ABSTRACT
Background: Vasospastic angina (VA), or Prinzmetal’s angina, is characterized by symptoms of coronary angina caused by coronary vasospasm, usually in the absence of atherosclerotic changes. It typically presents with chest pain, which can be accompanied by transient electrocardiographic changes, if visualized during the attack. It can also rarely present with severe manifestations of acute myocardial angina, ventricular fibrillation, or cardiac arrest.
Case presentation: We present a case of a 50-year-old Caucasian male who initially presented to the hospital with chest pain and was diagnosed with VA. Later, he was brought to the hospital by emergency medical services later with ventricular fibrillation, despite normal coronary anatomy on angiogram. He was managed with placement of an intra–cardiac defibrillator (ICD) for secondary prevention. The patient continued to have recurrent episodes of ventricular fibrillation with associated ICD shocks, and had multiple admissions to the hospital with similar presentations. Symptoms and arrhythmia improved after optimizing antianginal therapy.
Conclusions: Ventricular fibrillation can be an uncommon but severe manifestation during VA crises. In cases with normal coronary vasculature, it is important to recognize VA as a cause of recurrent ventricular fibrillation in order to optimize medical management for prevention of fatal arrhythmias.
1. Background
Vasospastic angina (VA), also known as Prinzmetal’s angina, or variant angina, is a clinical entity first described by Prinzmetal et al. in 1959 as ‘angina at rest due to coronary artery vasospasm with transient ischemic electrocardiogram changes that resolve with short–acting nitrates’ [Citation1]. It results from coronary vasospasm and can occur with or without coronary artery disease. It usually presents as chest pain at rest or at night that lasts longer than the typical chest pain in acute coronary syndrome (ACS). It can sometimes be associated with ACS, arrhythmias, and sudden cardiac death, or the clinical presentation can be variable leading to under diagnosis of the condition [Citation2].
Vasospastic angina can occur in patients with or without atherosclerotic vasculature. It can have focal or diffuse involvement of coronary arteries or epicardial microvasculature [Citation3]. Multiple mechanisms have been proposed for the pathogenesis of coronary artery spasm including vascular smooth muscle hyperactivity, altered autonomic system, endothelial dysfunction, low–grade inflammation, electrolyte abnormalities, and oxidative stress. Vascular smooth muscle hyperactivity is considered a key factor [Citation3]. The symptoms show diurnal variation, occurring more often at night. Increased fibrin formation and decreased fibrinolytic activity at night are considered to be the underlying mechanism [Citation4]. Hyperinsulinemia, some genetic factors, and systemic vasomotor disorders like migraine or Raynaud’s phenomenon have also been associated with increased risk for this condition [Citation5–7].
According to the Coronary Vasomotor Disorders International Study (COVADIS) Group, the diagnostic criteria for VA include spontaneous nitrate-responsive angina, spontaneous transient ischemic changes on electrocardiogram (ECG) without obvious causes of increased myocardial activity, and angiographic evidence of transient coronary artery spasm causing total or subtotal coronary artery occlusion [Citation8]. Diagnosis is usually made based on nitrate responsiveness associated with transient ischemic changes on ECG, as angiographic documentation of spasmodic episodes is often not possible due to its transient nature [Citation9].
Ventricular fibrillation (VF) refers to a pulseless abnormal cardiac rhythm consisting of rapid uncoordinated cardiac beats, which can lead to sudden cardiac death and cause mortality without immediate intervention [Citation10]. VF more commonly occurs in association with acute coronary disease and can be the first symptom of coronary artery disease (CAD) in up to half of the cases of sudden cardiac death [Citation11]. While CAD is the leading cause of life-threatening ventricular arrhythmias and cardiac arrest, coronary ischemia from other factors such as vasospasm has also been reported to lead to VF.
Ventricular arrhythmias have been described as the most common arrhythmia during VA crises and the prevalence of the arrhythmias are related to the duration of episodes, degree of ST‐segment elevation, presence of ST–T wave alternans, or the presence of >25% increase of the R wave [Citation4]. Some studies have shown up to 10% of VA presenting as sudden cardiac death and the prognosis of patients presenting with aborted sudden cardiac death has been reported to be poor [Citation12]. Because of the presence of fatal complications such as VF and cardiac arrest, accurate diagnosis, and management of VA is crucial.
We present a case of a 50-year-old male presenting with recurrent episodes of VF who had transient ST elevations on ECG and non-occlusive coronary disease on cardiac catheterization. The arrhythmia in his case was felt to be related to coronary vasospasm.
