ABSTRACT
A pseudoaneurysm of the splenic artery (SAP) is a rare entity which is associated with pancreatitis in 52% of cases. In the presence of pancreatitis, the enzymatic damage to the wall of splenic artery results in pseudoaneurysm formation. The clinical presentation is variable and ranges from asymptomatic to hemodynamic instability. The diagnosis of SAP is challenging in the presence of peripancreatic fluid collection or pseudocyst, where CT abdomen can miss small pseudoaneurysms. Angiography is a useful modality to establish a definitive diagnosis. We present a 49-year-old male with a history of recurrent pancreatitis due to alcoholism who presented with acute abdominal pain and was found to have acute pancreatitis. Abdominal CT scan showed a peripancreatic fluid collection and hyperdense lesion at the splenic hilum, which was diagnosed as SAP on angiography. A transcatheter embolization was performed with complete resolution of symptoms thereafter.
1. Introduction
Splenic artery pseudoaneurysm (SAP) is a rare, but lethal entity which can result in life-threatening hemorrhage if left untreated[Citation1]. About 58% of cases with hemorrhage present also with hemodynamic instability[Citation1]. In the current literature, the total reported cases of SAP are less than 200, making it a diagnostic challenge [Citation1,Citation2]. The most common etiologies of SAP are pancreatitis (52%, both acute [6%] and chronic [46%] pancreatitis), abdominal trauma (29%), post-surgical complications (3%), and peptic ulcer disease (2%)[Citation1]. The clinical presentations range from an incidental finding on cross-sectional radiological imaging to hemodynamic instability, which can be misdiagnosed due to other etiologies, especially given the rarity of SAP. Regardless of symptoms, prompt recognition and repair of SAP are recommended because of the high risk of mortality due to sudden rupture.
2. Case presentation
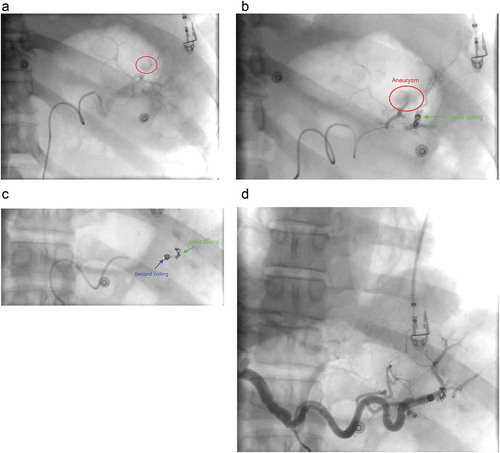
A 49-year-old Caucasian male with a history of recurrent pancreatitis complicated by a large peripancreatic fluid collection, presented with acute abdominal pain for one day. It was described as 8 out of 10, constant in the epigastrium and left upper quadrant, non-radiating, worse with movement, and partially relieved with analgesics. It was also associated with nausea and one episode of nonbloody, nonbilious emesis. He had 2–3 episodes of loose bowel movements; however, he denied melena or hematochezia. Social history was notable for one pack per day tobacco use, occasionally smoking of marijuana, and alcohol abuse. He had two previous episodes of acute pancreatitis in the past year. Physical examination showed unremarkable vitals, a soft non-distended abdomen with epigastric tenderness, and audible bowel sounds. Laboratory workup was only remarkable for a lipase of 425 U/L. Computed tomography (CT) scan of the abdomen () revealed peripancreatic fat stranding and multiple acute peripancreatic fluid collections, including a 5.6 × 5.1 cm collection between the spleen and the greater curvature of stomach, an 8.3 × 1.8 cm collection extending medially along pancreatic tail and wall of the stomach, and a 3.8 × 2.1 cm heterogeneous collection between the spleen and tail of the pancreas. Due to the nature and locations of the fluid collections found, an abdominal CT angiography () was done which showed a hyperdense component between the spleen and pancreatic tail with suspicion for either clot or pseudoaneurysm of the splenic artery. For further investigation of the hyperdense component, a catheter angiogram of the splenic artery was performed which demonstrated a rounded area of late arterial phase opacification, with staining consistent with a pseudoaneurysm of the splenic artery. A transcatheter embolization () was then successfully performed by placing the embolization coil both distally and proximally to the blood vessel supplying the pseudoaneurysm. Complete resolution of pseudoaneurysm was confirmed with a postembolization angiogram, after which the patient’s symptoms completely resolved.
Figure 1. Computed tomography (CT) scan of abdomen (a) transverse view, (b) coronal view both showing hyper-dense lesion surrounded with peripancreatic fluid collection at the hilum of the spleen (red circle).
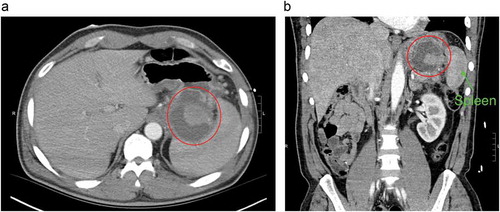
Figure 2. Computed tomography angiogram of abdomen (a) transverse view, (b) coronal view both showing hyper-dense lesion surrounded with peripancreatic fluid collection at the hilum of the spleen (red circle). The arrow shows the branch of the splenic artery feeding pseudoaneurysm.
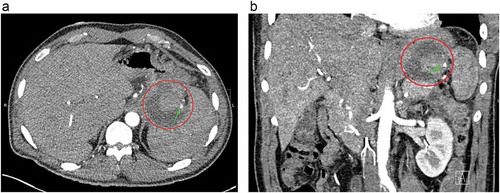
Figure 3. Transcatheter angiogram of abdomen showing (a) pseudoaneurysm (red circle), (b) placement of initial embolization coil in the branch of splenic artery distal to pseudoaneurysm, (c) placement of second embolization coil in the branch of splenic artery proximal to pseudoaneurysm, (d) Angiogram post-embolization with complete resolution of the pseudoaneurysm.
