ABSTRACT
Nocardiosis is an infection caused by the gram-positive bacterium Nocardia, which typically manifests as an isolated pulmonary or systemic disease. Of note, Nocardia has a predilection for the central nervous system (CNS) involvement, which is more commonly seen in systemic nocardiosis rather than as an isolated CNS infection. According to the Centers for Disease Control and Prevention, the estimated incidence of nocardiosis is only 500 to1000 cases in the USA every year, with cases mostly found in immunocompromised people, although infection in the immunocompetent may also occur. Here, we present a case of an immunocompromised patient who presented with neurologic symptoms and intracranial lesions initially concerning metastatic disease. Upon further investigation, the patient was found to have CNS nocardiosis with bacteremia. This is an extremely rare presentation given the lack of concurrent pulmonary and cutaneous involvement. The insidious onset and rarity of nocardiosis can result in a delayed or missed diagnosis. Early recognition is crucial as this is a potentially life-threatening illness. After obtaining adequate culture specimens, empiric treatment must be started expeditiously, keeping in mind the diversity of the Nocardia species and their antimicrobial resistance patterns.
1. Introduction
Nocardiosis is a rare infection caused by aerobic, gram-positive, weakly acid-fast, branching, rod-shaped bacterium of the Nocardia genus [Citation1]. These bacteria are widely found in soil, and human infections occur by inhalation or direct inoculation of the skin [Citation2]. Nocardiosis is typically regarded as an opportunistic infection with cell-mediated immunodeficiency being a risk factor; however, up to one-third of infected patients are immunocompetent. The presentation of nocardiosis varies, but pulmonary nocardiosis is the most common presentation in up to two-thirds of cases with cutaneous nocardiosis as the presentation in most other cases. Nocardia can involve the central nervous system (CNS) in 44% of cases; an isolated CNS presentation is found in as few as 9% of cases [Citation3]. In CNS nocardiosis, the disease can be clinically silent, and if symptoms do arise, they may be thought to be due to mass lesions rather than infection [Citation2]. Thus, CNS nocardiosis may be misdiagnosed as a neoplasm initially, especially given the rarity of nocardiosis. We present a case of isolated CNS nocardiosis with bacteremia in an immunocompromised patient who presented with dizziness and blurry vision.
2. Case presentation
A 73-year-old female presented to the emergency department with a one-day history of dizziness and blurry vision. She had a past medical history of rheumatoid arthritis for which she took 15 mg of methotrexate weekly and corticosteroids as needed, breast cancer status post lumpectomy and chemotherapy/radiation therapy in 2000, hypertension, and diabetes mellitus type 2. Patient came in with a chief complaint of dizziness with room spinning. This dizziness had been going on for one day along with a blurry vision. It started when she woke up and was unrelenting and caused nausea and non-bilious, non-bloody vomiting. She denied fever, chills, chest pain, dyspnea, abdominal pain, headache, and speech difficulty. Physical exam was unremarkable for her and had intact cranial nerve exams and normal strength and sensation throughout. Dix-Hallpike exam did reproduce vertigo but no nystagmus was seen. The HINTS test showed no saccade on head impulse, no nystagmus, and no skew was seen with a test of skew. Her labs were also unremarkable other than hyperglycemia of 355 and hemoglobin A1c of 10.2%. However, her systolic blood pressure (SBP) was elevated as high as the 220s mm Hg; due to concern for a hypertensive emergency, the patient was started on a cardene drip. This hypertensive urgency was likely due to the patient not taking her home medications hydralazine and diltiazem. She had been dizzy, confused and had emesis prior to presenting to the hospital. Her blood pressure responded well to controlling her nausea and restarting oral medications.
