ABSTRACT
Hypercoagulability has been found in patients diagnosed with the novel coronavirus 19 (COVID-19) and has been identified as a major cause of morbidity and mortality. Herein, we report the challenge in managing a patient presenting with a 5 day history of COVID-19 diagnosis, complicated by deep venous thrombosis, pulmonary embolism and ischemic stroke in the setting of atrial septal aneurysm, presumed patent foramen ovale and paradoxical embolism, identified to have clots in transit on echocardiogram. The application of anticoagulation was felt to be high risk. The patient was transferred to a tertiary facility where the patient underwent thrombus aspiration and was eventually complicated by hemorrhagic conversion of the stroke.
1. Introduction
Ever since the SARS-CoV-2 pandemic in December 2019, the medical community has been challenged with the wide spectrum of manifestations that SARS-CoV-2 presents with. While the most common presentation of the disease is hypoxic respiratory failure, many patients were also found in hypercoagulable states, presumably having deep venous thrombosis with microemboli in pulmonary vasculature. Arterial thrombosis leading to cardiovascular complications have been reported [Citation1–4], but reports regarding systemic arterial thrombosis have been few, with Kashi et al. reporting increased incidence of critical limb ischemia in intensive care unit patients. A systematic review by Tan et al. showed an acute ischemic stroke incidence of 1.2% in COVID-19 with a mortality rate of 38% [Citation5]. Here, we present a case of an elderly female patient presenting with pulmonary emboli and a large vessel stroke due to a presumed paradoxical embolism in the setting of COVID-19 pneumonia.
2. Case description
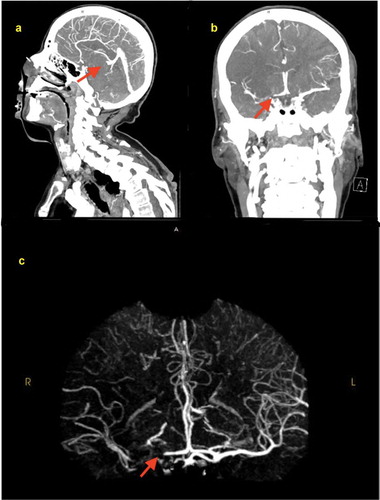
A 77-year-old female patient with a past medical history of chronic obstructive pulmonary disease, hypertension, hyperlipidemia who presented to our emergency room (ER) due to worsening of respiratory distress. Four days prior to admission, the patient was hospitalized for management of community acquired pneumonia and upper gastrointestinal (GI) bleed and was later discharged to a rehab center. During a physical therapy session at the rehab facility, the patient developed severe shortness of breath and was found to be hypoxic with an oxygen saturation (SpO2) of 75%, she was placed on a nasal cannula. Presenting vitals in the ER showed a blood pressure of 152/83 mmHg, pulse 137 bpm, respiratory rate of 18/min and SpO2 97% on a 15 litre nonrebreather, and the patient was found to be COVID-19 positive using the RT-PCR test. Initial computed tomography (CT) of the chest pulmonary embolism protocol found multiple small bilateral pulmonary emboli as well as elevated right ventricular to left ventricular ratio of 1.4 indicating right heart strain (). The patient was noted to have expressive aphasia and left sided flaccid paralysis, and an initial CT scan of the head without intravenous (IV) contrast showed no evidence of acute intracranial abnormality. A magnetic resonance imaging (MRI) of the head with and without gadavist intravenous (IV) contrast () done later on Day 1 found a large acute right middle cerebral artery (MCA) territory infarct. The patient was started empirically on IV dexamethasone, antibiotic therapy, aspirin, atorvastatin, and therapeutic enoxaparin. On Day 2, a CT angiography of the head and neck with IV contrast showed an abrupt vessel cut off of the right internal carotid artery at the level of the supraclinoid right ICA, and nonvisualization of the distal right internal carotid artery as well as the right M1 due to a non-occlusive thrombus ().
