ABSTRACT
Background: Coronary artery calcification (CAC) is a pathological deposition of calcium in the intimal and medial layer of the arterial wall. A plethora of therapeutic calcium debulking techniques is available for the treatment of CAC, including orbital or rotational atherectomy, excimer lasers, cutting, and scoring balloons, which are associated with a soaring rate of complication and low efficacy. To this end, in 2016, the Food and Drug Administration (FDA) posited that shockwave intravascular lithotripsy (S-IVL) technique can be employed with minimal complication.
Methods: A retrospective review of cases received lithotripsy for calcified coronary artery disease was performed by using online data from PubMed, Embase, and the Cochrane Central Register of Controlled Trials. The available search results were downloaded into an Endnote library and analyzed into two phases.
Results: Out of 24 participants from case reports and series, Majority were found to be Male. There was no significant difference found in the mortality of patients undergoing IVL for the stenosis of the left main stem, left anterior descending, left circumflex artery, or diagonal branch. The mortality was found to be high among 6 patients with prior comorbidities and underwent more than 3 cycles of IVL (OR 37,95% Cl 1.54–886.04, P 0.02). Out of 24 patients, 2 (8.33%) patients developed complications such as vessel dissection (OR 3.4, 95% Cl 17.87–64.68, P 0.4).
Conclusion: Shockwave intravascular lithotripsy (S-IVL) may be used in cases of the calcified disease to gain vessel lumen in order to deploy drug-eluting stents with PCI. The success of the DES implantation of IVL can be 100% with a minimal complication rate.
1. Introduction
Coronary artery calcification (CAC) is a pathological deposition of calcium in the intimal and medial layer of the arterial wall [Citation1]. The presence of calcified plaques in coronary arteries is an independent risk factor for failure to recanalize [Citation2]. The burden of CAC disease is also a risk factor for major adverse cardiac events (MACE), and mortality outcomes in the future [Citation3]. A plethora of therapeutic calcium debulking techniques is available for the treatment of CAC, including orbital or rotational atherectomy, excimer lasers, cutting, and scoring balloons [Citation4]. While these techniques can variably be employed, a soaring rate of procedural complications, along with low efficacy, has been reported with the aforesaid debulking modalities. Of these complications, the most sinister are coronary artery dissection and perforation, which can portend exceedingly poor treatment outcomes. Thus, an alternative treatment modality, ideally one that elicits a minimal degree of complications, is warranted. To this end, in 2016, Food and Drug Administration (FDA) posited that shockwave intravascular lithotripsy (S-IVL), a technique similar to the one used in nephrolithiasis, can be employed with minimal complications [Citation5].
Since the advent of S-IVL, numerous studies, such as the observational studies DISRUPT-1, DISRUPT-OCT, DISRUPT-II, and a vast concoction of cases have substantiated the efficacy of S-IVL in a variety of CAC disease on a case-to-case basis [Citation6–8]. We sought to retrospectively review the efficacy and safety profile at an individual case level.
2. Methods
2.1. Search strategy
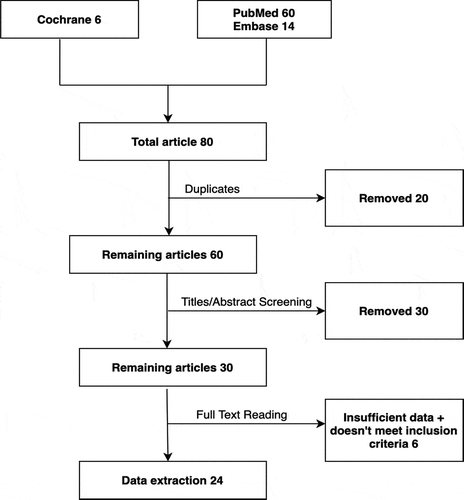
Online databases including PubMed, Embase, and the Cochrane Central Register of Controlled Trials were retrospectively searched from January 2020 to inception. The search strategy followed Preferred Reporting Items for Systematic Reviews and Meta-Analyse (PRISMA)” guidelines by using MeSH and keywords like ‘shockwave intravascular lithotripsy,’ ‘coronary lithotripsy,’ ‘right coronary artery,’ ‘left coronary artery,’ ‘acute coronary syndrome,’ ‘ST-elevation myocardial infarction,’ and ‘calcified coronary artery disease,’. The search items were combined using Boolean operators (‘OR’; ‘AND’). No filters including language, country of publication, and type of articles, including abstracts and posters, were applied. The references of individual case reports were sifted to find any relevant cases. The available results were downloaded into an EndNote library. The full search strategy is shown in the PRISMA diagram ().