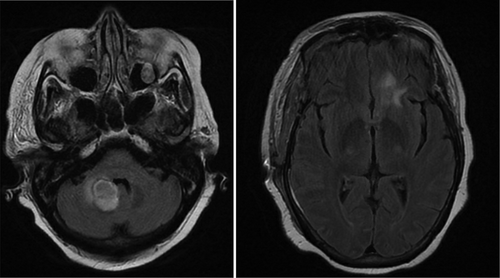
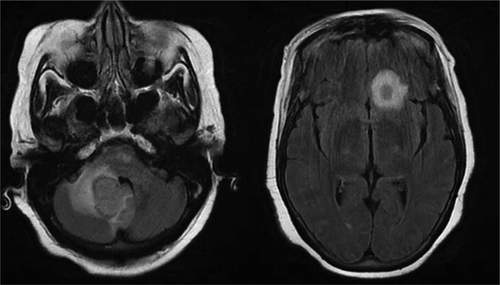
The patient also underwent head and neck computerized tomography (CT) and computerized tomography angiography (CTA) scans for concern for stroke, but these were unremarkable. In order to rule out stroke, magnetic resonance imaging (MRI) of the brain was ordered, which revealed two small ring-enhancing lesions with surrounding edema up to 1.3 cm x 1.1 cm ( left) in the left frontal lobe and right middle cerebellar peduncle ( right). These lesions were concerning for metastasis especially in the setting of her history of breast cancer. A chest/abdomen/pelvis CT scan revealed a focal spiculated nodular density in the right middle lobe of the lung measuring 1.5 cm x 2.0 cm x 1.1 cm, which increased the concern for metastasis. Oncology and neurosurgery were consulted and neurosurgery deemed surgery unfavorable. A bronchoscopy was performed and biopsies of the lung lesion were obtained via transbronchial biopsy. However, the biopsy came back negative for malignant cells. During the hospital stay, the patient’s mental status deteriorated with delirium. In addition, she was fixated on only her blood glucose level and seemed unconcerned about her brain mass and refused to answer questions, physical exam and labs. It was unclear whether she was cognitively at baseline and a repeat MRI of the brain was done, which showed an interval increase in the size of the previous two lesions to 2.7 cm x 2.4 cm for the right cerebellar peduncle lesion ( left) and 1.4 cm x 1.0 cm from 0.6 cm for the left frontal lesion ( right). In addition, at least four new lesions with surrounding edema were now present in the pons, right temporal lobe, and left superior frontal vertex. Due to the appearance of new lesions and the development of fever, infectious etiology was suspected and infectious disease was consulted. One of the two blood cultures obtained came back positive for gram-positive branching bacilli that was speciated as Nocardia farcinica. Neurosurgery was reconsulted for a brain biopsy; Biopsy and culture of one of the intracranial lesions produced purulent material and grew Nocardia farcinica on acid-fast bacteria (AFB) culture. The previous lung biopsy was used to perform an AFB smear but came back negative. The patient was started on imipenem/cilastatin 500 mg intravenously every 6 hours and TMP-SMX (trimethoprim-sulfamethoxazole) 5 mg/kg intravenously every 8 hours. Susceptibility testing showed intermediate resistance to imipenem, but susceptibility to TMP-SMX and moxifloxacin. Thus, imipenem/cilastatin was discontinued and moxifloxacin 400 mg daily was added to the TMP-SMX. The patient’s mental status did not improve as expected. This was not due to failure of treatment but rather from hyponatremia caused by the syndrome of inappropriate antidiuretic hormone secretion and hypervolemic hyponatremia caused by intravenous antibiotic infusion. The patient was clinically hypervolemic with bilateral 2+ lower extremity swelling to her hips and her serum osmolality was low at 270 mOsm/kg. It was thought to be due to administration of hypotonic fluids (the antibiotic sulfamethoxazole–trimethoprim was initially given in 5% dextrose solvent then given in 5% dextrose with 0.45% normal saline. It could not be concentrated or increased any further in tonicity per pharmacy). Her urine studies were collected when the serum sodium was near its lowest, 118 mmol/L. Her urine osmolality was 722 mOsm/kg and the urine sodium was 150 mmol/L. This was consistent with SIADH likely due to her intracranial lesion. Ultimately, it was thought the hypotonic fluids were exacerbated by the state of SIADH leading to a decline in her serum sodium. It improved at an appropriate rate with the use of tolvaptan. Besides the serum sodium, there were no other abnormalities in her serum chemistry. The patient was given one 30 mg dose of tolvaptan and 2 mg of bumetanide, but overcorrection of sodium occurred to 129 mmol/L and was stabilized with dextrose 5% in water. With a resolution of the hyponatremia, the patient’s mental status improved. A repeat MRI was performed on the day 10 of antibiotics, which showed a decrease in all intracranial lesions with no new lesions. The right cerebellar peduncle lesion had decreased from 2.8 cm to 2.3 cm ( left) and the left frontal lesion decreased from 1.0 cm x 1.4 cm to 0.4 cm x 0.9 cm ( right). The patient was discharged to a long-term acute care facility with intravenous TMP-SMX 320 mg every 8 hours and intravenous moxifloxacin 400 mg daily for 1 year with the option of switching to oral formulations after 6 weeks of intravenous therapy.
