ABSTRACT
Heart failure is a complex clinical syndrome associated with high mortality and morbidity, creating a major public healthcare problem. It has a variety of etiologies, including substance abuse. Cocaine-induced cardiotoxicity is caused by direct effects of inhibition of sodium channels and indirect effects by inhibiting catecholamine uptake leading to increased sympathetic activity. Management is through the cessation of cocaine use and implantation of guideline-directed medical therapy for heart failure with the exception of beta-blockers as their safe usage is still controversial due to the risk of the unopposed alpha-adrenergic activity. Dexmedetomidine (Precedex) and Benzodiazepines (i.e., midazolam) are options for patients that demonstrate signs and symptoms of acute cocaine intoxication. If the actions of benzodiazepines fail to achieve hemodynamic stability, nitroglycerin may be used (especially in patients with cocaine-associated chest pain and hypertension). Cardiac transplantation is recommended for those who have demonstrated severe cardiovascular disease from cocaine. We present a 43-year-old male with a long-standing history of cocaine use who developed cardiomyopathy and severe acute decompensated heart failure found to have an ejection fraction of <20% admitted to the intensive care unit. He required inotropic support with milrinone and mechanical ventilation. He was later extubated and then discharged with an outpatient evaluation for a cardiac transplant.
KEYWORDS:
1. Introduction
Heart failure (HF) is a major healthcare and public health concern that contributes to a magnitude of morbidity and mortality and affects almost more than 37.7 million individuals across the world. Due to the aging population, the prevalence of individuals with heart failure continues to increase in the USA, with an estimated total of 6.2 million Americans in the period between 2013 and 2016 [Citation1]. Each year, there are estimated 870,000 newly diagnosed individuals with heart failure in the USA alone [Citation2]. Symptoms and chronicity of this condition severely affect the quality of life. According to the Framingham heart study, the heart failure mortality rate is approximately 10% at 30 days after diagnosis, 20–30% at one year and 45–60% at 5 years [Citation3, Citation4–9].
2. Case report
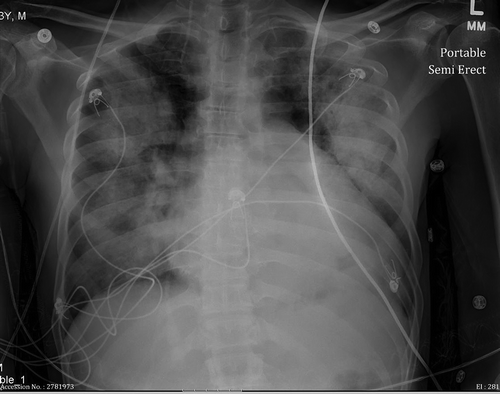
A 43-year-old male with a past medical history of hypertension, cocaine abuse, and congestive heart failure presented to our hospital with worsening shortness of breath. He also reported bilateral leg swelling, orthopnea and a dry cough the week before his presentation. He otherwise denied any other significant symptoms like chest pain, palpitations, or paroxysmal nocturnal dyspnea. The patient reported that he has been sniffing and shooting cocaine for more than 10 years. He also reported smoking tobacco. However, he denied any alcohol use. His physical exam was remarkable for tachypnea with a rate of 26 breaths/min, tachycardia with a rate of 105 BPM, BP 100/70 mmHg, and oxygen saturation of 80% on room air. Heart exam showed an S3 gallop with no audible murmur. Lung exam showed bilateral basal crackles as seen in , and his lower extremities were remarkable for 2+ edema below the knees.
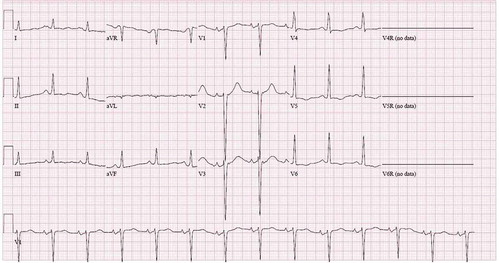
EKG showed sinus rhythm with evidence of LVH by voltage criteria and T wave inversion in the lateral leads as seen in .
His labs on admission were remarkable for Na 126, BNP > 5000, creatinine 1.9, BUN 34, AST 140, and ALT 66. The patient was initially placed on BIPAP and was given Lasix 80 mg IV twice daily and admitted to ICU for further management.
