ABSTRACT
Legionella pneumophilia remains an important cause of pneumonia that can cause substantial clinical morbidity and mortality. We describe a case involving a 67-year-old woman with legionella pneumonia who developed diffuse alveolar hemorrhage. We also highlight the pitfalls of commonly used legionella urine antigen testing. Furthermore, we explore the role of high-dose steroid therapy as a treatment option for patients with diffuse alveolar hemorrhage secondary to legionella pneumonia who continue to deteriorate in spite of adequate antimicrobial therapy.
1. Case report
A 67-year-old woman with a history of Crohn’s disease (with history of iritis and erythema nodosum) on Azathioprine presented to the hospital with a 2-day history of fever, poor appetite, fatigue, dizziness, and cough. On initial evaluation, she was noted to have a fever of 39.2°C, heart rate of 102, blood pressure 122/67 mm Hg, and oxygen saturations of 91% on room air.
Figure 1. Lung imaging on admission (Hospital Day 0), Hospital Day 1, Day 2, and Day 7. It demonstrates progressively worsening right-sided infiltrates from Day 0 to Day 2 (CT chest available on Day 0 and Day 2). Significant improvement in infiltrates noted on Day 7.
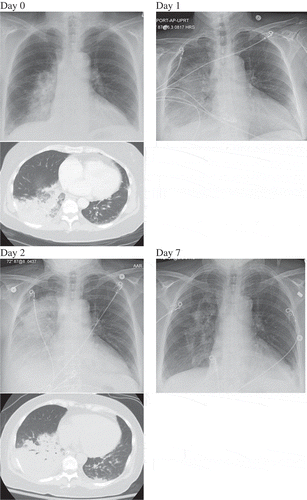
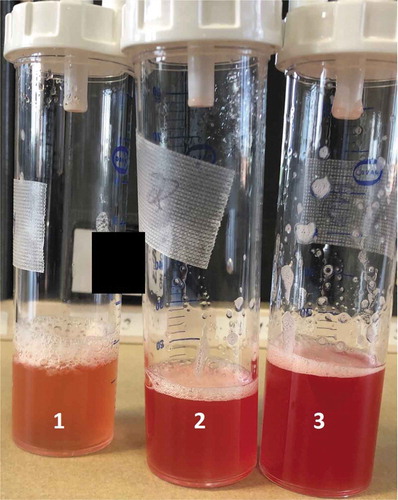
Figure 2. Bronchoalveolar lavage showing progressive hemorrhage in the absence of airway inflammation and purulence.
Initial laboratory data were significant for hyponatremia with sodium of 131 mmol/L, hypokalemia of 3.1 mmol/L, increased anion gap of 19 with bicarbonate of 20 mmol/L and lactate of 4.2 mmol/L. Chest x-ray and CT chest showed diffuse right-sided pneumonia (). Streptococcal and Legionella urine antigen and peripheral blood cultures were negative.
The patient was admitted for severe sepsis secondary to community-acquired pneumonia and was treated with intravenous ceftriaxone and azithromycin and fluid resuscitated. Unfortunately, the patient continued to become increasingly hypoxic initially requiring high flow oxygen and eventually required intubation and mechanical ventilation. An arterial blood gas done prior to intubation demonstrated hypoxia with respiratory alkalosis. Repeat chest x-ray showed rapidly worsening right lung opacities now encompassing much of the right lung. Post intubation, the patient required FiO2 of 1 and PEEP of 18 to maintain adequate oxygenation. A presumptive diagnosis of adult respiratory distress syndrome complicating community-acquired pneumonia was made. She was ventilated with low tidal volumes and required deep sedation and neuromuscular blockade. She developed hypotension requiring vasopressors. Antibiotics were changed to vancomycin, meropenem, and doxycycline (Azithromycin was discontinued due to persistently prolonged QTc >500).