Figure 1. CT chest pulmonary embolism protocol on day 1. (a): Multiple small bilateral pulmonary emboli (yellow arrows in a). Elevated RV to LV ratio of 1.4 indicative of right heart strain. (b): Bilateral lower lobe patchy consolidation. Small patchy ground glass opacity in the right upper lobe (Red arrows in b). Extensive emphysema
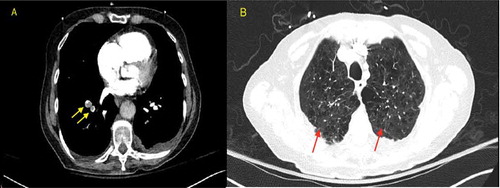
Figure 2. MRI brain with and without IV contrast on day 1
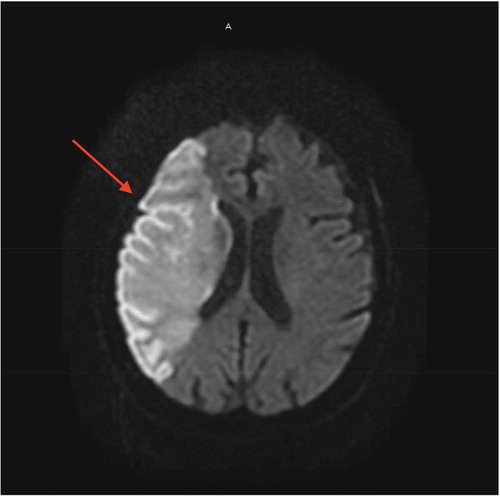
Figure 3. CT angiography of the head and neck with IV contrast
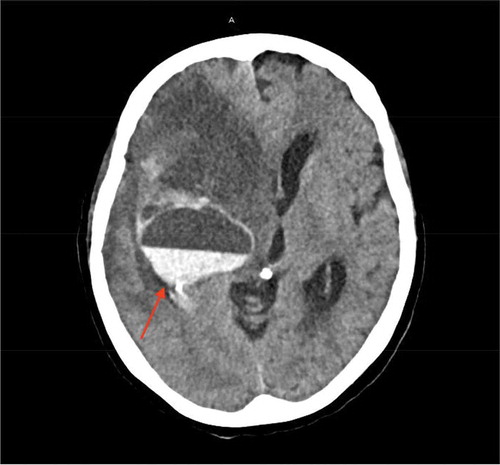
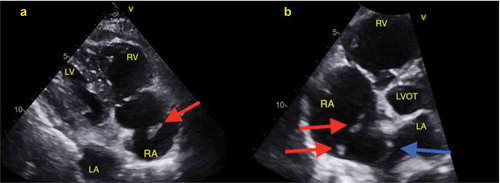
The patient was transferred to an acute care facility, and a transthoracic echocardiogram () showed moderately dilated right ventricle with moderately reduced systolic function, right atrial thrombus in transit and hypermobile atrial septal aneurysm. The thrombus was managed with thrombectomy using the Inari FlowTriever device () and was started on continuous IV infusion of unfractioned heparin. On day 3, a venous doppler of the lower extremities showed a thrombus in the right femoral vein of the distal thigh, gastrocnemius veins, posterior tibial, and peroneal veins. At this point, COVID-19 related hypoxic respiratory symptoms were mild and required only nasal cannula for supplemental oxygen. Patient remained hemodynamically stable until day 10 her condition was complicated by an upper GI bleeding in the form of melanotic bowel movement, she subsequently underwent esophagogastroduodenoscopy (EGD) and found a clot in the anterior bulb, which was treated with epinephrine, clot removal and cautery of a non-bleeding vessel. She was then treated with pantoprazole IV infusion and transfused two units of packed red blood cells (PRBC), while anticoagulation therapy was held. An interdisciplinary decision was made to restart anticoagulation on day 13. On Day 18, the patient had a hemorrhagic conversion of right middle cerebral artery ischemic infarct which was detected on CT of the head (), and heparin infusion was discontinued. Neurosurgery evaluated the patient and she was deemed not a candidate for any surgical intervention. Given her grave prognosis, the patient was transitioned to hospice care on Day 20.
Figure 4. Two-dimensional transthoracic echocardiogram on day 2. (a): Subcostal view; showing echogenic nobile thrombus in the right atrium. RV: Right ventricle; RA: Right atrium; LA: Left atrium; LV: Left ventricle. Red arrow: mobile right atrial thrombus in transit. (b): Apical Four-chamber view. LVOT: Left ventricle outflow tract; LA: Left atrium; RV: Right Ventricle; RA: Right atrium; Red arrows showing right atrial thrombi. Blue arrow: Atrial septal aneurysm
3. Discussion
The novel coronavirus pandemic has undoubtedly been a challenge to the medical community, and with the growing number of COVID-19 cases, our knowledge of the disease grows and our approach to management changes rapidly. Most cases present with severe acute respiratory syndrome and would require oxygen support.