He was rapidly deteriorating and found to be in severe respiratory distress with worsening mental status, and the decision was made to intubate the patient. Transthoracic echocardiogram showed dilated cardiomyopathy with severely decreased global left ventricular systolic function with ejection fraction <20%. The patient was then started on a milrinone drip with significant hemodynamic improvement. He was successfully extubated and transferred to telemetry and eventually discharged with a cardiology follow-up appointment for a possible heart transplant.
3. Discussion
Cocaine is a highly addictive stimulant that alters human behavior through the limbic system’s activity, a structure in the brain involved in motivation, emotion, learning, and memory. The nucleus accumbens (NA) is a specific area within the limbic system that receives connections through dopaminergic neurons. When stimulated, the accumulation of dopamine at the NA causes euphoria, conditioning the brain to establish a reward pathway in association with a stimulant. Cocaine inhibits dopamine transport protein (DAT) embedded within presynaptic neurons of the NA, forming a reward pathway; thus, explaining the drug’s highly addictive nature and potential for abuse [1, 5]. Although the overall incidence of recreational cocaine use has been declining over the years within the United States, the global prevalence of cocaine is still approximately 0.4%. Many studies have provided significant evidence explaining the relationship between cocaine use and the onset of cocaine-induced morbidity (including cardiovascular, neurovascular, psychiatric, and infectious illnesses) and mortality over time.[Citation2,Citation3,Citation4,Citation6,Citation7]
Cocaine intoxication disrupts the homeostasis in nearly all organ systems throughout the human body; however, the cardiovascular system is responsible for most adverse effects. [8] The major cardiovascular complications associated with patients that use cocaine include myocardial infarction (MI), aortic dissection (AD), heart failure and cardiomyopathies, stroke, excessive hypertension, chest pain, and arrhythmia [9]. Cocaine can give rise to both acute and chronic cardiovascular disease, prompting adequate understanding of the pathophysiology so that prudent diagnostic and therapeutic action can be taken [Citation10,Citation11]
Direct cardiotoxic effects of cocaine include inhibition of sodium channels to produce a local anesthetic effect (similar to class I antiarrhythmic drugs) [Citation12,Citation13]. Inhibition of sodium currents decreases myocardial action potential (phase 0 depolarization) and intracardiac conduction, prolonging the QRS interval and thus, causing arrhythmia and progression to sudden cardiac death [Citation14]. These direct effects on myocardial conduction and contractility cause decreased left ventricular function, therefore, providing insight into the pathophysiology associated with cocaine-induced cardiomyopathy [Citation10,Citation15,Citation16]. Maintenance of calcium ion homeostasis [Citation17], inhibition of potassium channels on cardiomyocytes [Citation18], genetics [Citation19], alcohol consumption [Citation20], and ventricular hypertrophy [Citation21,Citation22] are other pathophysiologic factors that may play a significant role in cocaine-induced cardiomyopathy.
The patient in the case presentation demonstrated EKG findings showing tachycardia, bilateral enlargement, and QTC interval of 503 msec. His echocardiogram showed an ejection fraction of less than 20%, a severely dilated left atrium and left ventricle, and moderate to severe mitral regurgitation, severe tricuspid regurgitation, and global hypokinesis. These findings support the pathophysiological effects studied in cocaine-induced cardiomyopathy; however, the direct impacts of use-dependent sodium channel inhibition on cardiomyocytes may have contributed to the most significant factor resulting in the patients’ decrease myocardial contractility and ejection fraction. This suggests that controlling electrolyte abnormalities (especially sodium) may improve prognosis in patients suffering from cocaine-induced cardiomyopathy. It remains unclear whether or not controlling use-dependent sodium inhibition (phase 0 depolarization) in cardiomyocytes provides any significant improvement in the prognosis of patients suffering from cocaine-induced cardiomyopathy [Citation23–25].
