ABSTRACT
A 33-year-old previously healthy man from Mexico who presented with massive hemoptysis, fevers, chills and found to have cavitary lesions in the right upper lobe of lung was highly suspicious for tuberculosis. The patient was treated with vancomycin, ceftriaxone, azithromycin and placed on isolation for suspected tuberculosis. Sputum AFB stains were negative and blood cultures grew Group A Streptococcus [GAS]. Antibiotics were narrowed down to ampicillin-sulbactam and the patient was discharged with significant clinical improvement. Strep A pyogenes is a rare cause of cavitary hemorrhagic pneumonia but is associated with high mortality. Clinical suspicion and early diagnosis are crucial in saving the patient.
1. Introduction
Among the various causes of pneumonia associated with hemoptysis and upper lobe cavitary lung lesion, mycobacterium tuberculosis is one of the main differentials, especially in patients from endemic areas for tuberculosis[Citation1]. Occasional cases of cavitary pneumonia occur due to Streptococcus pneumoniae, Hemophilus influenzae or Staphylococcus aureus. Group A streptococcus [GAS] is rarely described as a cause for this entity [Citation2]. GAS is a gram-positive coccus in chains which is ubiquitous mostly associated with skin and soft tissue infections, pharyngitis, and toxic shock syndrome. Approximately 10% of GAS infections present as pneumonia [Citation3]. Even though rare, it is important to have an early diagnosis of GAS as it can be rapidly progressing and fatal because of its presentation as hemorrhagic pneumonia and sepsis [Citation4].
2. Case report
A 33-year-old previously healthy man presented to the emergency department with complaints of acute hemoptysis, fevers, chills, pleuritic chest pain and shortness of breath for a duration of 3 days. He immigrated from Mexico 17 years ago with no recent travel. He also denied any history of smoking, vaping, or incarceration.
On initial examination, temperature was 39.6°C, heart rate 139 per minute, blood pressure 98/67 mm Hg, oxygen saturation of 95% on room air with increased vocal fremitus and decreased breath sounds over the right upper lobe. He was holding a cup filled with 100–150 mL of blood. He was in moderate respiratory distress requiring up to 3 liters of oxygen via nasal cannula. On cardiovascular examination, there was regular rhythm, no murmurs, rubs or gallops, and abdomen was non-distended, soft non-tender and without organomegaly. No rashes were seen upon skin examination.
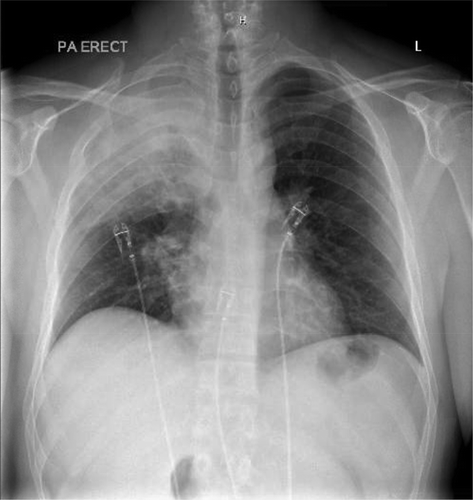
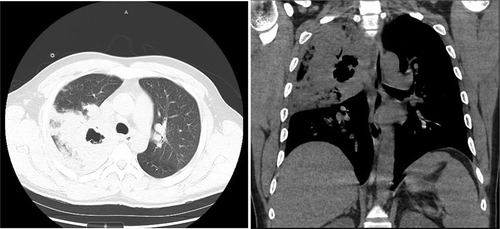
Laboratory findings were significant for Hemoglobin 15.3 g/dL, WBC count of 1.9k/µL, platelet count 83,000/µL, lactic acid 3.3 mmol/L, CRP 338.7 mg/L. Urine streptococcus and legionella were both negative. Influenza type B antigen was positive. HIV testing was negative. On the date of admission, blood cultures were drawn which later grew GAS pyogenes. Sputum gram stain showed rare gram-positive cocci in chains. Repeat blood cultures were negative after antibiotic treatment. Three sputum AFB stains were done 8 hours apart all of which were negative and did not show any acid-fast bacilli. Mycobacterium tuberculosis DNA PCR was also negative. shows the chest x ray depicting the right upper lobe pneumonia. The cavitation is well visualised on the Chest CT in .
Figure 2. Chest CT without contrast showing right upper lobe cystic cavitation measuring approximately 3 cm in diameter. It also shows prominent precarinal and right hilar lymph nodes
Echocardiogram: Left ventricle systolic function is normal with an estimated ejection fraction of 65%. There is mild concentric hypertrophy with grade 2 diastolic dysfunction and a normal right ventricle.
