ABSTRACT
Neuroleptic malignant syndrome is a potentially fatal neurological condition secondary to antipsychotic medication. It is characterized by distinctive clinical findings and autonomic disturbances. NMS has not been associated with Takotsubo cardiomyopathy (TCM).
TCM is an abnormal response to physiological stressors resulting from the autonomic abnormalities which at times can mimic myocardial infarction (MI). We present a unique case of a 54-year-old female with bipolar disease presenting with lithium and haloperidol-induced NMS complicated by TCM. The purpose of this case is to make clinicians aware of this rare association.
1. Introduction
NMS is an uncommon condition, usually associated with hyperthermia and muscle rigidity in the presence of raised creatine phosphokinase (CPK) levels. Patients also have several autonomic symptoms, such as tachycardia, increased blood pressure, urinary retention, hypersalivation, and sweating. NMS dysautonomia has not been associated with left ventricular systolic dysfunction (LVSD), such as TCM. We have presented a rare association of Takotsubo cardiomyopathy with the neuroleptic malignant syndrome and have highlighted the importance of medication adjustments during the management of NMS in such conditions.
2. Case presentation
A 54-year-old woman with a past medical history of anxiety, hypothyroidism and bipolar disorder presented to the emergency room (ER) for evaluation of a change in mental status for 2 days. Her bipolar disorder had been well controlled for many years on lithium and trifluoperazine. However, she was recently started on perphenazine due to a shortage of trifluoperazine. She was noted to be extremely agitated and dehydrated in the ER.
On arrival, she was afebrile with normal blood pressure and slightly tachycardic (90 bpm). For the agitation, she received two doses of Haldol 5 mg. Subsequently, she became catatonic with diffuse muscle rigidity. On examination, she was oriented to person but not to place, time or events.
Her blood pressure rose to 170/85 mmHg, her heart rate increased (122/minute) and her temperature rose to 105 F. Her cardiac, chest and abdominal examinations were unremarkable.
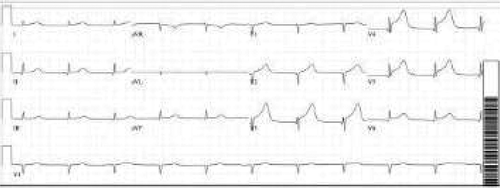
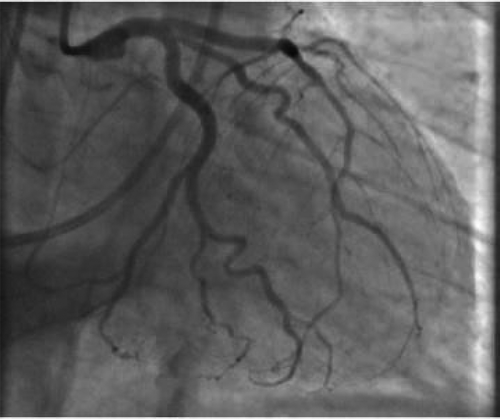
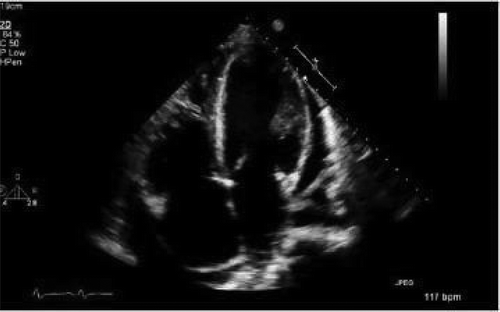
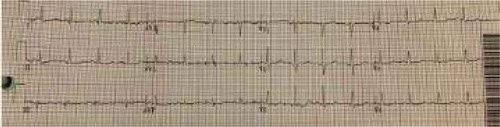
After admission, in the general ward, the patient complained of chest pain and ST-segment elevation was noted on EKG. () A rapid response was called. The patient was ultimately intubated and shifted to the cardiac unit where her coronary angiography was done, which came out normal. (). However, echocardiography showed her left ventricular ejection fraction (LVEF) to be 20–25%. Echocardiography showed good movement of the basal wall and severe hypokinesis of the rest of the walls consistent with Takotsubo cardiomyopathy. () She required the insertion of IABP and heparin infusion. Over time her blood pressure improved and IABP was removed subsequently.