2. Case presentation
A 50-year-old Caucasian male with a medical history of gastroesophageal reflux disease (GERD), hypertension, and chronic smoking presented to the emergency department with chest discomfort. His vitals on presentation were a temperature of 98.4 F, blood pressure (BP) of 159/89 mmHg, pulse rate of 72/minute, respiratory rate of 22/minute, and oxygen saturation of 95% on room air. He did not report any significant family history of coronary artery disease or cardiomyopathies. His blood troponin levels were within normal limits and he did not have any new changes on his ECG. He was admitted to the hospital and an echocardiogram was done which showed normal left ventricular function with trace valvular insufficiencies and mildly dilated aortic root (). Acute coronary syndrome (ACS) was ruled out and the patient was referred for an outpatient exercise stress echocardiogram, which showed no evidence of ischemia. There were no changes to his medication regimen during this encounter.
Figure 1. ECHO from the first presentation showing normal cardiac function with no wall motion abnormalities
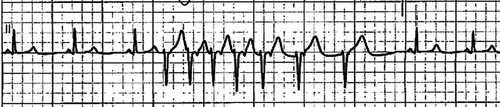
Six months later, he called emergency medical services (EMS) with episodes of stabbing chest pain associated with shortness of breath, lightheadedness, and dizziness. The patient was found to be in VF arrest en-route and was defibrillated by the EMS. On arrival, his mental status returned to baseline and the patient was conversant. His vital signs were within normal limits, and an ECG showed normal sinus rhythm but his blood work revealed elevated troponin levels that peaked at 11.74 ng/ml. An emergency cardiac catheterization was done which did not show any evidence of obstructive lesions, or focal or diffuse vascular spasms except for mild right coronary artery plaque. A computerized tomography (CT) angiogram of his chest did not show aortic aneurysm or dissection. He had frequent episodes of non–sustained ventricular tachycardia on telemetry as an inpatient (). A single chamber implantable cardiac defibrillator (ICD) was placed with settings at ventricular tachycardia (VT) monitor zone 160 and VFib zone 200 for secondary prevention of VF. He was discharged on diltiazem 180 mg daily and isosorbide mononitrate 60 mg daily.
Figure 2. Telemetry strip on the second admission showed nonsustained ventricular tachycardia (NSVT)
About one month later, he again presented to the hospital with spontaneous ICD shock following an episode of transient chest pain at rest, with no notable precipitating factors. An interrogation of his ICD was done which revealed VF episodes that led to ICD firing. Subsequently, he was started on sotalol and his diltiazem was switched to amlodipine 5 mg. His ICD was reprogrammed after evaluation by an electrophysiologist.
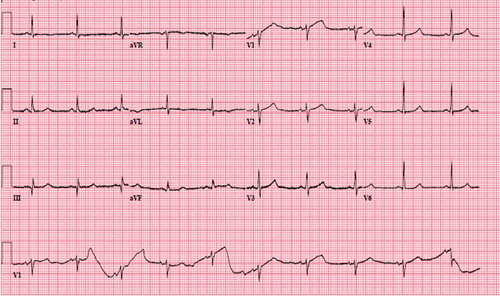
Seven months after discharge, the patient came to the emergency department complaining of sudden substernal chest discomfort, which he rated at 10 out of 10 in intensity. His vital signs on presentation were temperature of 98.6 F, BP 166/93 mmHg, pulse rate of 69/min, respiratory rate of 48/min, and an oxygen saturation of 93% on room air. His chest discomfort improved with sublingual nitroglycerine and morphine. An ECG revealed ST elevations in lead I and aVL with reciprocal ST–T wave changes in inferior leads (). He underwent emergency cardiac catheterization with concerns for ACS, which revealed no focal coronary spasms, and non–occlusive disease with a left ventricular ejection fraction (EF) of 50–55% and mild hypokinesia in the anterolateral wall. His labs showed an elevated troponin level of 2.58 ng/ml (normal <0.03). A complete blood count, basic metabolic panel, serum electrolytes, and lipid panel were all within normal limits. A repeat ECG showed resolution of prior ECG changes (). The patient was discharged on aspirin 81 mg once a day, atorvastatin 40 mg once a day, increased amlodipine to 10 mg, sotalol 120 mg twice a day, and nitroglycerin 0.4 mg as needed for chest pain. Of note is his Imdur had been stopped a few days prior to his presentation as he had not had any recurrent` chest pain and had started using sildenafil citrate as needed for erectile dysfunction.