2.2. Study selection
We exclusively selected case reports or case series. Two authors (Y.S and S.B) independently reviewed the abstracts, titles, and types of studies that meet eligibility criteria during phase 1. The Disagreement was resolved by consensus with a third author (W.U). The second phase of the search included full-text review of articles to enable identification of items for data extraction based on the inclusion criteria. Irrelevant articles at this stage were excluded with due justification, as shown in . The inclusion criteria mandated the fulfillment of one of the following: 1) Severe CAC disease with chronic or acute stenosis with calcium angle >230 with failure of PCI without IVL. 2) S-IVL at least one or multiple cycles used for revascularization or to implant drug-eluting stent (DES). 3) The CAC disease included the left coronary artery and its branches with or without the involvement of their branches.
3. Results
We included 24 patients (Case reports n = 17; Case Series n = 2). The mean age of the included population was 71 ± 9 years; with 67% males. The baseline demographic and procedural characteristics are shown in . In comparison of vascular site, there was no significant difference in the mortality of patients undergoing IVL for the stenosis of the left main stem (9.1% vs. 16.7%, OR 0.94, 95% CI 0.04–6.4, p = 0.5), left anterior descending (OR 0.8, 95% CI 0.036–6.9, p = 0.5), left circumflex artery (11.1% vs. 20%, OR 0.82, 95% CI 0.036–6.9, p = 0.61), or diagonal branch (13.6% vs. 0%, OR 0.27). Among 6 patients with S-IVL Cycles>3 with underlying comorbid conditions, mortality was reported in 3 patients up to 50% in this subset (OR 37,95% Cl 1.54–886.04, P 0.02). Out of 24 patients, 2 (8.33%) patients developed complications such as vessel dissection (OR 3.4, 95% Cl 17.87–64.68, P 0.4) ()
Table 1. Baseline characteristic of included population, and procedural characteristics
Table 2. Association of mortality and complication with number of S-IVL cycles
4. Discussion
Intravascular lithotripsy (IVL) is a modern derivative of traditional lithotripsy used for kidney stones, that can be implicated for severe calcified coronary artery disease. One of the cornerstone features of IVL is its pulsatile ultrasonic fracture of both intimal and medial vessel wall calcification, thereby providing a luminal area gain to deploy stent [Citation9]. IVL can be potentially helpful in management in severely calcified coronary artery disease including complex chronic total occlusion or left main coronary disease [Citation10]. The proposed indications of S-IVL are shown in .
Table 3. Coronary intravascular lithotripsy indications
5. Mechanism of action
The novel technique of S-IVL is designed to disrupt the calcified plaques through localized sonic pressure waves to improve the outcomes and minimize complications. This system consists of a rechargeable generator, connector cable, intuitive catheter, and 2 shockwave emitters placed inside an expandable balloon. The treatment is initiated by guiding the balloon catheter through a 6Fr sheath, positioning it at the site of stenosis, and inflating it up to 4 atm pressure [Citation9]. On activating the system, the battery-powered generator delivers a series of electrical pulses via connector cable into the lithotripsy emitters which convert it into mechanical energy (sonic pressure waves). The combined solution of saline and contrast inside the balloon facilitates the transfer of sonic pressure waves through the soft tissues into the intimal and medial calcium deposits. These acoustic waves deteriorate the calcium deposits by creating multiple microfractures and eventually increase vessel compliance ().
