ABSTRACT
Tumor lysis syndrome (TLS) is an oncological emergency characterized by a classic tetrad of hyperuricemia, hyperkalemia, hyperphosphatemia, and hypocalcemia. Risk assessment and prophylactic therapy is critical in preventing this oncological emergency. Treatment of established TLS involves aggressive hydration, electrolyte management, and the use of hypouricemic agents.
1. Introduction
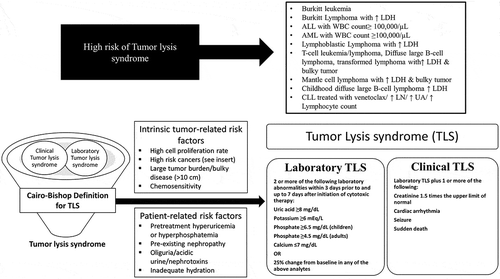
Tumor lysis syndrome (TLS) is a common, but life-threatening oncological emergency that frequently requires inpatient management. TLS occurs because of the rapid lysis of proliferating tumor cells. With increased availability of highly effective cytotoxic therapies, this condition is now more widely observed in patients with hematologic cancers and solid tumors [Citation1,Citation2]. TLS is a constellation of metabolic derangement characterized by hyperuricemia, hyperkalemia, hyperphosphatemia, and hypocalcemia () [Citation1,Citation2]. It is frequently associated with uric acid nephropathy and/or nephrocalcinosis. For the purpose of this narrative review, PubMed was searched for articles published in the last 20 years using the MeSH search term for ‘Tumor Lysis Syndrome’. The search was limited to English language articles, involving human subjects.
2. Clinical overview of tumor lysis syndrome
TLS most commonly occurs after initiation of cytotoxic chemotherapy in patients with acute lymphoblastic leukemia and high-grade lymphomas like Burkitt’s lymphoma [Citation1,Citation2]. Although rare, it may also be seen with chronic leukemias and solid tumors. It typically presents during or within 1 week after initiation of chemotherapy. TLS may rarely present in the absence of cytotoxic therapy (known as spontaneous TLS) [Citation2].
3. Pathophysiology, clinical features and risk assessment of tumor lysis syndrome
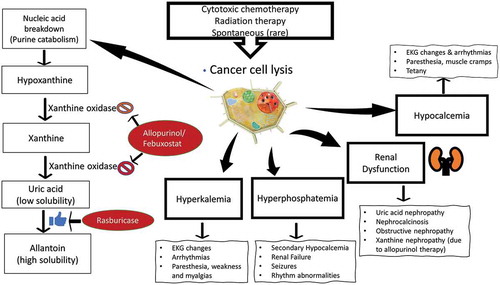
Rapid breakdown of tumor cells, with leakage of intracellular content, leads to a classic tetrad of metabolic derangement: hyperuricemia, hyperkalemia, hyperphosphatemia, and hypocalcemia. Increased nucleic acid breakdown and overwhelming purine catabolism result in hyperuricemia which is the hallmark of TLS. Clinical signs and symptoms reflect underlying metabolic derangement (). TLS is comprised of two components: clinical TLS and laboratory TLS (). The most widely used diagnostic criteria for TLS is the Cairo & Bishop criteria [Citation1]. This was later revised in 2011 by Howard et al. [Citation2]. Elevated serum lactate dehydrogenase (LDH) is an important biomarker of rapid cell turnover and is important for risk assessment of TLS. Malignant cells have a higher concentration of phosphorus [Citation3]. Thus, with rapid tumor cell lysis hyperphosphatemia ensues. This leads to secondary hypocalcemia. When the calcium phosphate product (calcium concentration × phosphate concentration) exceeds 60 mg2/dL2, there is an increased risk of nephrocalcinosis. Both nephrocalcinosis (calcium phosphate deposition), and acute uric acid nephropathy can lead to acute kidney injury. However, with the widespread use of hypouricemic agents (allopurinol, & Rasburicase) nephrocalcinosis has become the major cause of acute kidney injury in TLS [Citation4].
Symptoms usually reflect underlying metabolic derangements. It may include nausea, vomiting, diarrhea, lethargy, myalgia, muscle cramps, paresthesia, tetany, seizures, cardiac arrhythmias, syncope and even death. Tumors most associated with TLS include aggressive Non-Hodgkin’s lymphoma (especially Burkitt’s, & diffuse large B cell lymphoma), acute lymphoblastic leukemia, and acute myeloid leukemia [Citation5–Citation7]. TLS is less commonly associated with anaplastic large cell lymphoma, T-cell or B-ALL, chronic lymphocytic leukemia (CLL), neuroblastoma, breast cancer, germ cell tumors, soft tissue sarcoma, medulloblastoma, and Merkel cell carcinoma (). Risk assessment is critical for the management of TLS [Citation5]. Risk stratification system is broadly based on tumor burden, type of malignancy, treatment, expected response, and baseline renal function[Citation5]. Some of the high-risk features include elevated white blood cell (WBC) count (≥100,000 per µL), elevated serum lactate dehydrogenase (LDH) level (≥2 times the ULN), and treatment with agents like venetoclax, obinutuzumab, and Dinaciclib.
