ABSTRACT
Good syndrome (GS) is a rare paraneoplastic syndrome seen before or after diagnosis of thymoma, and its treatment, and is characterized by hypogammaglobulinemia. Rarely, pure white cell aplasia (PWCA) can also be seen which can present as recurrent neutropenia. We describe a 64-year-old man with recurrent sinus infections and previous thymectomy for stage 1 type B2 thymoma presenting with chronic diarrhea and recurrent neutropenia necessitating serial hospitalizations despite repeated antimicrobial treatment. Immunoglobulin levels, including IgM, IgA, IgD, and IgE were undetectable. Flow cytometry also showed absent B cells. Patient was initiated on immunoglobulin replacement therapy with consequent significant clinical improvement. Despite thymectomy, patients can develop thymoma-associated paraneoplastic syndromes, including GS.
1. Introduction
Good syndrome (GS) is a rare cause of late adult-onset immunodeficiency syndrome associated with thymoma. Though its etiology and pathogenesis are unknown, patients with GS are at risk of recurrent sinopulmonary infections, diarrhea, and other paraneoplastic syndromes [Citation1]. First described in 1954 by Robert Good, it currently does not have recognized diagnostic criteria [Citation2]. Due to its rare occurrence, patients are usually not diagnosed until late in the course of the disease. Rarely, GS can manifest as pure white cell aplasia (PWCA), consisting of agranulocytosis and absent B cells in the bone marrow [Citation3,Citation4]. We describe a case of a 64-year-old man with recent thymectomy presenting with recurrent neutropenic fevers and chronic diarrhea.
2. Case
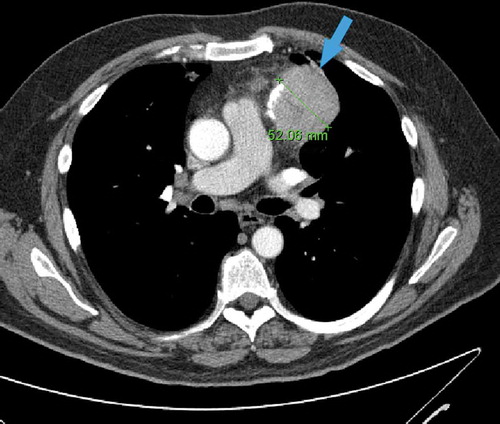
A 64-year-old man originally presented with nasal congestion, cough, and orthopnea. Chest X-ray showed an incidental lung mass. Computed Tomography (CT) of the chest showed a large anterior mediastinal mass () and the patient underwent thymectomy. Acetylcholine receptor auto-antibody testing was negative. Post-operative course was complicated by Haemophilus influenzae pneumonia and non-specific colitis after endoscopic evaluation of diarrhea.
Figure 1. Axial contrast-enhanced chest CT scan showing a 52 mm round left anterior mediastinal mass with small foci of calcification (arrow)
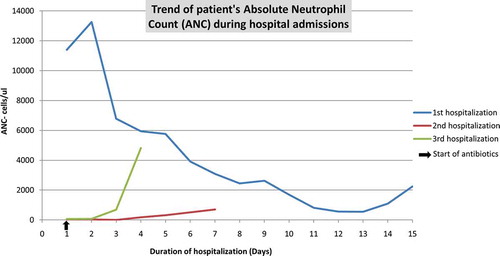
The following year, the patient was hospitalized for severe non-granulomatous colitis mimicking inflammatory bowel disease causing large bowel obstruction which responded to endoscopic decompression, corticosteroid therapy, and empiric antimicrobial therapy. Soon thereafter, the patient presented in gram-negative septic shock with bacteremia due to perforated sigmoid diverticulitis in the context of severe neutropenia. He underwent Hartmann procedure with colostomy. Neutropenia recovery was seen with IV antibacterial treatment. Four months later, recurrent colitis and rhinovirus upper respiratory tract infection along with profound neutropenia led to another hospitalization. HIV screening test, stool ova and parasites, stool culture and Clostridium difficile toxin, and PCR were negative. Bone marrow biopsy was consistent with regenerative benign marrow. Neutropenia recovery was again observed with antibacterial treatment ().