Figure 3. EKG showing sinus bradycardia, ST elevation in lateral leads (I, aVL), ST depression in inferior leads (III, aVF)
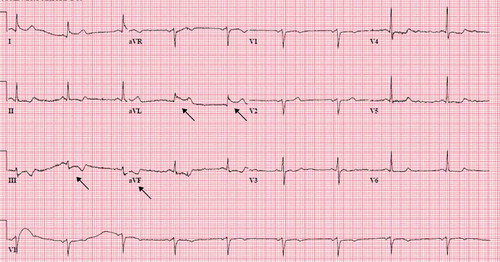
Figure 4. EKG following resolution of chest pain showing normalization of ST elevation in lateral leads (I, aVL) and ST depression in inferior leads (III, aVF)
On follow up, the patient has not had any more hospitalization or further episodes of chest pain or ICD shocks on the current medication regimen.
3. Discussion
The incidence or prevalence of VA is not so well defined. It is estimated that VA is responsible for about 2% of hospital admissions for unstable angina [Citation7]. Various studies have shown genetic and other factors predisposing patients to a higher incidence in certain populations, such as Japanese individuals compared to Caucasians [Citation13]. It has been commonly seen in males in the age group of 50–60 years [Citation7]. Major cardiovascular risk factors like hypertension, diabetes mellitus, hypercholesterolemia, and increased body mass index may not contribute to coronary vasospasm [Citation13]; however, smoking and history of hypertension are known risk factors [Citation14,Citation15]. Therefore, based on clinical presentation, risk factors, and family history, it is difficult to attribute anginal presentations to vasospastic angina without ruling out coronary artery disease (CAD).
Our patient belongs to the common age and sex group for VA. He also has cardiovascular risk factors such as smoking and hypertension, which predispose to VA [Citation14,Citation15]. Other triggers that are reported to lead to VA such as substance use (cocaine, marijuana, alcohol, ephedrine–based products, amphetamines) and magnesium deficiency were absent in his case [Citation7,Citation16–18]. He was taking Sildenafil infrequently, but no temporal association could be established with use of the medication and VA in his case. He did not have any history of percutaneous coronary intervention in the past. During his initial presentation, based on negative troponin levels and normal ECG findings, ACS was ruled out and he was scheduled for outpatient stress testing.
Classically, VA presents as recurrent angina at rest lasting for 5–15 minutes that is relieved with short-acting nitrates and has transient ST–segment elevation of at least 2 mm in at least two consecutive leads [Citation13]. Exercise tolerance is usually preserved. The presentation may alternate with hot and cold phases, frequent recurrent angina, and remission for weeks and months respectively [Citation7]. Our patient had a normal exercise stress echocardiogram as an outpatient, which shows that coronary etiology of chest discomfort may be missed in cases of VA. Case series have reported short silent crisis periods of coronary spasms [Citation4], the presence of which is a possibility in our case leading up to his hospital presentation.
The diagnosis of vasospastic angina makes it challenging because of its transient features. It may not always be possible to get a 12–lead ECG at the time of anginal symptoms. The ST changes (elevation or depression) usually return to baseline after the resolution of symptoms as demonstrated in our patient [Citation7]. Stress ECG can be considered for risk stratification regarding the presence of CAD and if normal, a 24–hour ambulatory ECG can be done to capture the diurnal pattern of vasospastic anginal episodes [Citation7,Citation19]. Our patient had a normal stress echocardiogram and subsequently no evidence of coronary narrowing on cardiac catheterization. It could be argued that CT coronary angiography would have shown a characteristic pattern of vasospastic angina as pointed out by Kang KM et al. [Citation20].
Our patient did not have any ST wave changes during his initial hospital presentation, but had ST depressions on ECG at the time of his subsequent hospitalization, which further highlights the risk of missing the diagnosis in view of a normal electrocardiogram and stress echocardiogram. In patients with normal arteries in coronary angiography with high suspicion for VA, provocative tests with an intravenous or intracoronary injection of methylergonovine or ergonovine or acetylcholine can be done. A decrease in luminal diameter by 75–99% during provocative testing can be considered diagnostic for coronary artery vasospasm [Citation19]. It is, however, not clear when to pursue such provocative testing, which is a class IIB recommendation by the American Heart Association/American College of Cardiology, and a class IIA recommendation by the European Society of Cardiology. Given ECG changes, chest pain and cardiac catheterization results, our patients presentation was felt to be consistent with severe vasospastic disease and additional provocative testing was not undertaken. The risk-benefit balance in our case is not clear in terms of the diagnostic yield from such testing.