Figure 1. (left) Initial magnetic resonance imaging (MRI) right cerebellar peduncle lesion. (right) Initial MRI left frontal lesion
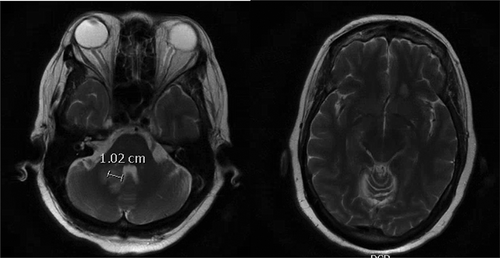
Figure 2. (left) Repeat magnetic resonance imaging (MRI) (12/19/19) showing an increase in the size of the right cerebellar peduncle lesion with surrounding edema with mass effect causing partial displacement of the fourth ventricle. (right) Repeat MRI showing increase in left frontal lobe lesion that has also increased in size with surrounding edema
3. Discussion
Nocardia are a group of soil-dwelling, aerobic, gram-positive bacteria that are diverse with more than 80 species. Thirteen Nocardia species have been identified as pathogenic, including Nocardia asteroids, N. farcinica, N. nova, N. brasiliensis and N. abscessus [Citation1]. Nocardiosis can affect multiple sites including the CNS, pulmonary, or extra-pulmonary sites such as cutaneous or lymphocutaneous tissues. Nocardiosis affects only the pulmonary system in about 40% of cases and affects two or more sites in 32% of cases. Of these cases, 44% had some CNS involvement, but CNS only involvement was only in 9% of cases [Citation3]. Nocardia bacteremia is so extremely rare that in the last 20 years, only 227 cases have been reported in English language literature [Citation4]. As there is a propensity for Nocardia to be attracted to the CNS, and if symptoms are suggestive of the presence of CNS disease or pulmonary nocardiosis is present, brain imaging is required to exclude CNS involvement in immunocompetent and immunocompromised patients. Cutaneous nocardiosis is much less likely to disseminate, so brain imaging should be decided to conduct case-by-case basis in immunocompetent patients [Citation5].
A brain abscess is a collection of pus within the brain parenchyma that can result from surgery, trauma, infection, direct inoculation, hematogenous spread from a different site, or from contiguous spread [Citation6]. The incidence of brain abscesses can be up to 8% of all intracranial masses in developing countries and 1% to 2% in developed countries [Citation7]. CNS nocardiosis often presents as a single abscess or multiple brain abscesses, with locations typically being supratentorial. These abscesses have a higher mortality rate than other etiologies of brain abscesses. Abscesses due to CNS nocardiosis result in mortality rates of 20% in immunocompetent patients and 55% in immunocompromised patients, with mortality rates up to 66% if multiple abscesses are present; mortality rates due to other brain abscesses are as low as 5% to 10% [Citation8].
CNS nocardiosis can often mask itself as a metastatic lesion as in our case and multiple other cases [Citation9]. This is because symptoms of CNS nocardiosis are often nonspecific such as headache, seizures, focal neurologic deficits, fever and can even present without any signs of infection [Citation2]. The diagnosis of CNS nocardiosis is more complicated due to blood cultures being positive in only 38% of cases despite the assumption that spread is hematogenous [Citation4]. Therefore, it is crucial to consider CNS nocardiosis in patients with brain lesions, especially those who are immunocompromised such as patients with acquired immunodeficiency syndrome (AIDS), organ transplant recipients, those on immunosuppressive therapy, or those with other opportunistic infections.
Diagnosis of CNS nocardiosis should begin with brain imaging. A CT scan may be sensitive enough to show lesions but is not enough for a diagnosis. In our case, the CT scan did not detect any lesions. A brain MRI may be needed to fully exclude the diagnosis. On MRI, a nocardial brain abscess characteristically presents as a hyper-enhanced lesion with multiple concentric rims and surrounding edema [Citation10]. A brain lesion with perilesional edema compounded with symptoms that correlate with a brain mass lesion rather than infection often causes the nocardial brain abscess to masquerade as a metastatic brain lesion. But diffusion-weighted imaging (DWI) and an apparent diffusion coefficient (ADC) can be helpful in differentiating the two types of abscesses. A brain abscess would show up as a homogeneously hyperintense lesion in DWI, but a hypointense lesion on ADC due to restriction of the Brownian motion of water molecules in the conglomeration of microorganisms, macromolecules, and inflammatory cells that make up an abscess [Citation11]. However, a definitive diagnosis of CNS nocardiosis requires a culture of the lesion that identifies the organism, usually with an acid-fast stain. A presumptive diagnosis may be made if filamentous branching rods are visualized. Early treatment is advantageous and has been associated with improved clinical outcomes in some patient populations (e.g., those with HIV) [Citation12].
