ABSTRACT
Introduction
Mycophenolate Mofetil (MMF), although a widely used immunosuppressant; an increasing concern of MMF induced Primary Central Nervous System Lymphoma (PCNSL) are being reported. Timely diagnosis and management of MMF induced PCNSL can play a vital role in improved outcomes.
Case Presentation
Eighty-one-year-old female with history of Eosinophilic Granulomatosis with Polyangiitis (EGPA) presented with word finding difficulty, right-hand weakness and right foot clumsiness. EGPA had been stable with MMF for 6 years. Physical examination revealed weakened right-hand grip, positive right-sided dysdiadokokinesia and right foot drop. MRI-brain identified three enhancing solid lesions – in right parietal, left insular and left mid brain extending into the left thalamus. Brain biopsy revealed a focally dense lymphoid infiltrate with CD20 positive B cells, with large atypical cells resembling Hodgkin Reed-Sternberg cells. With concern for immunosuppression related PCNSL, MMF was stopped. Patient was treated with 8 weeks of rituximab therapy for its least toxic profile and concomitant benefit in EGPA. On a 2 month follow up MRI-brain, near total resolution of the intracranial lesion was observed. Patient still had some residual right lower extremity incoordination, however, strength and speech normalized with resolution of dysdiadokokinesia. Patient was advised to discontinue MMF indefinitely and remains on low dose prednisone daily.
Conclusion
MMF is an inhibitor of Inosine Monophosphate Dehydrogenase which prevents T- and B-cell proliferation. PCNSL is a potential complication of chronic immunosuppression with this medication. Discontinuation of the drug along with immunosuppressive therapies have been the effective therapeutic options till date.
1. Background
Immunodeficiency-associated lymphoproliferative disease (IALD) is a known complication of immunosuppressive therapy. IALD can rarely be restricted to the central nervous system, and can be designated as primary central nervous system (PCNS) IALD [Citation1,Citation2]. These immunosuppressive therapies are used in the treatment for organ transplant as well as autoimmune diseases such as Systemic Lupus Erythematosus (SLE), Rheumatoid Arthritis (RA), Myasthenia Gravis (MG), and Sjogren Syndrome [Citation1–4]. The immunosuppressant mycophenolate mofetil (MMF) is a commonly selected drug for these autoimmune disorders due to its favorable toxicity [Citation3]. However, there is limited literature concerning PCNS IALD developing in patients being treated for Eosinophilic Granulomatosis Polyangiitis (EGPA). Considering the increasing use of MMF for chronic immunosuppression, we describe a case of caution in which a patient developed PCNS IALD after several years of MMF therapy for EGPA.
2. Case description
An 81-year-old Caucasian female presented to the hospital with a three-month history of worsening right-sided weakness, decreased coordination with acute onset word-finding difficulty. She carried a six-year diagnosis of EGPA (with initial symptoms of adult-onset asthma, chronic sinusitis, and nasal polyps). She was maintained on long term immunosuppressive therapy due to cardiac involvement requiring mitral valve replacement (eosinophilic infiltration of valve with subsequent fibrosis). Patient had been initially treated with steroids and azathioprine, but was later transitioned to MMF one-gram twice daily due to liver dysfunction from azathioprine. Induction therapy with cyclophosphamide was considered, however, her five factor score [Citation4] at the time was only one with no neurological, renal or GI involvement. Therefore, azathioprine and MMF were only used as steroid sparing agents in order to try to decrease the dose of steroids and not as induction agents. For the past six years, she had a good control of her symptoms related to EGPA while on MMF.
At this presentation, her right lower extremity weakness and incoordination had necessitated the use of a walker for 1 month. Additionally, she had acutely developed word-finding difficulties. Patient otherwise denied other neurological complaints or history of fever, chills, weight loss, and changes in appetite.