3. Discussion
The most common etiology of SAP is pancreatitis. The reported mechanism of SAP is autodigestion of the splenic artery due to pancreatic proteolytic enzymes, which results in structural disruption of the elastic tissue planes in the vessel wall and subsequent pseudoaneurysm formation [Citation3,Citation4]. The formation of a pseudoaneurysm may take up to 1–4 months after an episode of pancreatitis[Citation1]. In a case series including 157 patients with SAP, abdominal pain was the most common presenting symptom (50%) followed by abdominal hemorrhage (30%), and then nausea, back pain, and chest pain (20%)[Citation1]. Abdominal CT scan with contrast is the preferred imaging modality to establish diagnosis, which helps in the identification of small pseudoaneurysms due to luminal enhancement. The diagnosis of SAP is challenging in the presence of a peripancreatic fluid collection or pseudocyst, where CT of the abdomen can miss smaller sized pseudoaneurysms[Citation5]. Abdominal angiography is a useful diagnostic tool to establish a definitive diagnosis when suspicion of SAP is high. The pseudoaneurysm should be suspected if the angiogram shows a hyperdense lesion within the peripancreatic fluid collection. The added advantage of angiography is its ability to perform transcatheter embolization at the time of evaluation. With the advancement of radiological imaging, such as CT angiography or magnetic resonance imaging (MRI), the rate of incidental detection of SAP has been rising, even in asymptomatic patients.
In our case, the patient developed SAP due to recurrent attacks of pancreatitis. The diagnosis was not clear with the initial abdominal CT scan due to the presence of concomitant peripancreatic fluid collection. Further investigation with catheter angiogram assisted in both confirmation of the diagnosis and allowed for therapeutic intervention. Untreated SAP could result in sudden rupture of pseudoaneurysm leading to life-threatening hemorrhage which can be intraperitoneal, retroperitoneal, or into pancreatic duct itself. The patients with hemorrhage into the pancreatic duct usually present with hematemesis, melena, or hematochezia [Citation1,Citation6]. The mortality due to hemorrhage from ruptured SAP is as high as 50%. The reported size of the ruptured pseudoaneurysm ranges from 0.3 cm to 17 cm.1 There is no correlation of the size of pseudoaneurysm with rupture in current literature.
The natural history of asymptomatic SAP is unclear. Compared to the splenic artery aneurysm, SAP is more prone to sudden rupture and results in high morbidity and mortality[Citation7]. Regardless of symptoms, repair of pseudoaneurysms is recommended in all patients with incidentally detected SAP [Citation1,Citation2,Citation7]. Transcatheter embolization is a promising treatment of choice in the hemodynamically stable patient with or without intact SAP. The selective embolization of both the proximal and distal ends to the feeding artery is recommended for effective thrombosis of pseudoaneurysm[Citation4]. This ‘sandwich’ technique results in the preservation of the splenic artery blood flow through collaterals from the surrounding blood vessels. The advantage of this approach is a high success rate, minimal rate of complication, and shorter hospital stay as compared to surgical exploration. In the presence of a pancreatic pseudocyst, the failure rate of this approach may be high due to the fragility of soft tissue structure, making embolization challenging [Citation1]. In patients with poor candidacy for transcatheter embolization, splenectomy with or without distal pancreatectomy is the standard treatment of choice with no reported failure rate. The surgical exploration with ligation of splenic artery is used preferably in patients with hemodynamic instability due to sudden rupture of SAP.
4. Conclusion
A high index of suspicion is required to diagnose SAP. A hyperdense opacity surrounded by a fluid collection at the pancreatic tail on abdominal CT scan could suggest SAP and should be further investigated with diagnostic angiography for a more definitive diagnosis. Early repair of SAP should be pursued to prevent death due to massive hemorrhage from the sudden rupture of the pseudoaneurysm.
Authors contributions
Yousaf MN: Manuscript writing and figures formatting
Chaudhary FS, Amrat E: Review of manuscript and data and proofreading
Wolff MA, Sittambalam CD: Manuscript supervision
Disclosure statement
The authors declare that they have no competing interests or conflicts of interest.
References
- Tessier DJ, Stone WM, Fowl RJ, et al. Clinical features and management of splenic artery pseudoaneurysm: case series and cumulative review of literature. J Vasc Surg. 2003;38(5):969–974. [published Online First: 2003/11/07].
- Chia C, Pandya GJ, Kamalesh A, et al. Splenic artery pseudoaneurysm masquerading as a pancreatic cyst-A diagnostic challenge. Int Surg. 2015;100(6):1069–1071.
- Golzarian J, Nicaise N, Devière J, et al. Transcatheter embolization of pseudoaneurysms complicating pancreatitis. Cardiovasc Intervent Radiol. 1997;20(6):435–440. [published Online First: 24 January 2014].
- Uflacker RD. Successful embolization of a bleeding splenic artery pseudoaneurysm secondary to necrotizing pancreatitis. Gastrointest Radiol. 1982;7(1):379–382.
- Savastano S, Feltrin GP, Antonio T, et al. Arterial complications of pancreatitis: diagnostic and therapeutic role of radiology. Pancreas. 1993;8(6):687–692. [published Online First: 1993/11/01].
- Ammori BJ, Madan M, Alexander DJ. Haemorrhagic complications of pancreatitis: presentation, diagnosis and management. Ann R Coll Surg Engl. 1998;80(5):316–325.
- Abbey-Mensah G, Herskowitz MM, Walsh J, et al. Massive hematemesis from a splenic artery pseudoaneurysm presenting two years after penetrating trauma. Case Rep Radiol. 2018;2018:3.
