ABSTRACT
Over the years, Takotsubo Cardiomyopathy (TCMP) has become increasingly apparent, now comprising a significant portion of patients presenting with suspected acute coronary syndrome. The most common presentation of TCMP is ST segment elevation on EKG, troponin elevation, and apical ballooning in the absence of significant coronary artery disease as seen via cardiac catheterization. Although this is the most common presentation, it is important to highlight the less common variants of TCMP. In this article, we present a case series of patients presenting with the different variants of TCMP, followed by a literature review.
1. Introduction
Takotsubo cardiomyopathy (TCMP), also known as stress cardiomyopathy, ‘broken heart syndrome’, apical ballooning syndrome, has become a fairly more recognizable entity in cardiology as physicians become more acquainted with its clinical presentation. Epidemiologically, it is believed that TCMP occurs in approximately 1–2% of patient populations that present with clinical signs (ST changes/troponin elevation) and symptoms of acute coronary syndrome. Moreover, it is preferential for women as they have a 10-fold risk compared to men and even more specifically 5 fold higher risk in women over the age of 55 90% of cases of TC have a mean age of 65–67 [Citation1,Citation2]. The classic patient will be admitted for shortness and chest pain symptoms that mimic acute coronary symptoms (ie. dyspnea, diaphoresis, chest pain) following emotional or physical stressors. Workup will typically demonstrate nonspecific ST-T changes and modest troponin elevation mimicking acute coronary syndrome (ACS). Not surprisingly, it is often misdiagnosed as other clinical entities such as myocardial infarction, COPD exacerbation, influenza virus infection, etc. Cardiac catheterization, is the gold standard for diagnosis, as TCMP can be visually identified with the classic apical ballooning on left ventriculogram and less common variants including reverse type, mid-ventricular type, and the localized type.
2. Case 1
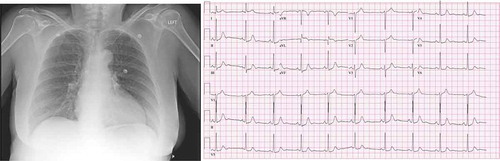
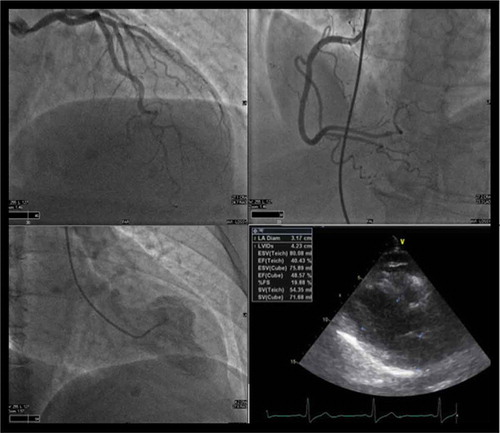
A 65 year old female with a past medical history of hypertension, anxiety, and cataract surgery 2 days prior who presented to our emergency department with complaints of chest pain onset 2 days prior at the time of her scheduled cataract surgery. She denied feeling stressed prior to or immediately after the procedure. The next day patient woke up with chest pain. She reported that pain rapidly worsened over those days following surgery. The pain was described as a chest pressure radiating to her left shoulder presenting at rest associated with coughing, diaphoresis, nausea, vomiting. Her symptoms were present at rest and worsened with exertion. The patient had no preceding fevers/chills, dizziness, headache. She denied any relieving factors. Vital signs on admission as follows: temperature 97 F, pulse rate 58, respiratory rate 19, blood pressure of 165/79, and oxyhemoglobin saturation of 97% on nasal cannula. EKG did not demonstrate any acute ST-segment elevation or depression (). Initial labs revealed a troponin elevation to 0.34. Age-adjusted D-dimer was negative. Chest x-ray demonstrated some increased cephalization of pulmonary vasculature (). In the ED, the patient was given aspirin, nitroglycerin, morphine, ondansetron, and heparin loading dose given as per ACS protocol. She was sent to a catheterization lab under the presumptive diagnosis of NSTEMI/Unstable Angina. Catheterization findings demonstrated proximal left circumflex lesion of 20%, ejection fraction of 50%, and focal LV dyskinesis suggestive of stress-induced cardiomyopathy. Follow-up transthoracic echocardiography () demonstrated EF of 40%; however, the images were inconclusive to evaluate for regional wall motion abnormalities. The patient was medically managed with instructions to continue aspirin, statin, and home regimen of lisinopril/HCTZ. Beta-blocker was not started as a patient’s heart rate was bradycardic. The patient was discharged home and recovered uneventfully.
