elucidates the importance of doing TEEs in patients with possible right sided endocarditis even in patients with negative TTE, as they have higher sensitivity for right sided vegetations, and, although TTEs are usually done first, in high-suspicion patients TEE studies could be considered as the first diagnostic tool due to its highest sensitivity, as it can detect smaller size vegetations and help define complications if present.
Disclaimer
This research was supported (in whole or in part) by HCA and/or an HCA affiliated entity. The views expressed in this publication represent those of the authors and do not necessarily represent the official views of HCA or any of its affiliated entities.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Loon JC, Chao C, Younger JF, et al. Eustachian valve endocarditis: case report and literature review. Australas J Ultrasound Med. 2017;21(1):29–35.
- Pellicelli AM, Pino P, Terranova A, et al. Eustachian valve endocarditis: a rare localization of right side endocarditis. A case report and review of the literature. Cardiovasc Ultrasound. 2005;3(1). DOI:10.1186/1476-7120-3-30
- Alves M, Faria R, Messias A, et al. Eustachian valve endocarditis: echocardiographic diagnosis in a critical care patient. Case Rep Crit Care. 2018;(2018:1–2.
- Román JA, Vilacosta I, Sarriá C, et al. Eustachian valve endocarditis: is it worth searching for? Am Heart J. 2001;142(6):1037–1040.
- Pabich WL, Grichnik K. Chapter 3. Anatomic variants and ultrasound artifacts. In: Mathew JP, Swaminathan M, Ayoub CM, editors. Clinical manual and review of transesophageal echocardiography, 2e. New York, NY: McGraw-Hill; 2010. [cited 2018 Oct 19]. Available from:: http://accessanesthesiology.mhmedical.com/content.aspx?bookid=417§ionid=40109060
- Edwards AD, Vickers MA, Morgan CJ. Infective endocarditis affecting the eustachian valve. Heart. 1986;56(6):561–562.
- Sawhney N, Palakodeti V, Raisinghani A, et al. Eustachian valve endocarditis: a case series and analysis of the literature. J Am Soc Echocardiography. 2001;14(11):1139–1142.
- Kottam A, Kaur R, Bhandare D, et al. Actinomycotic endocarditis of the eustachian valve: a rare case and a review of the literature. Tex Heart Inst J. 2015;42(1):44–49.
- Wong R-C-C, Teo SG, Yeo TC. An unusual right-sided endocarditis: a case report of eustachian valve endocarditis. Int J Cardiol. 2005;109(3):406407.