ABSTRACT
Tuberculosis and sarcoidosis are both granulomatous diseases centered on the lung but capable of myriad extrapulmonary manifestations. Because of substantial similarity in their presentations, these two entities can be notoriously challenging to differentiate. This can be particularly true of countries in which tuberculosis is rarely encountered because of a reflexive tendency to ascribe granulomatous inflammation in the lung to sarcoidosis, especially if the granulomas are non-necrotizing. However, as our case from a non-endemic country reminds, sarcoidosis can be comfortably diagnosed only after convincing exclusion of infectious causes of granulomas. Distinguishing these two diseases is of utmost importance as, despite their overlapping presentations, they have completely non-overlapping treatments which can lead to harm if erroneously applied. At the end of our discussion, we summarize the clinical features favoring one diagnosis over the other.
1. Introduction
Worldwide, tuberculosis is the commonest infectious granulomatous disease of the lung while sarcoidosis is its commonest noninfectious counterpart. These two causes of granulomatous inflammation have sufficiently different prevalence distributions across the globe such that usually areas familiar with one are less familiar with the other. Both are known for extrapulmonary manifestations, and overall they share enough presenting features to not infrequently create diagnostic confusion. The challenge of distinguishing tuberculosis from sarcoidosis is best recognized in regions endemic for the former, but herein we present a case to illustrate that this problem can likewise be encountered in a non-endemic area for tuberculosis. This diagnostic distinction is highly relevant because tuberculosis and sarcoidosis call for diametrically opposite treatment approaches. We discuss the thinking that underlies differentiating the two diseases in confounding cases such as ours and compare and contrast helpful clinical features.
2. Case presentation
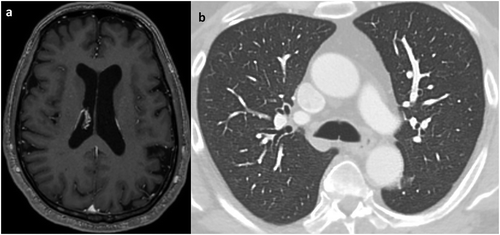
A 58-year-old Italian American man was referred to our institution by his primary care physician for two months of blurry vision, lightheadedness, and gait instability. During that time period, the patient had also noticed left hand weakness and occasional difficulty with word finding. He reported unintentional weight loss as well. His past medical history was significant for microscopic hematuria for which ureterocystoscopy with bladder biopsies had been performed six years previously and was negative for malignancy. He was subsequently noted to have recurrent right nephrolithiasis in the setting of an incompletely duplicated right collecting system and had undergone right ureteropelvic stenting on two occasions, most recently about one year prior to presentation. He was a former smoker who worked in the maintenance department of our institution. He had immigrated to the USA from the Calabria region of Italy approximately 35 years earlier and had last traveled back within the preceding two years. At the time of this admission, he had a blood pressure of 136/79 mmHg, a pulse of 88 beats/min, and an oxygen saturation of 99% while breathing room air. He was afebrile. His physical examination revealed several neurological abnormalities: slight gaze deviation, nystagmus, and mild left upper extremity weakness (4+/5). Routine laboratory evaluation was unremarkable. Computed tomography (CT) of the brain without intravenous contrast was consistent with multifocal vasogenic edema, prompting contrast-enhanced magnetic resonance imaging of the brain, which showed numerous ring-enhancing lesions involving both cerebral and cerebellar hemispheres as well as the basal ganglia, thalami, and brainstem ()). This pattern raised concern for metastatic malignancy. CT of the abdomen and pelvis with contrast ordered in search of a primary site demonstrated right hydronephrosis with ureteral strictures. Ureterocystoscopy was performed to exclude neoplasia, and multiple biopsies were taken, all of which were negative for malignancy. Following the urological procedure, the patient had his first fever of the admission: 39.7°C. After 48 hours of antibiotics, he defervesced. The search for a primary tumor site continued with CT of the chest without intravenous contrast, which revealed innumerable micronodules ()). There was no associated intrathoracic lymphadenopathy.