4. Prevention and treatment of tumor lysis syndrome
Monitoring, hydration, and hypouricemic therapy are the fundamental preventive measures in patients who are at risk for TLS[Citation5]. Intravenous hydration is the cornerstone of preventing TLS. Urine output and volume status should be closely monitored, and exogenous sources of potassium and calcium must be stopped. Choice of IV crystalloids should depend on the clinical circumstances and electrolyte status. Isotonic saline should be the initial hydration fluid if the patient is hypovolemic or hyponatremic. The goal of IV hydration is to induce a high urine output and improve glomerular filtration and renal perfusion. Urine output should generally be maintained within a range of 70 to 100 ml/m2 per hour. Care must be exercised in patients receiving concomitant steroids as they may have sodium retention and hypertension. In case of high-dose concomitant steroid therapy, 5% dextrose one-quarter normal (isotonic) saline should be considered. Patients with normal renal function generally do not require diuretics. Loop diuretics may be considered for augmentation of urine output after ruling out hypovolemia and obstructive uropathy. Urine alkalization (either with acetazolamide or Sodium Bicarbonate) may potentially promote uric acid excretion, but its role in the management of TLS is controversial due to the potential risk of calcium phosphate precipitation and worsening kidney injury and hypocalcemia [Citation8,Citation9]. IV hydration must be continued until the tumor burden has resolved and/or serum LDH is normalized. Depending on volume status and comorbidities, generally 2–3 L/m2 of IV crystalloids is administered daily. Urine output, uric acid, creatinine and serum electrolytes (potassium, calcium, and phosphorus) must be closely monitored especially in high-risk patients[Citation8].
5. The choice of hypouricemic therapy depends on the risk assessment
A: Allopurinol is the agent of choice in intermediate-risk patients. The usual dose of allopurinol is 100 mg/m2 every 8 h (maximum 800 mg per day) in adults and 50 to 100 mg/m2 every 8 h (maximum 300 mg/m2 per day) or 10 mg/kg per day in divided doses every 8 h in children [Citation6,Citation7]. Dose of allopurinol must be reduced in the setting of acute kidney injury or concomitant administration of azathioprine or mercaptopurine. Intravenous allopurinol must be given in patients who cannot tolerate oral route (dose: 200 to 400 mg/m2 per day, in one to three divided doses (maximum dose 600 mg per day). Xanthine nephropathy and nephrolithiasis is a rare complication of allopurinol therapy especially in patients with deficiencies in the purine salvage enzyme hypoxanthine-guanine phosphoribosyltransferase (HGPRT)[Citation10].
B. Rasburicase is recommended for high-risk patients or patients with impaired renal function Rasburicase is a recombinant urate oxidase (uricase) that act by converting uric acid to a highly soluble form allantoin. It is the treatment of choice for established TLS [Citation4,Citation11]. It is to be noted that rasburicase works by breaking down uric acid, while Allopurinol works by preventing uric acid formation. The dose of rasburicase is 0.2 mg/kg once daily for 5–7 days [Citation6,Citation7]. Length of treatment is generally based on clinical situation and/or response to the first dose. Some smaller studies have demonstrated the efficacy of 6 mg, single-dose therapy. In USA Rasburicase comes in 1.5 mg and 7.5 mg vials. Rasburicase is contraindicated in patients with G6PD deficiency[Citation12]. Some of the pertinent side-effects of rasburicase include methemoglobinemia, hemolysis, and anaphylaxis [Citation4,Citation12]. Since rasburicase works ex vivo, it can lead to spuriously low uric acid measurement [Citation13]. Hence, the blood sample should be collected in a pre-chilled tube and should be immediately placed on ice, and the assay should be completed within 4 h.
C. Febuxostat is a new, orally administered hypouricemic agent that selectively inhibits xanthine oxidase and may be used in patients who are allergic to allopurinol or who cannot tolerate allopurinol [Citation14]. Febuxostat has fewer drug–drug interactions [Citation15]. Benefits of febuxostat include (1) less drug–drug interaction; (2) no dose adjustment in patients with mild to moderate renal insufficiency; and (3) minimal effect on other enzymes involved in purine/pyrimidine metabolism. Compared to allopurinol, supporting evidence for the use to febuxostat is less robust.
Despite TLS prophylaxis, 3–5% patients develop laboratory and/or clinical evidence of TLS[Citation2]. Patients with established TLS should ideally be monitored in an intensive care setting with continuous telemetry. Serum electrolytes, creatinine, and uric acid should be monitored every 4–6 hours. Rasburicase should be continued until uric acid levels are normalized. Urine output should be closely monitored, and renal replacement therapy should be considered for worsening AKI, refractory volume overload, and worsening electrolyte abnormalities.