Half a year later, the patient presented yet again with recurrent colitis and profound neutropenia. Further laboratory work-up revealed severe hypogammaglobulinemia, undetectable levels of IgA (<5 mg/dL (range 70–320 mg/dL)), IgD (<5 mg/dL (range 50–300 mg/dL)), IgM (<1.2 mg/dL (range <14.11 mg/dL), as well as very low levels of IgG 109 mg/dL (range 600–1540 mg/dL)) were reported. Diagnosis of GS was entertained. Intravenous Immunoglobulin (IVIG) therapy was initiated with satisfactory clinical response. Since then, the patient has done well on scheduled IVIG out-patient replacement therapy.
3. Discussion
GS should be suspected in all adults presenting with recurrent infections in the setting of thymoma or post-thymectomy [Citation1]. The mean age at presentation is between 40 and 70 years with no gender preference [Citation1]. In GS, both T and B cells can be affected, leading to hypogammaglobulinemia, abnormal CD4/CD8 + T-cell ratio, low or absent B cells, or CD4 T lymphopenia [Citation5]. When presenting with an active infection, patients with thymoma usually have neutrophilic leukocytosis but neutropenia can be seen [Citation6]. Myasthenia gravis is more frequently associated with thymoma and is seen in 10–30% of cases whereas hypogammaglobulinemia is rarer and seen in 6–11% [Citation5,Citation7].
Neutropenia has been reported in patients with GS. PWCA, defined as agranulocytosis with absent myeloid precursors but preservation of other cell lines on bone marrow analysis, is very rare and can be seen in 1.1% of patients with thymoma [Citation4,Citation5]. PWCA has been commonly associated with mixed type AB thymoma, though our patient’s final histological type was B2 thymoma [Citation8]. Although most previously reported cases of thymoma-associated neutropenia were at the time of thymoma diagnosis, our patient presented with recurrent neutropenia several years post thymectomy [Citation5,Citation9–11]. Both autoimmunity to myeloid precursor cells and the presence of granulocyte-colony growth inhibition in serum have been described as mechanisms for PWCA [Citation5].
Significant morbidity and mortality have been reported with GS, mostly being infectious in etiology. More commonly, patients can have recurrent sinopulmonary infections with encapsulated bacterias including Haemophilus influenza, Streptococcus pneumoniae, and other microorganisms including Pseudomonas species, likely due to humoral immunity deficiency and hypogammaglobulinemia [Citation12,Citation13]. Diarrhea has also been reported in patients with GS due to autoimmune or bacterial colitis, and other cryptogenic etiologies [Citation7,Citation14], and at times, post thymectomy [Citation14–16].
The pathogenesis of GS remains unclear given the variable immune abnormalities seen in these patients. Presentation can be at the time of thymoma diagnosis or delayed years later. Several mechanisms have been proposed. Impaired immunity, loss of self-tolerance, and risk of autoimmunity due to thymus loss have been implicated [Citation17]. Thymectomy remains the mainstay of treatment for patients with stage 1 and 2 thymoma. Though it prevents locally invasive growth and metastasis, dysimmunity may be unavoidable and lead to immune deficiencies [Citation18,Citation19]. Treatment with IVIG has been shown to reduce hospitalizations, decrease infections, and improve long-term clinical outcomes in patients with GS [Citation18–20].
4. Conclusion
To date, established criteria for the diagnosis of GS have not been defined. A high index of clinical suspicion is needed in patients with thymoma or post thymectomy presenting with recurrent infections. Long-term clinical surveillance is therefore needed in patients with thymoma even after thymectomy. IVIG therapy should be initiated early in patients with hypogammaglobulinemia.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Kelleher P, Misbah SA. What is good’s syndrome? Immunological abnormalities in patients with thymoma. J Clin Pathol. 2003;56(1):12–16.