Physical exam demonstrated alert and oriented female, with vitals within normal limits. Neurological examination revealed symmetrical facial expression, a midline tongue protrusion, and an intact shoulder shrug. A mild weakness in right foot dorsiflexion and right-hand grasp was noted with otherwise intact muscle strength and reflexes. Gait assessment showed right-sided foot drop and difficulty with balance. Finger-to-nose test showed some mild dysmetria and right-sided dysdiadochokinesia.
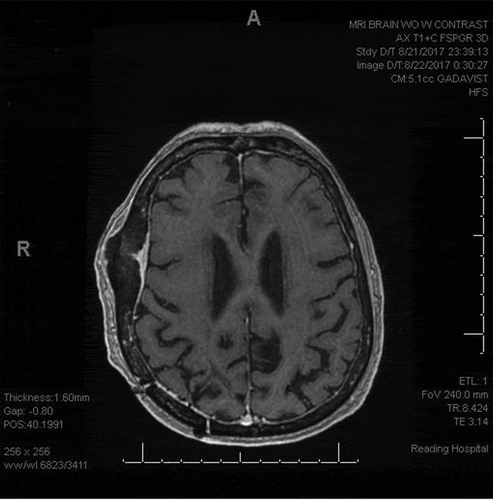
Magnetic resonance imaging (MRI) was done which revealed three solid enhancing lesions in the right parietal lobe, left insular lobe, and left midbrain extending into the left thalamus (,)). An inflammatory process related to patient’s EGPA was thought to be possible although the parenchymal lesions appeared more concerning for a neoplastic process. Computed tomography scan of the chest, abdomen, and pelvis did not show any acute findings. A spinal MRI followed by bone scan of spine showed degenerative disease without any discrete masses. Serologic testing for HIV was negative. ANCA serologies were negative. CRP was 0.22 mg/dL and eosinophil count was 0.29 × 10 3/µL. These findings made EGPA relapse less likely.
Figure 1. (a,b) Three enhancing solid mass lesions within the left insula, left midbrain-thalamus, and right parietal lobe with mild increased T2 signal with mild adjacent vasogenic edema. Focal meningeal thickening along the right lateral frontal temporal bone possibly secondary to a granulomatous process given the patient’s history of Churg-Strauss syndrome
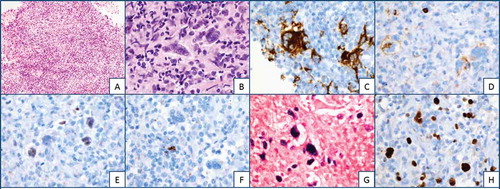
Patient further underwent craniotomy with biopsy of right parietal brain mass. The biopsy specimen () demonstrated a patchy but focally dense lymphoid infiltrate predominantly composed of small lymphocytes with admixed large atypical cells showing frequent multinucleation. These atypical cells resembled Hodgkin/Reed-Sternberg (H/RS) cells with abundant cytoplasm, large nuclei, and prominent nucleoli. Immunohistochemistry demonstrated positive CD30 staining, some CD15 staining, and dim Pax-5 and occasional dim CD20 expression. CD45 and CD10 staining were negative. The lymphoid infiltrate contained a mixture of CD3/CD5-positive T-cells and CD20-positive B-cells, with T-cells predominating. Background T-cells were negative for CD30. An in-situ hybridization (ISH) for EBV-encoded RNA (EBER) was positive in the H/RS cells and scattered small cells. The Ki-67 proliferation index was overall low, but significantly increased in the H/RS cells.