3. Case 2
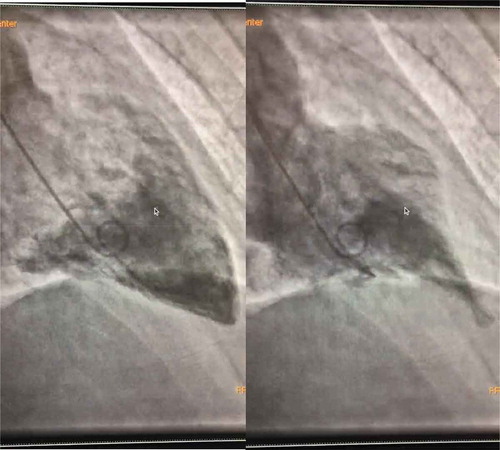
A 36-year-old Caucasian gentleman with no past medical history presented to the ED for management of nephrolithiasis. Upon arrival, the patient was given ketorolac and Hyoscyamine immediately after which he developed chest pressure, severe shortness of breath with desaturation to 83% on RA, hypertensive emergency with BP of 188/122, sinus tachycardia with HR as high as 130 bpm. At this time, CT chest with contrast was performed which showed left ventricular dilatation and bilateral airspace disease representing pulmonary edema. EKG revealed diffuse ST-T wave elevations, which prompted troponins that were peaked at 2.16. A follow-up echocardiogram as seen in and revealed diffuse hypokinesis, RVSP of 45 mmHg and LVEF of 35%. The patient was taken for cardiac catheterization and was found to have no coronary artery disease, however, did have ballooning of the basal myocardium with a hyperdynamic apex; findings consistent with reverse Takotsubo cardiomyopathy. This case illustrates one of the rare cases of reverse Takotsubo cardiomyopathy in the setting of anticholinergic toxicity. Hyoscyamine, which is used as an antispasmodic commonly, results in anticholinergic side effects on the cardiovascular system causing tachycardia which in this case resulted in reverse Takotsubo cardiomyopathy. Clinicians should remain cognizant of the effect of anticholinergic medications, particularly the cardiovascular system as it may result in the aforementioned findings.
4. Case 3
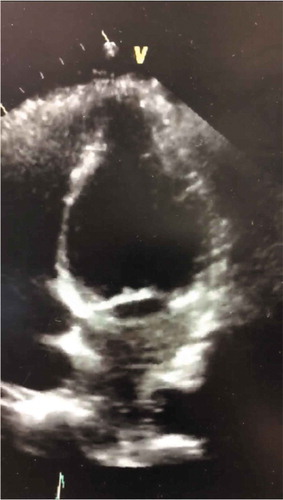
An 84-year-old female with a past medical history of hypertension and asthma presented to the emergency department with acute onset of severe shortness of breath with associated complaints of diaphoresis, productive (thick clear secretions) cough, and some nausea. Otherwise, she denied chest pain, palpitations, orthopnea, dizziness, fever, chills, or headache. She has a history of asthma attacks which occur about 1–2 times per year, and usually aborted with the use of rescue inhaler; however, at this time, the rescue inhaler offered minimal relief. The patient failed nebulizer treatments with EMS and subsequently was initiated on BiPAP therapy for the acute hypoxic respiratory failure. On arrival to the emergency department, patient was hemodynamically stable and remained on the BiPAP. On admission, labs were significant for POC troponin 0.69, troponin 0.58, BNP 2200. CXR revealed moderate cardiomegaly and mild central vascular prominence. EKG was concerning for ST elevations in lead 1, V2 and aVL, and T-wave inversion in lead III; no prior EKG available for comparison. Per patient, she followed with her PCP regularly and had an echocardiogram and stress performed recently, which she reports was normal (no records readily available on admission). In the ED, patient was given Solu-Medrol 125 mg IV x1, ASA 325 mg, initiated on Heparin drip, and Cardiology was consulted for further workup. Echocardiogram revealed left ventricular ejection fraction (LVEF) 40–45% with hypokinesis of the entire apical myocardium, findings were consistent with stress-induced cardiomyopathy. The left heart catheterization revealed moderate coronary artery disease, LVEF 40–45% with ballooning consistent with stress-induced cardiomyopathy. The patient was managed medically with beta-blockers, statin, and ASA. Patient respiratory distress resolved, and troponin levels trended down. She was discharged with optimal cardiac regimen, short steroid course, and recommendation to follow up with her PCP and cardiologist within 1–2 weeks. This case illustrates one of the rare presentations of Takutosubo cardiomyopathy in the setting of a severe asthma attack. The sympathetic response and subsequent catecholamine release which occurs during an asthma attack can contribute to the development of stress-induced cardiomyopathy or better known as Takutosubo cardiomyopathy.