Treatment of nocardial brain abscesses should always start with intravenous empiric coverage of two to three agents that include the preferred first-line agent TMP-SMX [Citation5]. Amikacin and/or imipenem are usually used as second or third-line [Citation13]. This is due to the varied Nocardia species and the different patterns of resistance and susceptibility of each, with N. farcinica being especially resistant. Due to the varying resistance patterns, susceptibility testing is important. No prospective study has been done to determine the antibiotic of choice; therefore, the antimicrobial choice has been based upon retrospective studies and experience. A 2010 retrospective review of 765 patients found that 42% were resistant to TMP-SMX and 61% were resistant to sulfamethoxazole [Citation3]. This study has not been replicated, and a second study showed only 2% resistance to TMP-SMX and/or sulfamethoxazole [Citation1]. Treatment is generally 3 to 6 weeks of intravenous therapy; patients can be switched to oral therapy with clinical improvement, and therapy should continue for at least 1 year [Citation14]. Furthermore, corticosteroids can be used, to reduce brain edema and mass effect, albeit with caution due to the risk of causing progression of infection [Citation15].
This CNS nocardiosis case had presented initially as concerning for a metastatic brain lesion in an immunocompromised patient. However, the diagnosis quickly changed with a negative lung biopsy and rapid growth of the brain lesions on imaging. It is crucial to differentiate between a nocardial brain abscess and metastatic brain tumor as early correct nocardiosis diagnosis improves clinical outcomes given the otherwise high mortality of this condition.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Brown-Elliott BA, Brown JM, Conville PS, et al. Clinical and laboratory features of the Nocardia spp. based on current molecular taxonomy. Clin Microbiol Rev. 2006;19(2):259–282.
- Beaman BL, Beaman L. Nocardia species: host-parasite relationships. Clin Microbiol Rev. 1994;7(2):213–264.
- Uhde KB, Pathak S, McCullum I Jr, et al. Antimicrobial-resistant nocardia isolates, USA, 1995-2004. Clin Infect Dis. 2010;51(12):1445–1448.
- Williams E, Jenney AW, Spelman DW. Nocardia bacteremia: A single-center retrospective review and a systematic review of the literature. Int J Infect Dis. 2020;92:197–207.
- Spelman D. Clinical manifestations and diagnosis of nocardiosis. In: Post TW, editor. UpToDate. Waltham, MA: UpToDate; 2020.
- Dohrmann PJ, Elrick WL. Observations on brain abscess. Review of 28 cases. Med J Aust. 1982;2(2):81–83.
- Bernardini GL. Diagnosis and management of brain abscess and subdural empyema. Curr Neurol Neurosci Rep. 2004;4(6):448–456.
- Baldawa A, Nayak N, Kukreja S, et al. Cerebral nocardiosis. Asian J Neurosurg. 2014;9(4):245.
- Patel H, Patel B, Jadeja S, et al. Central nervous system nocardiosis masquerading as metastatic brain lesions. IDCases. 2019;18;e00652(20):1865–1869.
- Chaudhari D, Renjen PN, Sardana R, et al. Nocardia Farcinica brain abscess in an immunocompetent old patient: a case report and review of literature. Ann Indian Acad Neurol. 2017;20(4):399–402.
- Bose B, Balzarini M. Diagnosis and treatment of nocardial brain abscess. Neurosurg Q. 2002;12(2):182–193.
- Uttamchandani RB, Daikos GL, Reyes RR, et al. Nocardiosis in 30 patients with advanced human immunodeficiency virus infection: clinical features and outcome. Clin Infect Dis. 1994;18(3):348–353.
- Schlaberg R, Fisher MA, Hanson KE. Susceptibility profiles of Nocardia isolates based on current taxonomy. Antimicrob Agents Chemother. 2014;58(2):795–800.
- Wilson JW. Nocardiosis: updates and clinical overview. Mayo Clin Proc. 2012;87(4):403–407.
- Mamelak AN, Mamalpam TJ, Obana WG, et al. Improved management of multiple brain abscesses: a combined surgical and medical approach. Neurosurgery. 1995;36(1):76–85.