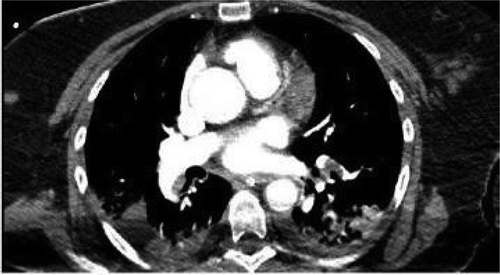
During her second day in CCU, she was found to have left upper extremity swelling which required evaluation with doppler, which was suggestive of subclavian vein thrombosis. Heparin was stopped due to the suspicion of heparin-induced thrombocytopenia (HIT). HIT antibodies and serotonin release assay (SRA) were sent and she was started on argatroban. She became hypoxic for which a computed tomography with pulmonary embolism (CTPE) protocol was done showing pulmonary embolism. () HIT assay came back negative. The initial CT scan of the head was unremarkable. Therefore, an MRI brain was done which showed multiple infarcts. She ultimately tolerated extubation but was nonverbal and bedridden due to multiple infarcts.
Blood work was significant for elevated creatinine levels of 1.32 mg/dL, blood urea nitrogen of 10 mg/dL and creatinine kinase levels of 9529 U/L. Complete blood count and liver function tests were unremarkable. During the rapid response, EKG showed ST-segment elevation in precordial leads, an x-ray showed bilateral infiltrates, BNP was 747 pg/ml, and a creatine kinase level of 9876 U/L. Troponins elevated to 9.05 ng/ml. Right heart catheterization showed a wedge pressure of 23 mmHg, a mean pulmonary artery pressure of 36, and a cardiac output of 2.3 L/min. A left heart catheterization showed normal coronaries but LVEF of 20–25% by left ventriculography. Subsequent brain MRI showed acute infarction in the subcortical white matter of the high right parietal lobe, and possible subcentimeter bifrontal foci of acute infarction.
Because of fever and a change in mental status, meningitis/encephalitis was high on the differential. Lumbar puncture (LP) was unsuccessful as the patient was extremely agitated. She was started on vancomycin, ceftriaxone, ampicillin, and acyclovir. She was initially admitted to the progressive care unit (PCU) with a working diagnosis of CNS infection versus neuroleptic malignant syndrome. She was also started on dantrolene (skeletal muscle relaxant) and bromocriptine (dopamine agonist) which were later discontinued. Her repeat EKG showed a resolution of ST elevation. () The patient was ultimately discharged to a rehabilitation center after a prolonged hospitalization. A 6 month follow up revealed an uneventful recovery, and the patient had no further episodes of Takotsubo cardiomyopathy.
3. Discussion
NMS is seen in 0.02 to 3% of patients taking neuroleptic medications [Citation1,Citation2]. It has been described in patients aged 9 months to 78 years [Citation1]. Several antiemetics and neuroleptic agents can precipitate NMS; most common are the high-potency antipsychotic medications like fluphenazine and haloperidol [Citation2]. Interestingly lithium by itself rarely causes NMS, but concomitant with other psychotropic drugs, especially the above mentioned higher-potency agents can precipitate NMS. Medical illnesses, like infections and stress from trauma and surgery, can also trigger NMS in the setting of lithium use [Citation1]. Our patient had a long-standing history of lithium use and received haloperidol for agitation. This combination incited the event of life-threatening hyperthermia, catatonia and laboratory findings consistent with NMS.