Figure 2. Biopsy specimen demonstrating a patchy but focally dense lymphoid infiltrate (a).Predominantly composed of small lymphocytes with admixed large atypical cells resembling Hodgkin/Reed-Sternberg cells (b). Immunohistochemistry demonstrating CD30 positive staining (c). CD 15 staining (d). Dim Pax-5 (e). Occasional dim CD 20 expression in H/RS cells (f). Lymphoid infiltrate containing mixture of CD3/CD5 positive T cells and CD-20 positive B cells, with predominating T cells. An in situ hybridization for EBV-encoded RNA (EBER) positive in H/RS cells (g). Ki-67 proliferation index is overall low, but significantly increased in H/RS cells (h)
MMF was discontinued following the biopsy due to concern for Primary CNS Immunodeficiency-associated lymphoproliferative disease (IALD). The patient was then started on rituximab therapy as the reversibility of this lymphoproliferation on discontinuation of immunosuppressants is very poorly defined. This therapy was selected as alternative therapies including methotrexate would be more toxic, and there was a concern for cognitive dysfunction with radiation therapy. Additionally, rituximab had a concomitant benefit in treating EGPA. Rituximab 375 mg/m2 weekly for 4 weeks of induction therapy lymphoma dose was used [Citation5]. This was planned to be continued for a total of 6–8 doses (for management of both EGPA and lymphoma) with interval clinical and radiologic monitoring.
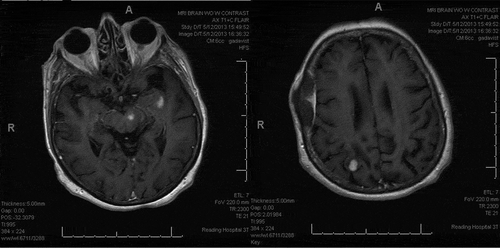
Following 8-weeks completion of rituximab therapy, repeat MRI showed near-total resolution of the enhancing intracranial lesions with minimal enhancement still evident within the heterogenous area involving lateral aspect of right occipital lobe (). Patient reported resolution of right-sided weakness and word-finding difficulties with significant improvement with her coordination. She remains on low dose prednisone and is in close follow-up with Rheumatology.
3. Discussion
IALD can present as a complication of immunosuppressants used in a variety of settings, including organ transplant recipients and patients with autoimmune disease. It can present in a variety of regions, however, a subset appears to be restricted to the central nervous system. Pathologically, these diseases are usually characterized by large B-cell morphologies, however additional variants include polymorphous and rarely can present as lymphoid granulomatosis. The pathology seen in this case is unique as it recapitulates a Hodgkin’s lymphoma like histologic pattern. This pattern has been rarely reported in intracranial lymphoproliferative diseases, including two primary CNS lymphomas (PCNSL) in the setting of HIV and in one PCNS IALD in a renal transplant patient [Citation5–7]. This is the first reported case of PCNS IALD with Hodgkin-like features reported in an autoimmune setting to our knowledge.
In recent years, MMF has been used as both induction and maintenance treatment in various autoimmune conditions and post-transplant cases. Despite the reported favorable safety profiles, case reports on MMF developing PCNS IALD are being reported [Citation1,Citation3,Citation8,Citation9]. The MMF prodrug mycophenolic acid (MPA) is a selective inhibitor of inosine monophosphate dehydrogenase, the rate limiting enzyme in de novo synthesis of guanosine nucleotides important in leukocyte production [Citation10]. MPA inhibits the subsequent proliferation of human T- and B-lymphocytes, but its effect appears to be more selective on T-lymphocytes. This compromise in T cell immune surveillance leads to a dysregulated B-cell proliferation in the setting of chronic antigen stimulation [Citation10]. This mechanism is particularly important in IALD as IALD is also characterized by Epstein Barr virus (EBV) reactivation [Citation3]. This results from the immunosuppressant therapy limiting the immune surveillance of cytotoxic T-cell activity [Citation4,Citation11]. The limited immune surveillance allows B-cells infected with dormant EBV to proliferate, causing mass like lesions [Citation4,Citation12]. These mechanisms may act synergistically.
In addition to these mechanisms, there is an additional risk of developing lymphoproliferative diseases in patients with chronic autoimmune conditions such as rheumatoid arthritis or systemic lupus erythematosus even when EBV negative [Citation4,Citation8,Citation13]. Our patient had an underlying autoimmune condition of EGPA, evidence of EBV infection, as well as treatment with the immunosuppressive MMF. Given these multiple independent risk factors, a direct association with MMF therapy cannot be confirmed. However, there is mounting evidence of association between PCNS IALD and MMF therapy in the literature (mostly in solid organ transplant patients receiving MMF). A study done by Crane et al. showed post-transplant lymphoproliferative disease (PTLD) arising in the setting of MMF had 11.4% chance of being PCNS vs 0.53% of PTLD arising with other therapies [Citation11]. This possible association was also questioned through small case series [Citation12], retrospective studies [Citation14] as well as clinical trial data [Citation15].
