ABSTRACT
With the advent of medical technology, coronary angiography is a common practice to evaluate patient for coronary artery disease. Normally, patients undergoing angiogram receive antiplatelets, anticoagulants, and platelet aggregation inhibitor agents. Glycoprotein IIb/IIIa receptor inhibitors are a type of platelets antiaggregant agents that can cause severe thrombocytopenia in very few cases. We present a case of a 69-year-old female who presented with chest pain, underwent an angiography and had two stents placed. She was administered tirofiban during angiogram that caused acute severe thrombocytopenia decreasing her platelets count from 224 to 2 k/mm3 within 1 day. Patients platelets gradually recovered after trial of steroid and platelets transfusion. Antiplatelets (Aspirin and Clopidogrel) were resumed; however, patient’s platelets remained stable.
1. Introduction
Glycoprotein IIb/IIIa receptors present on platelet membranes are responsible for platelet aggregation by crosslinking von Willebrand factor and fibrinogen [Citation1]. Glycoprotein IIb/IIIa receptors have been one of the major targets in the management of high risk acute coronary syndrome and in conjunction with angioplasty, especially for bailout during thrombotic complications [Citation2].
Tirofiban, a small, non-peptide and a highly specific Glycoprotein IIb/IIIa (GPI), is a competitive inhibitor of the platelet fibrinogen receptor and when administered intravenously, tirofiban inhibits ex vivo platelet aggregation in a dose and concentration-dependent manner. It is a risk factor for severe thrombocytopenia [Citation1,Citation3]. The pathogenesis for this phenomenon is not completely clear but is believed to be due to drug-dependent antibodies that adhere to platelets only in the presence of the offending drug [Citation1,Citation3–Citation6]. There is growing body of evidence to suggest that tirofiban alters the arrangement of glycoprotein receptors on the platelets, hence creating a new antigen which is then recognized and removed from the circulation due to immune-mediated reaction. This, in essence, is the disposal of thrombocytes which is the cause of the sudden and severe thrombocytopenia [Citation7]. Severe thrombocytopenia can set in immediately after exposure to Tirofiban [Citation6].
We hereby report a case of 69-year-old female with tirofiban-induced thrombocytopenia in which the platelet count dropped to within 12–24 h of administration with no other alternative explanation.
2. Case presentation
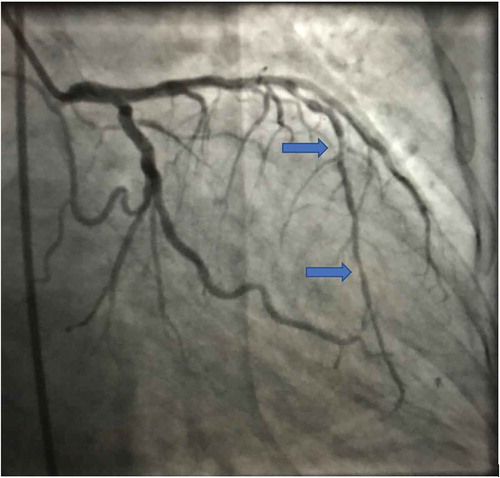
A 69-year-old female with past medical history significant for diabetes mellitus, coronary artery disease, and hypertension presented to the emergency department with complaints of chest pain that was substernal in nature, not associated with any radiation, diaphoresis, nausea, or vomiting. At the time of admission, patient physical examination was unremarkable. Her platelet levels were 224 k/mm3 at baseline and troponins (<0.01). She was initially started on aspirin 81 mg. She had an invasive angiogram () due to high pre-test probability the next day and was administered tirofiban infusion during the heart catheterization, she had two drug eluting stents placed in mid and distal portion of left anterior descending artery that were found to have 85% stenosis and 80% stenosis, respectively.
The next day after percutaneous coronary intervention, patient had developed ecchymosis and mild epistaxis, on further evaluation it was determined that her platelets had decreased from 224 to 2 k/mm3. Her antiplatelets were held due to acute severe thrombocytopenia. Hematology service was consulted, patient was started on steroids and given 2 units platelet transfusion. The peripheral smear ruled out thrombotic thrombocytopenic purpura (TTP) due to the absence of schistocytes.
In the subsequent days, as the patient’s platelets count recovered, aspirin and plavix were restarted. Her platelets continued to improve, and the patient was discharged with a platelet count of 114 k/mm3. After a week, repeat laboratory work revealed an improved platelet count to 442 k/mm3. The etiology of thrombocytopenia was concluded to be tirofiban induced as platelet count was not affected by antiplatelets when they were restarted and other alternative explanations for thrombocytopenia were ruled out.
3. Discussion
Thrombocytopenia is a prominent complication of GPIs with an incidence of severe thrombocytopenia (platelet count <50 k/mm3) to be 0.2–0.5% [Citation1,Citation3,Citation5,Citation8].