The pathogenesis of NMS can be explained by the class of medications associated with NMS, most of which are the anti-dopaminergic agents. Hyperthermia, dysautonomia, and dystonia in NMS are due to the blockade of central dopamine receptors in the hypothalamus [Citation3]. Catatonia and rigidity are due to inhibition of the nigrostriatal dopaminergic pathways [Citation3]. Another proposed mechanism for rigidity and unregulated vasomotor activity (labile blood pressure and tachycardia) in NMS is the increase in the sympathetic drive [Citation4]. Similarly, Becker et al first reported its association with myocardial infarction due to high catecholamine and sympathetic hyperactivity [Citation5]. Interestingly, TCM has also been shown to be linked to endothelial dysfunction and estrogen deficiency, which explains why it is more common in postmenopausal women. Findings from recently published data suggest that patients with TCM had an underlying endothelial dysfunction. Both increased sympathetic drive and endothelial dysfunction during menopause are due to decreased estrogen levels, thus increasing the risk of TCM in menopausal women [Citation6].
It is unclear how NMS causes TCM as the literature is very scarce and only one case has been reported so far by Oomura et al [Citation7]. But the possible mechanism for TCM in NMS is hypothesized to be the autonomic dysregulation in NMS.
TCM is also called stress cardiomyopathy or apical ballooning syndrome due to its association with stress and characteristic findings on echocardiography, respectively. It has been reported with a variety of stressors and is characterized by reversible LVSD, EKG findings similar to MI, but clean coronary arteries on angiography [Citation8,Citation9]. The proposed mechanism is the excess of circulatory catecholamine, coronary artery spasm, and microvascular dysfunction [Citation10]. Approximately 44% of patients can have ST-segment elevation, mostly in the anterior precordial leads, which cannot be distinguished from ST-elevation MI [Citation11]. Approximately 10% of TCM patients can complicate into cardiogenic shock [Citation9]. Our patient’s high catecholamine state potentially caused TCM with ST-elevation and the resulting cardiogenic shock.
Thus, TCM can be regarded as one potential complication of NMS related to autonomic dysfunction [Citation7].
Other features of NMS are hyperthermia and rigidity which occur along with mental status changes [Citation12].
This NMS tetrad typically lasts 1–3 days and present in about 97 to 100% of patients [Citation12]. Diagnosis of NMS is made by these symptoms in the setting of associated medication use and raised CK levels. Based on clinical and laboratory findings, an international multispecialty consensus group released diagnostic criteria for the diagnosis of NMS in 2011 [Citation13]. These criteria also emphasize the exclusion of alternative diagnoses before labeling someone with NMS, with each item given a priority score based on its importance in NMS diagnosis [Citation13]. TCM, on the other hand, requires the presence of a stressor and characteristic echocardiographic findings of LVSD with apical akinesia for diagnosis. It is important to rule out CAD by angiography before labeling someone with TCM.
NMS management includes dantrolene, which decreases CK levels and body temperature [Citation14]. Bromocriptine has also been found to hasten clinical response [Citation15]. All patients should be given supportive care with intravenous fluids and electrolytes. Renal function should be monitored and managed accordingly. Complete recovery is reduced by 6 days with dantrolene and 5 days with bromocriptine if given in addition to supportive care alone [Citation15]. There is evidence of reduced mortality of about 8.6% with dantrolene and 7.8% with bromocriptine, compared with supportive care (21%) [Citation16]. TCM in NMS is treated just like any other TCM, where the management of the responsible stressor is of paramount importance [Citation8]. Treatment of TCM should be individualized depending upon the TCM related complications such as for LVSD. Anticoagulation is sometimes needed for associated thrombosis in the ventricle [Citation8]. Patients with cardiogenic shock usually recover with transient use of intra-aortic balloon pump as in our case.
Patients with TCM usually have a good prognosis and the heart function returns to normal over the course of a few months. However, the risk of severe complications in the hospital is similar to patients with acute MI [Citation9]. NMS takes about two weeks to normalize and the slow and careful resumption of the neuroleptic agents should be considered. However, it can have devastating complications such as widespread thrombosis as occurred in our case where the patient had multiple ischemic infarcts, venous thrombosis, and pulmonary embolism.