Management of electrolyte abnormalities should be based on the following premise [Citation2,Citation7]:
A. Hyperkalemia. It is the most life-threatening electrolyte abnormality due to the risk of fatal cardiac arrhythmias.
Continuous telemetry
Frequent monitoring of serum potassium (every 4–6 hours)
Avoid exogenous potassium intake
Potassium lowering agents (Patiromer, & sodium polystyrene sulfonate)
Administration of IV Insulin-Glucose, and inhaled beta-agonists (albuterol)
IV Calcium administration to prevent cardiac arrythmias.
Hemodialysis or hemofiltration.
B. Hyperphosphatemia and Hypocalcemia
Intravenous hydration and continuous telemetry
Frequent monitoring of serum phosphate and calcium (every 4–6 hours)
Eliminate exogenous phosphate intake
Phosphate binder therapy
Avoid nephrocalcinosis by lowering serum phosphate level before treating hypocalcemia (calcium phosphate product >60 mg2/dL2 increases the risk of calcium phosphate precipitation). However, symptomatic hypocalcemia with EKG changes/arrhythmias should be promptly treated.
Renal replacement therapy
6. Conclusion
TLS is a life-threatening oncological emergency characterized by a classic tetrad of hyperuricemia, hyperkalemia, hyperphosphatemia, and hypocalcemia. With the ever-expanding armamentarium of personalized cancer therapy, the risk of TLS is increasing. Risk assessment and prophylactic therapy are critical in preventing this oncological emergency. Hospitalists who are co-managing oncological patients should be aware of this important condition and should maintain a high index of suspicion in preventing and managing this condition. The care of patients with TLS requires an interdisciplinary approach with close collaboration between hospitalists, oncologists, and nephrologists.
Disclosure statement
The authors have no financial relationships relevant to this article to disclose.
References
- Cairo MS, Bishop M. Tumour lysis syndrome: new therapeutic strategies and classification. Br J Haematol. 2004;127(1):3–11.
- Howard SC, Jones DP, Pui CH. The tumor lysis syndrome [published correction appears in N Engl J Med. 2018 Sep 13;379(11):1094]. N Engl J Med. 2011;364(19):1844–1854.
- Brown RB, Razzaque MS. Phosphate toxicity and tumorigenesis. Biochim Biophys Acta Rev Cancer. 2018;1869(2):303–309.
- Lopez-Olivo MA, Pratt G, Palla SL, et al. Rasburicase in tumor lysis syndrome of the adult: a systematic review and meta-analysis. Am J Kidney Dis. 2013;62(3):481–492.
- Cairo MS, Coiffier B, Reiter A, et al., TLS Expert Panel. Recommendations for the evaluation of risk and prophylaxis of tumour lysis syndrome (TLS) in adults and children with malignant diseases: an expert TLS panel consensus. Br J Haematol. 2010;149(4):578–586. .
- Coiffier B, Altman A, Pui CH, et al. Guidelines for the management of pediatric and adult tumor lysis syndrome: an evidence-based review [published correction appears in J Clin Oncol. 2010 Feb 1;28(4):708]. J Clin Oncol. 2008;26(16):2767–2778.
- Jones GL, Will A, Jackson GH, et al. British committee for standards in haematology. Guidelines for the management of tumour lysis syndrome in adults and children with haematological malignancies on behalf of the British committee for standards in haematology. Br J Haematol. 2015;169(5):661–671.
- Abu-Alfa AK, Younes A. Tumor lysis syndrome and acute kidney injury: evaluation, prevention, and management. Am J Kidney Dis. 2010;55(5 Suppl 3):S1–S19.
- Van den Berg H, Reintsema AM. Renal tubular damage in rasburicase: risks of alkalinisation. Ann Oncol. 2004;15(1):175–176.
- LaRosa C, McMullen L, Bakdash S, et al. Acute renal failure from xanthine nephropathy during management of acute leukemia. Pediatr Nephrol. 2007;22(1):132–135. .
- Bose P, Qubaiah O. A review of tumour lysis syndrome with targeted therapies and the role of rasburicase. J Clin Pharm Ther. 2011;36(3):299–326.
- Sonbol MB, Yadav H, Vaidya R, et al. Methemoglobinemia and hemolysis in a patient with G6PD deficiency treated with rasburicase. Am J Hematol. 2013;88(2):152–154.
- Ueng S. Rasburicase (Elitek): a novel agent for tumor lysis syndrome. Proc (Bayl Univ Med Cent). 2005;18(3):275–279.
- Mayer MD, Khosravan R, Vernillet L, et al. Pharmacokinetics and pharmacodynamics of febuxostat, a new non-purine selective inhibitor of xanthine oxidase in subjects with renal impairment. Am J Ther. 2005;12(1):22–34.
- Stamp LK. Safety profile of anti-gout agents: an update. Curr Opin Rheumatol. 2014;26(2):162–168.