5. Case 4
An 81-year-old Caucasian male with a history of diagnosis of myasthenia gravis 4 weeks prior presented to our facility with fall, respiratory distress, and subacute progressive weakness. On arrival, he was found to be in ventricular tachycardia on electrocardiogram (ECG). Initial point of care troponin was 0.08. He was started on intravenous amiodarone after which he developed a high-grade Mobitz 2 heart block that progressed to complete heart block with hypotension. He subsequently had a cardiac arrest and required chest compressions and epinephrine. He achieved a return of spontaneous circulation (ROSC) and subsequently intubated. He was started on norepinephrine and transferred to the intensive care unit. Transthoracic Echocardiogram (TTE) demonstrated an initial ejection fraction of 35%. He was started on intravenous pyridostigmine and methylprednisolone by neurology who suspected myasthenic crisis complicated by suspected myocardial infarction with transient type 2 heart block. As the patient clinically improved, he was extubated and eventually evaluated with cardiac catheterization. Catheterization demonstrated unobstructed coronary vasculature and significant diminished left ventricular ejection fraction of 25% and apical anterior, apical inferior, and apical lateral wall hypokinesis. Transesophageal echo at the end of the hospital course demonstrated EF = 40%. ECG demonstrated resolution of type 2 mobitz; however, new persistent QT prolongation, T-wave inversions in anterolateral leads were noted. The patient was discharged uneventfully with plans for potential implantable cardioverter-defibrillator at a later time.
6. Discussion
6.1. Definition
In the year 1990, Sato et al. first observed the classic appearance of stress-induced cardiomyopathy and coined the term ‘Takotsubo’ which was named due to similarity between the ballooning of the left ventricle during systole and the octopus pots used by local Japanese fishermen [Citation3]. Since then, many other names have also been used including apical ballooning syndrome and broken heart syndrome.
Takotsubo syndrome and its subtypes constitute an acute process of reversible heart failure that is not attributed to coronary artery disease. Several diagnostic criteria have been proposed including the modified criteria by Mayo clinic, the Japanese Takotsubo Cardiomyopathy group, and the Takotsubo Italian network. The most comprehensive diagnostic criteria is by the European Society of Cardiology which is as follows:
Transient regional wall motion abnormalities of the left and/or right ventricular (RV) myocardium, which are frequently, but not always, preceded by a stressful trigger (emotional or physical).
Regional wall motion abnormalities usually extending beyond a single epicardial vascular distribution, often resulting in circumferential dysfunction of the ventricular segments involved (apical and/or mid-LV or basal segments).
Absence of culprit atherosclerotic coronary artery disease including acute plaque rupture, thrombus formation and coronary dissection, or other pathological conditions to explain the pattern of temporary LV dysfunction observed (e.g., hypertrophic cardiomyopathy, viral myocarditis).