4. Conclusion
NMS associated TCM should be suspected in patients presenting with rigidity and fever and the appearance of an acute MI. It is important to avoid dantrolene in such patients as it can worsen the cardiomyopathy. TCM is reversible if the NMS is managed appropriately. NMS be complicated by widespread thrombosis and hence should be managed promptly.
Author Contributions
Waqas Ullah and Zain Ali wrote the manuscript. Muhammad Arslan Cheema did the data extraction and analysis. Ammar Ashfaq and Maryam Mukhtar did the reference arrangement and data mining. Vincent Figueredo and Mamoon Ur Rashid did the critical review.
Disclosure Statement
No potential conflict of interest was reported by the authors.
References
- Levenson JL. Neuroleptic malignant syndrome. Am J Psychiatry. 1985 Oct.
- Chandran GJ, Mikler JR, Keegan DL. Neuroleptic malignant syndrome: case report and discussion. Can Med Assoc J. 2003 Sep 2;169(5):439–442.
- Henderson VW, Wooten GF. Neuroleptic malignant syndrome A pathogenetic role for dopamine receptor blockade? Neurology. 1981 Feb 1;31(2):132.
- Gurrera RJ. Is neuroleptic malignant syndrome a neurogenic form of malignant hyperthermia? Clin Neuropharmacol. 2002 Jul 1;25(4):183–193.
- Caroff SN, Mann SC. Neuroleptic malignant syndrome. Med Clin North Am. 1993 Jan;77(1):185–202.
- Pelliccia F, Kaski JC, Crea F, et al. Pathophysiology of Takotsubo syndrome. Circulation. 2017 Jun 13;135(24):2426–2441.
- Oomura M, Terai T, Sueyoshi K, et al. Reversible cardiomyopathy as the autonomic involvement of neuroleptic malignant syndrome. Int Med. 2004;43(12):1162–1165.
- Sato H. Takotsubo-type cardiomyopathy due to multivessel spasm. In: Kodama K, Haze K, Hon M, editors. Clinical aspect of myocardial injury: from ischemia to heart failure. Tokyo: Kagakuhyouronsha; 1990. p. 56.
- Templin C, Ghadri JR, Diekmann J, et al. Clinical features and outcomes of Takotsubo (stress) cardiomyopathy. N Engl J Med. 2015 Sep 3;373(10):929–938.
- Wittstein IS, Thiemann DR, Lima JA, et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med. 2005 Feb 10;352(6):539–548.
- Parkkonen O, Allonen J, Vaara S, et al. Differences in ST-elevation and T-wave amplitudes do not reliably differentiate takotsubo cardiomyopathy from acute anterior myocardial infarction. J Electrocardiol. 2014 Sep 1;47(5):692–699.
- Velamoor VR, Norman RM, Caroff SN, et al. Progression of symptoms in neuroleptic malignant syndrome. J Nerv Ment Dis. 1994 Mar;182(3):168–173.
- Gurrera RJ, Caroff SN, Cohen A, et al. An international consensus study of neuroleptic malignant syndrome diagnostic criteria using the Delphi method. J Clin Psychiatry. 2011 Sep 1;72(9):1222.
- Tanii H, Taniguchi N, Niigawa H, et al. Development of an animal model for neuroleptic malignant syndrome: heat-exposed rabbits with haloperidol and atropine administration exhibit increased muscle activity, hyperthermia, and high serum creatine phosphokinase level. Brain Res. 1996 Dec 16;743(1–2):263–270.
- Rosenberg MR, Green M. Neuroleptic malignant syndrome: review of response to therapy. Arch Internal Med. 1989 Sep 1;149(9):1927–1931.
- Sakkas P, Davis JM, Janicak PG, et al. Drug treatment of the neuroleptic malignant syndrome. Psychopharmacol Bull. 1991;27(3):381–384.