Figure 1. (a) Representative T1-weighted axial image from magnetic resonance imaging of the brain with gadolinium shows numerous ring-enhancing lesions in both cerebral hemispheres (arrows). These lesions were particularly densely concentrated in the cerebellum of this patient (inset, lower right). (b) Chest computed tomography axial image at the level of the upper lobes demonstrates diffuse bilateral involvement with innumerable micronodules
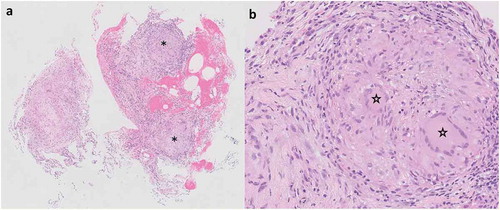
Despite the fevers, which were attributed to urological instrumentation, metastatic malignancy continued to be the top consideration in view of subacute neurological deficits and weight loss coupled with brain and lung lesions on imaging. Evaluation proceeded with bronchoscopy and transbronchial biopsies of the right upper lobe, which showed confluent non-necrotizing granulomatous inflammation (). Tissue stains for fungal organisms and acid-fast bacilli (AFB) were negative.
Figure 2. (a) Transbronchial biopsy specimen viewed at low magnification showing non-necrotizing granulomatous inflammation. Discrete granulomas are denoted by asterisks (Hematoxylin & eosin, original magnification x 20). (b) Higher magnification of one of the granulomas from (a) highlights the presence of multinucleated giant cells indicated by stars (Hematoxylin & eosin, original magnification x 40)
Based on the bronchoscopic biopsy, diagnostic momentum began to shift – understandably – away from widespread malignancy and towards sarcoidosis involving the lung and central nervous system. Several features of this case, however, made its attribution to sarcoidosis problematic. For one, concurrent with the release of lung biopsy results was the resumption of the patient’s fevers, spiking as high as 39°C, at a time when they could no longer be ascribed to a urological intervention. It is unusual for nodular lung disease due to sarcoidosis not to be accompanied by intrathoracic lymphadenopathy, which is typically symmetrical and bulky. Reactivation of granulomatous infections such as tuberculosis, in contrast, need not present with prominent lymph node involvement. Despite the classical association of infectious agents with formation of necrotizing granulomas, the absence of necrosis does not preclude an infectious etiology. Thus, skepticism regarding the provisional diagnosis of sarcoidosis was maintained. On the other hand, the patient was not at obvious risk for common granulomatous infections. He was believed to be immunocompetent: testing for the human immunodeficiency virus was negative, and his absolute CD4+ lymphocyte count was 521cells/µL (normal rage 441–1494cells/µL). Although he was born outside the USA and had recently visited his home country, Italy is not an endemic area for Mycobacterium tuberculosis (MTB). Blood interferon-gamma release assay was performed and was indeterminate. In the USA, he had not lived in or traveled to areas considered endemic for fungal infections. His occupation and hobbies did not include known exposures to environmental pathogens.
By this point the patient was smear- and polymerase chain reaction-negative for MTB in cerebrospinal fluid, three sputum samples, and bronchoalveolar lavage fluid (BALF). The most pressing question became whether glucocorticoid therapy would be appropriate for presumed symptomatic sarcoidosis with neurological involvement, which is an indication for urgent treatment. The pulmonary consultation team, however, advised caution given the potentially catastrophic consequences of administering glucocorticoids without antimicrobial agents in granulomatous infections. When MTB manifests with diffuse randomly distributed lung nodules, producing the so-called ‘miliary’ pattern in certain cases, it does so via hematogenous rather than endobronchial dissemination, resulting in a comparatively low yield of respiratory specimens. In contrast, involvement of distant organs can lead to positivity at extrapulmonary sites, notable among them the urinary tract [Citation1]. Therefore the recommendation was to expedite processing of a urine sample obtained at cystoscopy for AFB culture. Glucocorticoid therapy remained on hold pending mycobacteriology.
Fewer than ten days after the discovery of granulomatous lung inflammation, the urine AFB culture returned positive for MTB. Approximately one week later, BALF fluid culture likewise yielded MTB. All three sputum samples were ultimately finalized as showing no growth.