Figure 2. (a) showing components of IVL device. (b) showing mechanism of action of IVL and calcium fractures in a vessel by optical coherence tomographic view
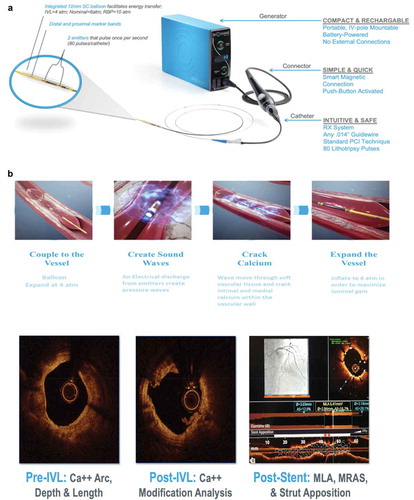
The system of S-IVL is capable of delivering 8 cycles of a shockwave with 10 pulses per cycle and one pulse every second which lasts for 1 microsecond [Citation9]. The IVL luminal gain and calcium fractures are shown in the Optical Coherence Tomographic view in ).
6. Efficacy of S-IVL
The coronary artery calcification is associated with the failure of percutaneous coronary interventions (PCI) and adverse cardiac events. The conventional approaches to treat these calcified plaques have several limitations due to procedural complications, recurrence, and poor clinical outcomes. In 2017, S-IVL received European CE mark approval for commercial use in the treatment of calcified plaques. The efficacy and safety of this alternative treatment approach are widely studied through multiple trials and observational studies. A multicentric prospective study, Disrupt CAD I, enrolled 60 patients with severely calcified vessels in seven countries. Their analysis demonstrated residual diameter stenosis of 13.3 ± 11.6%, and successful stent implantation in all patients [Citation7]. Another prospective trial, Disrupt CAD II, that studied 120 cases with extensive calcification from nine countries; also reported successful outcomes with residual stenosis of 7.8 ± 7.1% and stent delivery in 100% cases [Citation8]. A recent prospective observational study of 78 calcified lesions by Aksoy et al. has shown a significant reduction in mean diameter stenosis of up to 26.7 ± 4.3% after S-IVL therapy [Citation11]. Similar results are seen in a retrospective study by Wong et al who reported residual stenosis of <20% in all 26 cases [Citation12]. In addition, the acute luminal gain after S-IVL was 2.1 mm in Disrupt CAD I and 1.67 mm in Disrupt CAD II [Citation7,Citation8]. The study by Aksoy et al. also supports these findings as to the mean luminal gain after S-IVL and post-stenting were 0.89 mm and 1.87 mm, respectively, [Citation11]. Since severe calcification is an important predictor of restenosis after PCI, a pre-treatment with S-IVL can potentially increase the vessel diameter and ensure effective stent placement. The efficiency of S-IVL to disrupt the calcium plaques can be witnessed from several studies that report calcium fractures and reduced luminal calcium angle. An optical coherence tomography sub-study of Disrupt CAD I and Disrupt CAD II trials have shown calcium fractures in 42.9% and 78.7% of the plaques, respectively. The Disrupt CAD II also reported a significant reduction in luminal calcium angle from 266.3 ± 77.1 at baseline to 215 ± 69.4 post S-IVL and stenting (p < 0.0001) [Citation7,Citation8]. The wide-ranging benefits of S-IVL are further established through a recent meta-analysis by Burneikaite et al. which showed increased exercise capacity, improved NYHA class, and reduced frequency of angina [Citation13].
7. Complications
The complications of S-IVL can be categorized into procedural and post-procedural. The procedural complications may include slow flow, lack of reflow, distal embolization, perforation, and arterial dissection. There were four cases of coronary artery dissection in Disrupt CAD I trial and in the study by Aksoy et al. whereas only two patients experienced Type B and C dissection in Disrupt CAD II study [Citation9,Citation11]. Our review also showed procedural complications in 2 patients out of 24, the most common reported complication was coronary artery dissection. The clinical success of S-IVL depends on the residual diameter and major cardiac adverse events (MACE) therefore assessment of death, myocardial infarction, and coronary revascularization is critical. In Disrupt CAD I, MACE was 0% at 30 days and 8.5% at 6 months with three MI events and two cardiac deaths [Citation7]. In our review, among six patients with prior comorbidities, 3 had cardiac mortality. The Disrupt CAD II trial reported a relatively higher rate of 7.6% MACE at 30 days with MI in 8 patients, cardiac death in 1 patient, and coronary revascularization in 1 patient [Citation8]. On the other hand, Aksoy et al. reported 0 cases of in-hospital MACE.