- Good RA. Agammaglobulinemia: a provocative experiment of nature. Bull Univ Minn Hosp Minn Med Fdn. 1954;26:1–19.
- Degos L, Faille A, Housset M, et al. Syndrome of neutrophil agranulocytosis, hypogammaglobulinemia, and thymoma. Blood. 1982;60(4):968–972.
- Uy K, Levin E, Mroz P, et al. Complication of thymoma: pure white cell aplasia in good’s syndrome. Case Rep Hematol. 2019;2019:1024670. Published 2019 Oct 13.
- Kelesidis T, Yang O. Good’s syndrome remains a mystery after 55 years: A systematic review of the scientific evidence. Clin Immunol. 2010;135(3):347–363.
- Verne GN, Amann ST, Cosgrove C, et al. Chronic diarrhea associated with thymoma and hypogammaglobulinemia (Good’s syndrome). South Med J. 1997;90(4):444–446.
- Mao ZF, Mo XA, Qin C, et al. Incidence of thymoma in myasthenia gravis: a systematic review. J Clin Neurol. 2012;8(3):161–169.
- Dong JP, Gao W, Teng GG, et al. Characteristics of good’s syndrome in China: a systematic review. Chin Med J (Engl). 2017;130(13):1604–1609.
- Weir AB 3rd, Dow LW. Response of agranulocytosis to thymectomy in a patient with thymoma and chronic lymphocytic leukemia. Med Pediatr Oncol. 1989;17(1):58–61.
- Akinosoglou K, Melachrinou M, Siagris D, et al. Good’s syndrome and pure white cell aplasia complicated by cryptococcus infection: A case report and review of the literature. J Clin Immunol. 2014;34(3):283–288.
- El-Galaly TC, Gregersen H, Bukh A. Good’s syndrome with lymphopenia and neutropenia. Ugeskr Laeger. 2011;173(4):280–281.
- Tarr PE, Sneller MC, Mechanic LJ, et al. Infections in patients with immunodeficiency with thymoma (Good syndrome). Report of 5 cases and review of the literature. Medicine (Baltimore). 2001;80:123–133.
- Malphetes M, Gerard L, Galicier L, et al. Good syndrome: an adult-onset immunodeficiency remarkable for its high incidence of invasive infections and autoimmune complications. Clin Infect Dis. 2015;61(2):e13–e19.
- Qu J, Lü X, Gao Q, et al. a rare cause of refractory chronic diarrhea and recurrent pneumonia in a Chinese patient after thymectomy. Clin Vaccin Immunol. 2013 Jul 1;20(7):1097–1098.
- Ohuchi M, Inoue S, Hanaoka J, et al. Good syndrome coexisting with leukopenia. Ann Thorac Surg. 2007;84:2095–2097.
- Liu K, Cowlishaw JL. Beware of the patient with thymectomy: good’s syndrome in a patient presenting with diarrhea. ACG Case Rep J. 2013;1(1):33–35. Published 2013 Oct 8.
- Zdrojewicz Z, Pachura E. The thymus: a forgotten, but very important organ. Adv Clin Exp Med. 2016;25(2):369–375.
- Detterbeck FC, Zeeshan A. Thymoma: current diagnosis and treatment. Chin Med J (Engl). 2013;126(11):2186–2191.
- Joven MH, Palalay MP, Sonido CY. Case report and literature review on Good’s syndrome, a form of acquired immunodeficiency associated with thymomas. Hawaii J Med Public Health. 2013;72(2):56–62.
- Wang CH, Chan ED, Perng CL, et al. Intravenous immunoglobulin replacement therapy to prevent pulmonary infection in a patient with Good’s syndrome. J Microbiol Immunol Infect. 2015;48(2):229–232.