The patient was initially treated with ceftriaxone and azithromycin. Once the influenza test was positive, vancomycin and oseltamivir were also started. Once the blood culture showed GAS, antibiotics were narrowed down to ampicillin/sulbactam and on the fourth day, he was discharged with a resolution of hemoptysis, improvement in leukopenia, thrombocytopenia, and no recurrence of fevers. Six weeks later, his sputum culture grew mycobacterium gordonae, but the patient remained asymptomatic and did not require any further treatment.
3. Discussion
The most common clinical presentation of GAS infections are skin and soft tissue infections. GAS causing pneumonias accounts for only around 10% of GAS infections[Citation1]. Very few case reports of Streptococcus pyogenes pneumonia were published in the last three decades [Citation5]. GAS pneumonia was found to have a 38% case fatality rate which is extremely high compared to the 12% case fatality rate of other GAS invasive infection [Citation6]. It is even known to cause death within 12 hours due to massive pulmonary bleeding, despite aggressive supportive care [Citation7]. Case fatality rate of GAS pneumonia in previously healthy individuals was still 18%. Older age and male sex were found to be associated with increased mortality. Eighty-one percent of GAS pneumonia were also found to be community acquired [Citation6]. Most cases were acquired after a viral outbreak of influenza. This becomes even more important with the rise in influenza cases.
Our patient had GAS pneumonia complicated by sepsis secondary to influenza type B. However, the most common bacteria causing post influenza pneumonia are Streptococcus pneumoniae, Hemophilus influenza and Staphylococcus aureus [Citation8]. Cases where Streptococcus pyogenes group A caused bacteremia and hemorrhagic pneumonia were rare to find. Clinical features include rapidly progressing pneumonia, empyema in 40% of patients, and bacteremia in 15%[Citation9]. Blood cultures have been shown to be effective in diagnosing about 75% of patients with GAS pneumonia [Citation5]. Parenteral penicillin is the treatment of choice [Citation9,Citation10].
In a patient from an endemic area for tuberculosis, with upper lobe cavitary pneumonia, it would be appropriate to rule out tuberculosis. However, in our patient, Mycobacterium gordonae is likely a contaminant [10]. Mycobacterium gordonae, like other nontuberculous mycobacteria, is found mostly in tap water systems. It is generally viewed as a contaminant, not requiring treatment, when isolated from clinical specimens. It is still crucial to follow the cultures as presumptive therapy for tuberculosis may be initiated pending speciation. Although the imaging in this patient was classic for tuberculosis, he had a more acute presentation which should alert us to think of other differentials.
4. Conclusion
In patients presenting with upper lobe cavity and hemorrhagic pneumonia from a tuberculosis endemic region, while tuberculosis and other bacterial infections are being ruled out, an appropriate antibiotic treatment that covers GAS pneumonia should be started since the delay of treatment has a high fatality rate.
Disclosure statement
The authors report no conflict of interest.
References
- Gadkowski LB, Stout JE. Cavitary pulmonary disease. Clin Microbiol Rev. 2008;21(2):305‐333.
- Parkar AP, Kandiah P. Differential diagnosis of cavitary lung lesions. J Belg Soc Radiol. 2016;100(1):100.
- Stockmann C, Ampofo K, Hersh AL. Evolving epidemiologic characteristics of invasive group a streptococcal disease in Utah, 2002-2010. Clin Infect Dis. 2012;55(4):479‐487.
- Santagati M, Spanu T, Scillato M, et al. Rapidly fatal hemorrhagic pneumonia and group A streptococcus serotype M1. Emerg Infect Dis. 2014;20(1):98–101.
- Akuzawa N, Kurabayashi M. Bacterial pneumonia caused by streptococcus pyogenes infection: a case report and review of the literature. J Clin Med Res. 2016;8(11):831‐835.
- Muller MP, Low DE, Green KA, et al. Clinical and epidemiologic features of group a streptococcal pneumonia in Ontario, Canada. Arch Intern Med. 2003;163(4):467‐472.
- Tamayo E, Montes M, Vicente D, et al. Streptococcus pyogenes pneumonia in adults: clinical presentation and molecular characterization of isolates 2006-2015. PLoS One. 2016;11(3):e0152640.
- Morris DE, Cleary DW, Clarke SC. Secondary bacterial infections associated with influenza pandemics. Front Microbiol. 2017;8:1041. Published 2017 Jun 23.
- Stevens DL. Streptococcus pyogenes: group A β-hemolytic streptococcus. [ Accessed Available at: 2014 Oct 20]. http://www.antimicrobe.org/b239.asp.
- Arnow PM, Bakir M, Thompson K, et al. Endemic contamination of clinical specimens by mycobacterium gordonae. Clinl Infect Dis. Aug 2000;31(2):472–476.