4. Conclusion
As MMF becomes further incorporated into standard therapy for various autoimmune conditions and in post-transplant patients, vigilance must be employed against development of lymphomas and patients must be informed of such potential risks. Discontinuation of the drug along with chemotherapy or immunosuppressive therapies have been the effective therapeutic options till date. Given the wide variability in immunosuppression regimens, further investigation into potential drug interactions and strategies for prevention of PCNS from immunosuppressive therapies are warranted.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Dasgupta N, Gelber AC, Racke F, et al. Central nervous system lymphoma associated with mycophenolate mofetil in lupus nephritis. Lupus. 2005;14(11):910–913.
- Lai GGY, Koo YX, Tao M, et al. Use of rituximab in combination with high-dose methotrexate in the treatment of primary central nervous system lymphoma in a mycophenolate mofetil treated patient with lupus nephritis. Acta Oncol. 2011;50(1):144–145.
- Finelli PF, Naik K, DiGiuseppe JA, et al. Primary lymphoma of CNS, mycophenolate mofetil and lupus. Lupus. 2006;15(12):886–888.
- Guillevin L, Pagnoux C, Seror R, et al. The five-factor score revisited: assessment of prognoses of systemic necrotizing vasculitides based on the French Vasculitis Study Group (FVSG) Cohort. Medicine (Baltimore). 2011;90(1):19–27.
- Hauptrock B, Hess G. Rituximab in the treatment of non-Hodgkin’s lymphoma. Biologics. 2008;2(4):619–633.
- Tsang HHL, Trendell-Smith NJ, Wu AKP, et al. Diffuse large B-cell lymphoma of the central nervous system in mycophenolate mofetil-treated patients with systemic lupus erythematosus. Lupus. 2010;19(3):330–333.
- Svobodova B, Hruskova Z, Rysava R, et al. Brain diffuse large B-cell lymphoma in a systemic lupus erythematosus patient treated with immunosuppressive agents including mycophenolate mofetil. Lupus. 2011;20(13):1452–1454.
- Kitchin JES, Washenik K. Rediscovering mycophenolic acid: A review of its mechanism, side effects, and potential uses. J Am Acad Dermatol. 1997;37(3):5.
- Omiya R, Buteau C, Kobayashi H, et al. Inhibition of EBV-induced lymphoproliferation by CD4+ T cells specific for an MHC class II promiscuous epitope. J Immunol. 2002;169(4):2172–2179.
- Kleinschmidt-DeMasters BK, Damek DM, Lillehei KO, et al. Epstein Barr virus-associated primary CNS lymphomas in elderly patients on immunosuppressive medications. J Neuropathol Exp Neurol. 2008;67(11):1103–1111.
- Crane GM, Powell H, Kostadinov R, et al. Primary CNS lymphoproliferative disease, mycophenolate and calcineurin inhibitor usage. Oncotarget. 2015;6:32.
- Sola-Valls N, Rodríguez C Ny, Arcal C, et al. Primary brain lymphomas after kidney transplantation: an under-recognized problem? J Nephrol. 2014;27(1):95–102.
- O’Neill BP, Vernino S, Dogan A, et al. EBV-associated lymphoproliferative disorder of CNS associated with the use of mycophenolate mofetil. Neuro Oncol. 2007;9(3):364–369.
- Snanoudj R, Durrbach A, Leblond V, et al. Primary brain lymphomas after kidney transplantation: presentation and outcome. Transplantation. 2003;76(6):930–937.
- Grinyó J, Charpentier B, Pestana JM, et al. An integrated safety profile analysis of belatacept in kidney transplant recipients. Transplantation. 2010;90(12):1521–1527.