Cessation of cocaine use is the first-line treatment for cocaine-induced cardiomyopathy, leading to significant improvement in systolic functioning; however, recurrence of cardiomyopathy is associated with relapse [Citation26]. Additional medical therapy for cocaine-induced cardiomyopathy and heart failure is consistent with the published guidelines for managing heart failure (except for beta-blockers). Dexmedetomidine (Precedex) is a sympatholytic drug that acts as an agonist on α2-adrenergic receptors in certain areas of the brain to abolish sympathetic nerve activity, thereby counteracting increased heart rate and blood pressure caused by the effects of cocaine [Citation27,Citation28]. Benzodiazepines (i.e., midazolam), an effective first-line medication for patients that demonstrate signs and symptoms of acute cocaine intoxication (hypertension, arrhythmia, and tachycardia) [Citation29]. If the actions of benzodiazepines fail to achieve hemodynamic stability, nitroglycerin may be used (especially in patients with cocaine-associated chest pain and hypertension) [Citation19,Citation25]. Other drugs that have been proven to be efficacious against cocaine intoxication include calcium channel blockers (verapamil), antiplatelet and antithrombin agents (aspirin, heparin, glycoprotein IIb/IIIa inhibitors, and clopidogrel) [Citation11], morphine [Citation30], and soil surrounding the coca plant (via bacteria that utilize cocaine esterase to hydrolyze cocaine) [Citation31].
Due to unopposed α-adrenergic receptor stimulation, the use of β-blockers was traditionally contraindicated [Citation32]. However, multiple studies highlighted the safety of beta-blockers and their potential benefits in cocaine-induced chest pain or cardiomyopathy since β-blockers represent the mainstay treatment for ischemic heart disease, heart failure (HF), and cardiomyopathies. Therefore, beta-blocker use in such patients is still controversial [Citation33,Citation34]. Cardiac transplantation for those who have demonstrated severe cardiovascular disease from cocaine is eligible while maintaining cocaine abstinence and concerns for relapse remain minimal [Citation11].
Disclosure of potential conflicts of interest
No potential conflict of interest was reported by the author(s).
References
- Virani S, Salim S Virani Search for more papers by this author, Alonso, A., Alvaro Alonso Search for more papers by this author, Benjamin, E., Emelia J. Benjamin Search for more papers by this author, … Al., E. Heart disease and STROKE STATISTICS-2020 Update: A report from the American Heart Association; 2020 Jan 29 [cited 2021 Feb 24]. Available from: https://www.ahajournals.org/doi/10.1161/CIR.0000000000000757
- Ziaeian B, Fonarow GC. Epidemiology and aetiology of heart failure. Nat Rev Cardiol. 2016;13(6):368–378.
- Bytyçi I, Bajraktari G. Mortality in heart failure patients. Anatol J Cardiol. 2015;15(1):63–68.
- Dugo E, Barison A, Todiere G, et al. Cardiac magnetic resonance in cocaine-induced myocardial damage: cocaine, heart, and magnetic resonance. Heart Fail Rev. 2020. Advance online publication. DOI:10.1007/s10741-020-09983-3
- Saland KE, Hillis LD, Lange RA, et al. Influence of morphine sulfate on cocaine-induced coronary vasoconstriction. Am J Cardiol. 2002;90:810–811.
- Menon DV, Wang Z, Fadel PJ, et al. Central sympatholysis as a novel countermeasure for cocaine-induced sympathetic activation and vasoconstriction in humans. J Am Coll Cardiol. 2007;50:626–633.
- Wood SK, Narasimhan D, Cooper Z, et al. Pre- vention and reversal by cocaine esterase of cocaine-induced cardiovas- cular effects in rats. Drug Alcohol Depend. 2010;106:219–229.
- Olds J, Milner P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J Comp Physiol Psychol. 1954 Dec;47(6):419–427. PMID: 13233369.
- Cormack JR, Orme RM, Costello TG. The role of alpha2-agonists in neurosurgery. J Clin Neurosci. 2005;12(4):375–378. PMID 15925765. S2CID 79899746.
- Nutt DJ, King LA, Phillips LD. Independent scientific committee on D. Drug harms in the UK: a multicriteria decision analysis. Lancet. 2010;376(9752):1558–1565.
- Farrell M, Martin NK, Stockings E, et al. Responding to global stimulant use: challenges and opportunities. Lancet. 2019;394(10209):1652–1667.
- Mladěnka P, Applová L, Patočka J, et al. TOX-OER and CARDIOTOX Hradec Králové researchers and collaborators. Comprehensive review of cardiovascular toxicity of drugs and related agents. Med Res Rev. 2018 Jul;38(4):1332–1403.