Li et al. first described hypercoagulation in patients with Sars-CoV-2 infection [Citation6]. It was theorised that hypercoagulability occurs mainly through the activation of the NOX2 leading to an increase in reactive oxygen species and subsequently leading to arterial vasoconstriction, platelet activation, clotting activation, as reported by Violi et al [Citation7]. Another theory discusses the involvement of antiphospholipid syndrome requiring therapies through means other than heparin, such as warfarin [Citation8]. Hypercoagulable states have been reported in around 50% of cases with severe COVID19 and with no established guidelines regarding pharmacologic prophylaxis against thromboembolism in COVID19 patients, with the use of anticoagulation has been evaluated on a case-by-case basis. In one study, the use of enoxaparin or direct oral anticoagulation (DOAC) has been shown to decrease risk of thromboembolism at the cost of increased risk of major bleeding [Citation4]. A reduction of incidence of thromboembolism to 5.5% with the use enoxaparin was evident in the Medenox trial [Citation9], and two cohort studies on COVID19 patients with prophylaxis found an incidence of venous thromboembolism up to 25% in the ICU, even with prophylaxis, symptomatic patients remained at 31% [Citation10] Thromboembolism has been mainly described as venous thromboembolism leading to pulmonary embolism, with some cases reporting right atrial thrombus formation [Citation10]. However, these cases have been reported in patients with severe illness with COVID19, and have attributed the thromboembolism due to disease severity and occurrence of a surge of proinflammatory markers known as ‘cytokine storm’ [Citation11]. Elevation of inflammatory markers such as D-Dimer as an index for high risk groups for venous thromboembolism and is sometimes used as a prognostic value [Citation12]. Stroke was involved in 3 times increased risk in mortality, but ischemic strokes were not found as a manifestation of the disease [Citation13]. In our patient, an infection with COVID19 has been mild, with the patient having only mild respiratory with resolution of symptoms with only nasal cannula oxygen support at the beginning. Inflammatory markers in our patients only showed an elevation with D Dimer peaking at 3.49 mcg/mL FEU, and CRP peaking at 6.27 mg/dL.
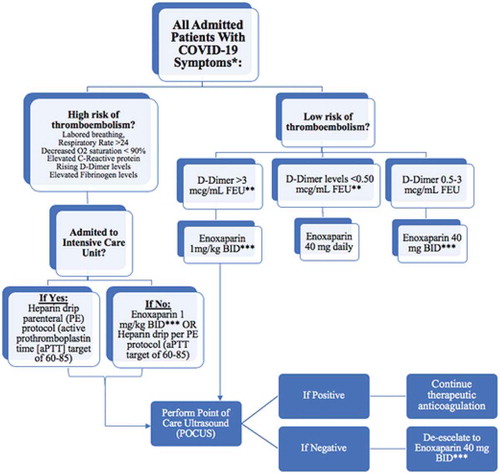
An algorithm was established and published in the European Society of Cardiology to identify individuals with high susceptibility to thrombotic complications, which includes elevation of markers such as D Dimer, CRP, and fibrinogen, respiratory rate over 24 breaths per minute, and dyspnea and oxygen saturation less than 90% (). The choice of anticoagulation in this study has been with the use of heparin or low molecular weight heparin, and they argued that heparin use has led to a degree of lowering inflammatory markers, including interleukin-6. This has also been shown in another study by Abbadi et al. which showed heparin use in hyperglycemia promoted to elevation of anti-inflammatory macrophages and inhibiting pro-inflammatory markers in this study such as tumor necrosis factor alpha and nitric oxidase [Citation14].