7.1. Limitation
The data on S-IVL is limited as it is an upcoming procedure, ongoing clinical trial including DISRUPT CAD-IV (NCT04151628) will give us a better look of efficacy and complications. More clinical trials comparing S-IVL with other calcium debulking procedures are needed for calcified coronary disease.
8. Conclusion
Coronary artery calcifications contribute significantly to the overall disease burden manifested by acute coronary syndrome (ACS), which mandates immediate medical attention and PCI. The S-IVL is a safe and effective treatment approach to disrupt the vascular calcifications through localized, circumferential sonic pressure waves. This modality when compared to conventional approaches, requires minimal training and has excellent outcomes of luminal widening, successful stent implantation, and reduced risk of major adverse cardiovascular events. The observations noted in this case-series will need to be confirmed in adequately powered randomized clinical trials in future.
Acknowledgments
We would like to acknowledge S-IVL company Ms. Suzzane Wallace for providing IVL procedural images used in this review.
Disclosure statement
we have no financial or any kind of conflict of interest. The images used in this review are only for the purpose of education.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
References
- Khan S, Li B, Salata K, et al. The current status of lithoplasty in vascular calcifications: a systematic review. Surg Innov. 2019;26:588–598.
- Robert AB, Davide C, Darren M, et al. State of the art: 40 years of percutaneous cardiac intervention. EuroIntervention. 2017;13:621–624.
- Yeoh J, Hill J. Intracoronary lithotripsy for the treatment of calcified plaque. Interv Cardiol Clin. 2019;8:411–424.
- Barbato E, Shlofmitz E, Milkas A, et al. State of the art: evolving concepts in the treatment of heavily calcified and undilatable coronary stenoses - from debulking to plaque modification, a 40-year-long journey. EuroIntervention. 2017;13:696–705.
- Inc SM. Clinical Evidence: PAD I; 2018.
- Ali ZA, Brinton TJ, Hill JM, et al. Optical coherence tomography characterization of coronary lithoplasty for treatment of calcified lesions: first description. JACC Cardiovasc Imaging. 2017;10:897–906.
- Brinton TJ, Ali ZA, Hill JM, et al. Feasibility of shockwave coronary intravascular lithotripsy for the treatment of calcified coronary stenoses. Circulation. 2019;139:834–836.
- Ali ZA, Nef H, Escaned J, et al. Safety and effectiveness of coronary intravascular lithotripsy for treatment of severely calcified coronary stenoses: the disrupt CAD II study. Circ Cardiovasc Interv. 2019;12:e008434.
- Forero MNT, Daemen J. The coronary intravascular lithotripsy system. Interv Cardiol. 2019;14:174–181.
- Yeoh J, Hill J, Spratt JC. Intravascular lithotripsy assisted chronic total occlusion revascularization with reverse controlled antegrade retrograde tracking. Catheter Cardiovasc Interv. 2019;93:1295–1297.
- Aksoy A, Salazar C, Becher MU, et al. Intravascular lithotripsy in calcified coronary lesions: a prospective, observational, multicenter registry. Circ Cardiovasc Interv. 2019;12:e008154.
- Wong B, El-Jack S, Newcombe R, et al. Shockwave intravascular lithotripsy for calcified coronary lesions: first real-world experience. J Invasive Cardiol. 2019;31:46–48.
- Burneikaitė G, Shkolnik E, Čelutkienė J, et al. Cardiac shock-wave therapy in the treatment of coronary artery disease: systematic review and meta-analysis. Cardiovasc Ultrasound. 2017;15:11.