- Richards JR, Le JK. Cocaine toxicity. [ Updated 2020 Oct 21]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan. Available from: https://www.ncbi.nlm.nih.gov/books/NBK430976/
- John WS, Wu LT. Trends and correlates of cocaine use and cocaine use disorder in the USA from 2011 to 2015. Drug Alcohol Depend. 2017;180:376–384.
- Schwartz BG, Rezkalla S, Kloner RA. Cardiovascular effects of cocaine. Circulation. 2010 Dec 14;122(24):2558–2569. PMID: 21156654.
- O’Leary ME, Hancox JC. Role of voltage-gated sodium, potassium and calcium channels in the development of cocaine-associated cardiac arrhythmias. Br J Clin Pharmacol. 2010;69(5):427–442.
- Hummel M, Unterwald EM. D1 dopamine receptor: a putative neurochemical and behavioral link to cocaine action. J Cell Physiol. 2002 April;191(1):17–27. PMID 11920678. S2CID 40444893.
- Glauser J, Queen JR. An overview of non-cardiac cocaine toxicity. J Emerg Med. 2007 Feb 01;32(2):181–186. 0736-4679. PMID 17307630.
- Hale SL, Alker KJ, Rezkalla SH, et al. Nifedipine protects the heart from the acute deleterious effects of cocaine if administered before but not after cocaine. Circulation. 1991;83:1437–1443.
- Brickner ME, Willard JE, Eichhorn EJ, et al. Left ventricular hypertrophy associated with chronic cocaine abuse. Circulation. 1991;84:1130–1135.
- Zimmerman JL. Cocaine intoxication. Crit Care Clin. 2012 Oct 01;28(4):517–526. 1557-8232. PMID 22998988.
- Havakuk O, Rezkalla SH, Kloner RA. The cardiovascular effects of cocaine. J Am Coll Cardiol (Review). 2017 July;70(1):101–113. PMID 28662796.
- Orr D, Jones I. Anaesthesia for laryngoscopy: a comparison of the cardiovascular effects of cocaine and lignocaine. Anaesthesia. 1968;23:194–202.
- Przywara DA, Dambach GE. Direct actions of cocaine on cardiac cellular electrical activity. Circ Res. 1989;65:185–192.
- Winecoff AP, Hariman RJ, Grawe JJ, et al. Reversal of the electrocardiographic effects of cocaine by lidocaine. Part 1. Comparison with sodium bicarbonate and quinidine. Pharmacotherapy. 1994;14:698–703.
- Hollander JE, Hoffman RS, Gennis P, et al. Cocaine-associated chest pain: one-year follow-up. Acad Emerg Med. 1995;2:179–184.
- Brogan WCIII, Lange RA, Kim AS, et al. Alle- viation of cocaine-induced coronary vasoconstriction by nitroglycerin. J Am Coll Cardiol. 1991;18:581–586.
- Willens HJ, Chakko SC, Kessler KM. Cardiovascular manifestations of cocaine abuse: a case of recurrent dilated cardiomyopathy. Chest. 1994;106:594–600.
- Pitts WR, Vongpatanasin W, Cigarroa JE, et al. Effects of the intracoronary infusion of cocaine on left ventricular systolic and diastolic function in humans. Circulation. 1998;97:1270–1273.
- Lange RA, Cigarroa RG, Flores ED, et al. Potentiation of cocaine-induced coronary vasoconstriction by beta-adrenergic blockade. Ann Intern Med. 1990;112:897–903.
- Catravas JD, Waters IW. Acute cocaine intoxication in the conscious dog: studies on the mechanism of lethality. J Pharmacol Exp Ther. 1981;217:350–356.
- Beckman KJ, Parker RB, Hariman RJ, et al. Hemodynamic and electrophysiological actions of cocaine. Effects of sodium bicarbonate as an antidote in dogs. Circulation. 1991;83:1799–1807.
- Arenas DJ, Beltran S, Zhou S, et al. Cocaine, cardiomyopathy, and heart failure: a systematic review and meta-analysis. Sci Rep. 2020;10(1):19795. Published 2020 Nov 13.
- Pham D, Addison D, Kayani W, et al. Outcomes of beta blocker use in cocaine-associated chest pain: a meta-analysis. Emerg Med J. 2018;35(9):559–563.