New and reversible electrocardiography (ECG) abnormalities (ST-segment elevation, ST depression, left bundle branch block, T-wave inversion, and/or QTc prolongation) during the acute phase.
Significantly elevated serum natriuretic peptide (B-type natriuretic peptide [BNP] or N-terminal pro-b-type natriuretic peptide [NTproBNP]) level during the acute phase.
Positive but relatively small elevation in cardiac troponin measured using a conventional assay (i.e., disparity between the troponin level and the amount of the dysfunctional myocardium present).
Recovery of ventricular systolic function on cardiac imaging at follow-up [Citation1].
Furthermore, Takotsubo cardiomyopathy has multiple subtypes. These subtypes are classified under two broad groups as follows.
6.2. Primary Takotsubo syndrome
Primary Takotsuboe syndrome occurs in patients in whom the presenting symptoms are the primary cause of the acute presentation. These patients may or may not have clearly identifiable stress triggers or comorbid conditions however these are not the primary cause for the catecholamine surge described in the pathophysiology section below.
6.3. Secondary Takotsubo syndrome
A large portion of cases occur in individuals who are undergoing physiological stress while hospitalized for other medical diagnoses leading to a catecholamine surge.
Under these two groups, multiple anatomic variants exist. The initial discovery of Takotsubo cardiomyopathy by Sato et al. with apical and mid-ventricular ballooning and hypokinesia with basal hypercontractility is now termed the classic variant. This variant is present in up to 80% of cases [Citation4]. The second most common are the atypical variants of inverted or reverse Takotsubo which has basal hypokinesia and apical hypercontractility as seen in the aforementioned case series as well [Citation5]. Even more rarer that the previously mentioned are the mid-ventricular type and the localized focal Takotsubo type.
6.4. Pathophysiology
All patients found to have Takotsubo cardiomyopathy share one factor in common which is the presence of a precipitating stressful event. This may lead to an augmented sympathetic response leading to a catecholamine surge which as been thought to play a central role in the development of this syndrome. Serum catecholamine levels in patients with Takotsubo cardiomyopathy are significantly elevated when compared to patients at rest and in patients who presented with heart failure secondary to myocardial ischemia [Citation6]. Per literature review, multiple hypotheses have been proposed to explain the variable appearance of the heart in response to the aforementioned stress response. Let us delve into the vascular and myocardial aspects of the development of Takotsubo cardiomyopathy. Firstly, let us explore the involvement of coronary vessel spasms which can be seen via diagnostic angiography as multivessel vasospasm which may be a result of the aforementioned catecholamine response. Histopathologic evaluation also revealed myocardial changes that could be attributed to infarction. Similarly, endomyocardial biopsies in the acute setting also showed disorganized proteins but no signs of cell death [Citation7].
Moving on, the augmented catecholamine response has also been thought to cause left ventricular outflow tract obstruction. This LV outflow tract obstruction was in approximately 25% of cases which highlighted the involvement of this dysfunction in the pathophysiology of Takotsubo [Citation8]. Lastly, it is important to highlight the direct effect of catecholamines on stunning the myocardium. In the basal myocardium, there is a more dense presence of sympathetic receptors compared to the apex and thus causes the classic appearance during a stress response.
6.5. Clinical presentation and diagnosis
Patients typically present with symptoms classic of an acute myocardial infarction which include acute and unstable substernal, pressure like chest pain, shortness of breath, palpitations, or arrhythmia. In the more severe cases involving severe left ventricular outflow tract obstruction, patients may also be in cardiogenic shock. Additionally, EKG changes classic of acute ST-segment elevation myocardial infarction are also seen but follow-up catheterization does not reveal coronary artery disease in the location of myocardial dysfunction. Important complications to keep in mind are as follows. Firstly, right ventricular involvement occurs from 18% to 34% of patients and has been associated with other complications such as lower EF, worsening heart failure, pleural effusions, and even longer hospital course. Other factors that were related to heart failure, cardiogenic shock, and longer hospital course were the presence of left ventricular outflow tract obstruction, acute mitral regurgitation, new-onset atrial fibrillation, thrombus formation due to akinetic myocardium, pericardial effusion, or ventricular-free wall rupture [Citation4,Citation9–Citation16].