3. Discussion
By classical dogma, the systemic granulomatous disease known as sarcoidosis can only be diagnosed after identifiable causes of granuloma formation have been excluded, primary among them granulomatous infections [Citation2]. With this as background, it has long been debated whether tuberculosis and sarcoidosis should be viewed as separate or related entities on the spectrum of granulomatous diseases. Theories attempting to crack the enigma of sarcoidosis pathogenesis include a potential role for MTB as at least one of the triggers of the aberrant immune response that produces sarcoid granulomas [Citation3]. The distinction is highly relevant clinically because treatment of sarcoidosis with anti-tuberculous therapy cannot be advocated at the present time; conversely, deleterious effects can be expected from glucocorticoid monotherapy for tuberculosis. For clinicians from endemic regions for MTB, this is a common dilemma [Citation4]. As our case illustrates, making the right call can be a challenge even in low-prevalence countries. Because of potentially overlapping presentations, tuberculosis and sarcoidosis are notorious mutual mimics. There are, however, clinical clues that favor one diagnosis over the other. It is useful to recognize, for example, that the sarcoidosis patient is typically less ill-appearing with relatively modest constitutional and respiratory symptoms in comparison to the tuberculosis patient [Citation5]. When faced with a patient with febrile episodes or one who is visibly unwell, it behooves the clinician to maintain a healthy dose of skepticism about the diagnosis of sarcoidosis even if it is otherwise appealing. Lung cavitation and hemoptysis – a classical combination in tuberculosis – are both rare in sarcoidosis at initial presentation. Prominent, diffuse intrathoracic lymphadenopathy favors sarcoidosis over tuberculosis, whereas pleural effusion is found much more commonly in tuberculosis than in sarcoidosis. Both tuberculosis and sarcoidosis are well-known for extrapulmonary manifestations. They can have very similar radiological features in many organs such as the brain and liver [Citation6,Citation7]. Neither can be effectively assessed for ‘activity’ using a single biomarker. As mentioned, the presence or absence of necrosis in granulomas is not a reliable differentiating factor. compares and contrasts the usual clinical presentation of tuberculosis with that of sarcoidosis.
Table 1. Comparison of the typical presenting features of tuberculosis versus sarcoidosis
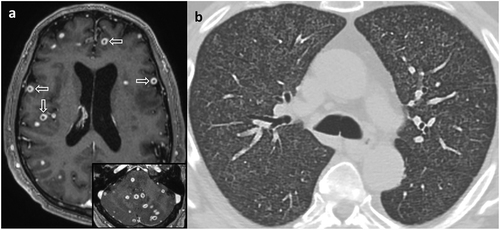
Our patient was started on RIPE (rifampin, isoniazid, pyrazinamide, and ethambutol) anti-tuberculous therapy. Sensitivity testing of his MTB isolate revealed susceptibility to all components of the regimen, so he completed the remainder of his year-long course for disseminated tuberculosis taking only isoniazid and rifampin. He improved clinically, and subsequent imaging demonstrated resolution of brain and lung abnormalities ().
Disclosure statement
No potential conflict of interest was reported by the authors.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
References
- Lewinsohn DM, Leonard MK, LoBue PA, et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention clinical practice guidelines: diagnosis of tuberculosis in adults and children. Clin Infect Dis. 2017;64:e1–33.
- Reich JM. On the nature of sarcoidosis. Eur J Intern Med. 2012;23(2):105–109.
- Rotsinger JE, Celada LJ, Polosukhin VV, et al. Molecular analysis of sarcoid granulomas reveals antimicrobial targets. Am J Respir Cell Mil Biol. 2016;55(1):128–134.
- Agrawal R, Kee AR, Ang L, et al. Tuberculosis or sarcoidosis: opposite ends of the same disease spectrum? Tuberculosis (Edinb). 2016;98:21–26.
- Gupta D, Agarwal R, Aggarwal AN, et al. Sarcoidosis and tuberculosis: the same disease with different manifestations or similar manifestations of different disorders. Curr Opin Pulm Med. 2012;18(5):506–516.
- Salahuddin M, Karanth S, Ocazionez D, et al. Clinical characteristics and etiologies of miliary nodules in the US: a single-center study. Am J Med. 2019;132:767–769.
- Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007;357(21):2153–2165.