Five different presentations of GPI-induced thrombocytopenia have been reported: (i) Acute Severe Thrombocytopenia (platelets <10 k/mm3) within 12 h of first dose administration, (ii) Acute Thrombocytopenia within 12 h of second dose administration, (iii) Delayed Thrombocytopenia (5–7 days after exposure), (iv) Anaphylaxis after first or second exposure, and (v) Pseudo thrombocytopenia [Citation1,Citation3].
Drug-induced thrombocytopenia is a diagnosis of exclusion and other alternative diagnoses should be carefully evaluated. Heparin-induced thrombocytopenia is similar to tirofiban-induced thrombocytopenia, both occurring within 1–4 h or 7–14 days, but this can be ruled out if the patient has had no previous exposure [Citation1]. Aspirin and clopidogrel have not reported to result in cases of isolated acute severe thrombocytopenia [Citation3]. Studies have shown clopidogrel to occasionally be associated with TTP, which typically encompasses microangiopathic hemolytic anemia, thrombocytopenia, neurologic abnormalities, fever, and renal dysfunction [Citation3]. Pseudo thrombocytopenia, which is clumping of platelets, can be excluded on a peripheral smear [Citation4]. Dual anti-platelet drugs are another potential cause of thrombocytopenia, however, its onset occurs after days or weeks unlike our patient’s case [Citation5].
To validate the hypothesis that tirofiban is, in fact, the offending agent, a case reported in 2007 employed ELISA and flow cytometry to confirm the drug-dependent antibodies were indeed formed only in the presence of tirofiban [Citation9].
Studies explain how crucial it is to understand the life-threatening consequences associated with intracoronary use of tirofiban [Citation3]. A platelet count before and after use of tirofiban at 2–6-hour intervals is strongly encouraged [Citation3,Citation4]. As the drug-dependent antibodies may persist for years, it is also essential for the patient to avoid the drug indefinitely in the future [Citation6].
Just like any case of drug-induced thrombocytopenia, recovery begins as soon as tirofiban is discontinued, and continues to get resolved over the next few days with early supportive care using steroids, Ig-G immunoglobulins, and platelet transfusion if necessary [Citation1,Citation3,Citation6]. Patients with severe thrombocytopenia without significant bleeding must not have platelet transfused due to potential risk of stent thrombosis or reinfarction [Citation10].
4. Conclusion
In conclusion, tirofiban-induced thrombocytopenia is a rare but serious complication. Tirofiban-induced thrombocytopenia should be managed early and carefully due to the concomitant risk of bleeding and stent thrombosis or reinfarction.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Rahman N, Jafary FH. Vanishing platelets: rapid and extreme tirofiban-induced thrombocytopenia after percutaneous coronary intervention for acute myocardial infarction. Tex Heart Inst J. 2010;37(1):109–112.
- Amsterdam EA, Wenger NK, Brindis RG, et al. AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: executive summary: a report of the American college of cardiology/American heart association task force on practice guidelines. Circulation. 2014;130(25):2354–2394.
- Yurtdas M, Yaylali YT, Aladag N, et al. Acute serious thrombocytopenia associated with intracoronary tirofiban use for primary angioplasty. Case Rep Med. 2014;2014:190149.
- Teke HU, Teke D. Profound thrombocytopenia related with tirofiban: will it be enough to only stop medicine? Platelets. 2013;24(4):335–337.
- Zhou X, Peng H, Yin Y, et al. Diffused alveolar hemorrhage: a rare and severe complication of tirofiban-induced thrombocytopenia. Int J Cardiol. 2016;206:93–94.
- George JN, Aster RH. Drug-induced thrombocytopenia: pathogenesis, evaluation, and management. Hematology Am Soc Hematol Educ Program. 2009;2009:153–158.
- Li Y, Xu Q, Guo X. Thromboembolic events secondary to tirofiban-induced thrombocytopenia being treated with thrombopoietin: a case report. Exp Ther Med. 2016;12(2):1177–1180.
- Bizzarri F, Frati G. Acute profound thrombocytopenia after treatment with tirofiban and off-pump coronary artery bypass grafting: is there a paradox? Ann Thorac Surg. 2009;88(3):1048. author reply.
- Clofent-Sanchez G, Harizi H, Nurden A, et al. A case of profound and prolonged tirofiban-induced thrombocytopenia and its correction by intravenous immunoglobulin G. J Thromb Haemost. 2007;5(5):1068–1070.
- Velibey Y, Golcuk Y, Ekmekci A, et al. Tirofiban-induced acute profound thrombocytopenia: what is the optimal approach to treatment? Platelets. 2015;26(2):197–198.