7. Management
Given that one of the diagnostic criteria for Takotsubo cardiomyopathy is spontaneous restoration of cardiac function, supportive care should aim to minimize any of the previously mentioned complications.
8. Conclusion
Although Takotsubo cardiomyopathy commonly takes a benign course, it is important for clinicians to be cognizant of the aforementioned variants as well as complications. This can potentially improve clinical outcomes but also optimize in hospital management of patients.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ghadri JR, Wittstein IS, Prasad A, et al. international expert consensus document on Takotsubo syndrome (part I): clinical characteristics, diagnostic criteria, and pathophysiology. Eur Heart J. 2018;39(22):2032–2046.
- Kurowski V, Kaiser A, von Hof K, et al. Apical and midventricular transient left ventricular dysfunction syndrome (tako-tsubo cardiomyopathy): frequency, mechanisms, and prognosis. Chest. 2007;132:809.
- Sato T, Hagiwara K, Nishikido A, et al. Takotsubo (ampulla- shaped) cardiomyopathy associated with microscopic polyangiitis. Intern Med. 2005;44:251–255.
- Eitel I, von Knobelsdorff-brenkenhoff F, Bernhardt P, et al. Clinical characteristics and cardiovascular magnetic resonance findings in stress (takotsubo) cardiomyopathy. JAMA. 2011;306:277–286.
- Ennezat PV, Pesenti-Rossi D, Aubert JM, et al. Transient left ventricular basal dysfunction without coronary stenosis in acute cerebral disorders: a novel heart syndrome (inverted Takotsubo). Echocardiography. 2005; (22):599–602. DOI:10.1111/j.1540-8175.2005.40046.x.
- Wittstein IS, Thiemann DR, Lima JA, et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med. 2005;352:539–548.
- Nef HM, Mollmann H, Kostin S, et al. Tako-Tsubo cardiomyopathy: intraindividual structural analysis in the acute phase and after functional recovery. Eur Heart J. 2007;28:2456–2464.
- El Mahmoud R, Mansencal N, Pilliere R, et al. Prevalence and characteristics of left ventricular outflow tract obstruction in Tako-Tsubo syndrome. Am Heart J. 2008;156:543–548.
- Elesber AA, Prasad A, Bybee KA, et al. Transient cardiac apical ballooning syndrome: prevalence and clinical implications of right ventricular involvement. J Am Coll Cardiol. 2006;47:1082–1083.
- Citro R, Rigo F, D’Andrea A, et al. Echocardiographic correlates of acute heart failure, cardiogenic shock, and in-hospital mortality in tako-tsubo cardiomyopathy. JACC Cardiovasc Imaging. 2014;7:119–129.
- Ohba Y, Takemoto M, Nakano M, et al. Takotsubo cardiomyopathy with left ventricular outflow tract obstruction. Int J Cardiol. 2006;107:120–122.
- Haghi D, Rohm S, Suselbeck T, et al. Incidence and clinical significance of mitral regurgitation in Takotsubo cardiomyopathy. Clin Res Cardiol. 2010;99:93–98.
- Syed FF, Asirvatham SJ, Francis J. Arrhythmia occurrence with takotsubo cardiomyopathy: a literature review. Europace. 2011;13:780–788.
- Pant S, Deshmukh A, Mehta K, et al. Burden of arrhythmias in patients with Takotsubo cardiomyopathy (apical ballooning syndrome). Int J Cardiol. 2013;170:64–68.
- Guevara R, Aguinaga-Meza M, Hazin MI, et al. Takotsubo cardiomyopathy complicated with acute pericarditis and cardiogenic shock. J Natl Med Assoc. 2007;99:281–283.
- Izumi K, Tada S, Yamada T. A case of Takotsubo cardiomyopathy complicated by ventricular septal perforation. Circ J. 2008;72:1540–1543.