Classically, the clinical presentations of VA are usually thought to be short, but sometimes may be associated with serious arrhythmias, as in our case. It is therefore important to recognize its presence so that it can be managed with an aim to prevent such life-threatening arrhythmias. Evidence from observational studies has indicated that patients with variant angina presenting with sudden cardiac death have a poorer prognosis than those without variant angina. As Kundu et al. have discussed, there is a lack of evidence regarding optimal management, as medical therapy may not be sufficiently protective in all cases [Citation21]. Our patient was managed on calcium channel blockers and anti-arrhythmics, but he continued to develop ICD shocks post-discharge. These resolved once medications were further optimized.
While some predictors such as age, hypertension, family history of sudden cardiac death, multivessel spasm, and left anterior descending artery spasm can point towards high-risk patients, it is difficult to stratify patients who are at high risk for sudden cardiac death [Citation21]. Our patient did not have any family history of cardiac arrest or cardiovascular diseases. Epidemiological studies have suggested genetic predisposition towards fatal arrhythmias such as VF, independent of other traditional risk factors [Citation11]. Our patient continued to develop VF requiring ICD firing to terminate the arrhythmias on multiple occasions. A better and more comprehensive understanding of the mechanisms for the development of VF in such cases is needed to explore further therapeutic options.
Management of VF is time-sensitive as it can cause sudden cardiac death if not immediately reverted and also involves diagnostic testing to delineate the cause of VF if possible. Defibrillation remains the mainstay of treatment to prevent significant mortality during acute episodes as well as to prevent further VF episodes. The literature on VF caused by coronary vasospasm is evolving, and a range of presentations from mild chest discomfort to myocardial infarction and life-threatening arrhythmias and sudden cardiac death have been reported [Citation13]. Our patient had recurrent hospital admissions () with chest pain and ST elevations noted on ECG leading to a coronary catheterization. Once ACS was ruled out, VFib was attributed to coronary vasospasm, which was further supported by recurrent episodes with potential anginal attacks but a decrease in the frequency of ICD shocks with the management of coronary vasospasm by calcium channel blockers and nitrates.
Calcium channel blockers and long-acting nitrates are effective and widely used in the prevention of coronary spasms. In patients with recurrent VF secondary to VA, implantable intra-cardiac defibrillator (ICD) is the standard of treatment with the goal of secondary prevention of VF [Citation22,Citation23]. The role of ICDs in secondary prevention of VF in patients with vasospasm is not entirely clear, but our patient did have recurrent VF as recorded by ICD. Two of the three patients reported by Eschalier et al, who had ICD placed for secondary prevention, reported recurrence of VF when the ICD was interrogated during follow-up [Citation24].
Our patient’s hospital presentation also mimicked acute myocardial infarction. His coronary angiogram was within normal limits and no revascularization procedure was performed. In a case reported by Naqvi et al. a patient with coronary artery vasospasm presenting with VF and cardiac arrest required insertion of a drug-eluting stent as well as a cardiac defibrillator [Citation9]. Placement of drug-eluting stents primarily for vasospastic angina can be an option in patients who have localized vasospasms refractory to medical management. Fiocca et al. also reported focal coronary spasm successfully treated with stent placement following which resolution of angina and arrhythmias was noted [Citation25].
Coronary artery vasospasm is an uncommon cause of ventricular fibrillation, which, if recurrent, may require placement of an ICD. It is important to recognize vasospastic angina as a cause of recurrent ventricular fibrillation in order to optimize management in a timely fashion.
Acknowledgments
We would like to acknowledge the help of Laurie J. Schwing MLS for her help and editorial support with the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Prinzmetal M, Kennamer R, Merliss R, et al. Angina pectoris. I. A variant form of angina pectoris; preliminary report. Am J Med. 1959;27:375–388.
- Murdoch D, Dhillon P, Niranjan S. Recurrent myocardial infarction secondary to Prinzmetal’s variant angina. Singapore Med J. 2015;56(5):e74–7.
- Picard F, Sayah N, Spagnoli V, et al. Vasospastic angina: a literature review of current evidence. Arch Cardiovasc Dis. 2019;112(1):44–55.