A bronchoscopy with bronchoalveolar lavage performed on hospital day 2 had findings consistent with diffuse alveolar hemorrhage (DAH) in the absence of airway inflammation and purulence (). Gram stain showed a rare white blood cell with no organisms. Bacterial cultures remained negative. Legionella PCR from bronchoalveolar lavage was positive. Legionella cultures were also obtained. Autoimmune workup was also obtained and except for weakly positive antinuclear antibody (1.2 U), decreased total complement (19 U/mL).
The patient was started on high-dose steroids with methylprednisolone 500 mg for 3 days (this was initiated prior to Legionella PCR being available to treat possible autoimmune-mediated DAH) followed by 1 mg/kg of prednisone with dramatic improvement in her respiratory symptoms as well as radiological improvement. She was successfully liberated from the ventilator on hospital day 4. Even though deemed unlikely culprit for the patient’s symptoms, Azathioprine was discontinued. The patient was discharged home after an 8-day hospital stay with cefdinir, doxycycline, and a tapering course of steroids while still awaiting finalized cultures. The patient had a radiological clearance of her pulmonary process during a 4-week post-hospital follow-up. Finalized bronchoalveolar cultures grew Legionella pneumophila serogroup 3. With the above data, a final diagnosis of diffuse alveolar hemorrhage secondary to legionella pneumonia was made.
2. Discussion
Legionnaire’s disease is a relatively uncommon cause of pneumonia that can cause substantial clinical morbidity and mortality. The most common species associated with this pneumonia is Legionella pneumophilia (L pneumophilia) although the urine antigen testing which is commonly used is poorly sensitive for non- L pneumophilia serogroup 1 or other species which can also cause infections. Transmission usually occurs by inhalation of aerosols or aspiration of water containing Legionella spp [Citation1].
The most commonly used diagnostic tool for Legionnaire’s disease is the detection of L pneumophilia antigen in the urine. Although its sensitivity can about 80–90% for L pneumophilia serogroup 1, sensitivity can be lower than 50% for disease caused by other strains. The culture and isolation of legionellae from clinical specimens is the diagnostic gold standard [Citation1]. As seen in our case above, the initial urine antigen testing was negative and bronchoalveolar lavage culture led to the isolation of non-L pneumophilia serogroup 1 species.
Diffuse alveolar hemorrhage (DAH) associated with Legionnaire’s disease is rare but described in previous publications [Citation2–Citation4]. Although treatment of non-immune-mediated causes of DAH is generally targeted to the management of the underlying etiology, in our patient mentioned above given worsening respiratory status in spite of aggressive antimicrobial therapy while immunologic and further workup was pending, high-dose steroid therapy was started with good clinical response. Similar response and improvement in clinical status after initiation of steroids was reported previously in another patient with DAH associated with Legionella [Citation3].
The takeaway from this case is two-fold. Clinicians need to mindful of the fact that the most commonly used urine testing for legionella is not very sensitive in the detection of non-L pneumonophilia subgroup 1 species. Testing for legionella with cultures of clinical specimens should still be considered if suspicion remains high. Secondly, in critically ill patients with Legionella associated DAH consideration of high-dose steroid therapy can be made on a case-by-case basis if the patient continues to have deterioration of clinical status in spite of appropriate and adequate antimicrobial therapy.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Phin N, Parry-Ford F, Harrison T, et al. Epidemiology and clinical management of Legionnaires’ disease. Lancet Infect Dis. 2014;14(10):1011–1021.
- Pataka A, Kotoulas SC, Sourla E, et al. Is it just an infection? Breathe (Sheff). 2018;14(3):e100–e104.
- Kashif M, Patel R, Bajantri B, et al. Legionella pneumonia associated with severe acute respiratory distress syndrome and diffuse alveolar hemorrhage - A rare association. Respir Med Case Rep. 2017;21:7–11.
- Sundar KM, Pearce MJ. Diffuse alveolar hemorrhage due to Legionella pneumonia. Sarcoidosis Vasc Diffuse Lung Dis. 2004;21(2):158–159.