- De Luna AB, Cygankiewicz I, Baranchuk A, et al. Prinzmetal angina: ECG changes and clinical considerations: a consensus paper. Ann Noninvasive Electrocardiol. 2014;19(5):442–453. .
- Suzuki S, Yoshimura M, Nakayama M, et al. A novel genetic marker for coronary spasm in women from a genome-wide single nucleotide polymorphism analysis. Pharmacogenet Genomics. 2007;17(11):919–930. .
- Shinozaki K, Suzuki M, Ikebuchi M, et al. Insulin resistance associated with compensatory hyperinsulinemia as an independent risk factor for vasospastic angina. Circulation. 1995;92(7):1749–1757. .
- Lanza GA, Maseri A. Coronary artery spasm. Curr Treat Options Cardiovasc Med. 2000;2(1):83–90.
- Ozdemir D, Kishor J, Hall JM, et al. Case of Vasospastic Angina Presenting with Inferior Lead ST-segment Elevation and Ventricular Fibrillation in the Absence of Coronary Obstruction: a Case Report. Cureus. 2019;11(12):e6332.
- Naqvi SY, Hanley A, Crowley J. Ventricular fibrillation due to coronary vasospasm. BMJ Case Rep. 2014;2014(feb03 1):bcr2013203253–bcr2013203253.
- Dresen WF, Ferguson JD. Ventricular Arrhythmias. Cardiol Clin. 2018;36(1):129–139.
- Glinge C, Sattler S, Jabbari R, et al. Epidemiology and genetics of ventricular fibrillation during acute myocardial infarction. Journal of Geriatric Cardiology : JGC. 2016;13(9):789–797.
- Ahn J-M, Lee KH, Yoo S-Y, et al. Prognosis of Variant Angina Manifesting as Aborted Sudden Cardiac Death. J Am Coll Cardiol. 2016;68(2):137–145. .
- Mishra PK. Variations in presentation and various options in management of variant angina. Eur J Cardiothorac Surg. 2006;29(5):748–759.
- Nobuyoshi M, Abe M, Nosaka H, et al. Statistical analysis of clinical risk factors for coronary artery spasm: identification of the most important determinant. Am Heart J. 1992;124(1):32–38. .
- Takaoka K, Yoshimura M, Ogawa H, et al. Comparison of the risk factors for coronary artery spasm with those for organic stenosis in a Japanese population: role of cigarette smoking. Int J Cardiol. 2000;72(2):121–126. .
- Satake K, Lee J-D, Shimizu H, et al. Relation between severity of magnesium deficiency and frequency of anginal attacks in men with variant angina. J Am Coll Cardiol. 1996;28(4):897–902.
- Forman MB, Blass M, Jackson EK. Variant angina in the setting of food-borne botulism. Clin Infect Dis. 2011;53(12):1300–1301.
- Stern S, Bayes De Luna A. Bayes de Luna A. Coronary artery spasm: a 2009 update. Circulation. 2009;119(18):2531–2534.
- Alexander KM, Veillet-Chowdhury MR, MacIntyre CJ, et al. A Shocking Development in a Young Male Athlete With Chest Pain. Circulation. 2016;133(8):756–763.
- Kang KM, Choi SI, Chun EJ, et al. Coronary vasospastic angina: assessment by multidetector CT coronary angiography. Korean J Radiol. 2012;13(1):27–33.
- Kundu A, Vaze A, Sardar P, et al. Variant Angina and Aborted Sudden Cardiac Death. Curr Cardiol Rep. 2018;20(4):26.
- Mitchell LB. Use of the implantable cardioverter-defibrillator in patients with coronary artery spasm as the apparent cause of spontaneous life-threatening ventricular tachycardia or ventricular fibrillation: crossing the spasm sudden death chasm. J Am Coll Cardiol. 2012;60(10):914–916.
- Meisel SR, Mazur A, Chetboun I, et al. Usefulness of implantable cardioverter-defibrillators in refractory variant angina pectoris complicated by ventricular fibrillation in patients with angiographically normal coronary arteries. Am J Cardiol. 2002;89(9):1114–1116. .
- Eschalier R, Souteyrand G, Jean F, et al. Should an implanted defibrillator be considered in patients with vasospastic angina? Arch Cardiovasc Dis. 2014;107(1):42–47. .
- Fiocca L, Di Biasi M, Bruno N, et al. Coronary vasospasm and aborted sudden death treated with an implantable defibrillator and stenting. Ital Heart J. 2002;3(4):270–273.