Figure 7. Atallah et al. formed a tailored algorithm/protocol for the management of coagulopathy in COVID-19 patients. *High bleeding risk patients are excluded. Also exclude patients with platelet count < 50,000; INR > 2. **FEU, fibrinogen equivalent unit. ***Adjust enoxaparin dose for renal failure
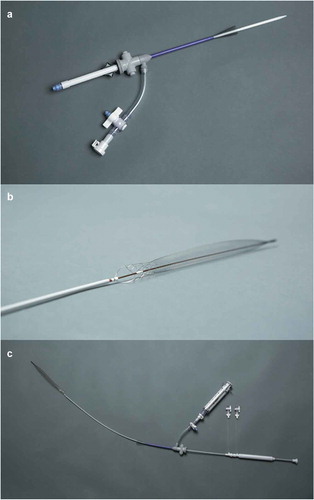
In our patient’s condition, an added difficulty of having a right atrial thrombus transit raised a challenge in the management decision. Literature review showed that the incidence of right heart thrombus in the setting pulmonary embolism occurs in 4% of cases, with a mortality rate of 28%, rising to 80–100% in untreated patients [Citation15]. Right heart thrombus presents as one of three types; Type A as a result of DVT, Type B originates from the heart’s atria or ventricles and is attached to the wall, and Type C highly mobile with resemblance to a myxoma [Citation16], with our patient likely presenting with Type A right heart thrombus. Several studies were done on the mortality of different treatment modalities for right heart thrombus; that is anticoagulation, thrombolysis, and surgical embolectomy, with results showing conflicting outcomes in each study. The latest study by Ganesh et al. in 2015 showed a mortality of 37.1%, 18.3%, 13.9%, respectively, with each management. In our case, a suspected atrial septal aneurysm was seen on TTE with patent foramen ovale (PFO), which is prevalent in 35% of the total population according to a study published in Circulation in 1998, and is associated with 2% of systemic arterial thrombi, which is the presumed cause of the large vessel ischemia in our patient’s case. The management used in our patient case for the right atrial thrombus was catheter directed thrombectomy using the inari FlowTriever (device shown in ). The inari aspiration was originally intended for use in peripheral vasculature, for the treatment of pulmonary embolism and for treating clot in transit in the right atrium. A study by Wible et al on the use of FlowTriever device for thrombectomy showed 71% reduction in intraprocedural oxygen requirement and 100% operative technical success [Citation17].
4. Conclusion
The use of anticoagulation for the development of thromboembolism has been weighed against the risk of hemorrhagic conversion of the ischemic stroke, and unfortunately in our patient, a fatal hemorrhagic conversion occurred. The question lies if such sequence of events could have been prevented with anticoagulation once C OVID-19 has been diagnosed. With limited guidance on best practices for COVID-19, we present this case with the hope that others may be vigilant of thromboembolic complications in their COVID patients and also that future investigation may demonstrate the therapeutic value of anticoagulation early in the course before a fatal cascade of clotting occurs.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Klok FA, Kruip MJHA, Van Der Meer NJM, et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res. 2020;191:148–150.
- Madjid M, Safavi-Naeini P, Solomon SD, et al. Potential effects of coronaviruses on the cardi ovascular system: a review. JAMA Cardiol. 2020;5:831.
- Driggin E, Madhavan MV, Bikdeli B. Cardiovascular considerations for patients, health care workers, and health systems during the coronavirus disease 2019 (COVID-19) pandemic. J Am Coll Cardiol. 2020. DOI:10.1016/j.jacc.2020.03.031
- Campbell CM, Kahwash R. Will complement inhibition be the new target in treating COVID-19 related systemic thrombosis? Circulation. 2020;141:1739–1741.
- Tan YK, Goh C, Leow AST, et al. COVID-19 and ischemic stroke: a systematic review and meta-summary of the literature [published online ahead of print, 2020 Jul 13]. J Thromb Thrombolysis. 2020;1–9. DOI:10.1007/s11239-020-02228-y.
- Taisheng L, Hongzhou L, Zhang W. Clinical observation and management of COVID-19 patients. Emerg Microbes Infect. 2020;9(1):687–690.
- Miesbach W, Makris M. COVID-19: coagulopathy, risk of thrombosis, and the rationale for anticoagulation. Clin Appl Thromb Hemost. 2020;26:1076029620938149.
- Zhang Y, Xiao M, Zhang S, et al. Coagulopathy and antiphospholipid antibodies in patients with COVID-19. N Engl J Med. 2020;382:e38.
- Violi F, Pastori D, Cangemi R, et al. Hypercoagulation and antithrombotic treatment in coronavirus 2019: a new challenge. Thromb Haemost. 2020;120(6):949–956.
- Terrigno VR, Tan JL, Singh D, et al. Right atrial thrombus in a patient with COVID-19. Cureus. 2020 Jul 28;12(7):e9441. . PMCID: PMC7451074.
- Cui S, Chen S, Li X, et al. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18(6):1421–1424.
- Trejo-Gabriel-Galán JM. Stroke as a complication and prognostic factor of COVID-19. Ictus como complicación y como factor pronóstico de COVID-19. Neurología. 2020;35(5):318–322.
- Samama MM, Cohen AT, Darmon JY, et al. A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in medical patients with enoxaparin study group. N Engl J Med. 1999;341(11):793–800.
- Poterucha TJ, Libby P, Goldhaber SZ. More than an anticoagulant: do heparins have direct anti-inflammatory effects? Thromb Haemost. 2017;117(3):437–444.
- Carson JL, Kelley MA, Duff A, et al. The clinical course of pulmonary embolism. N Engl J Med. 1992 May 7;326(19):1240–1245.
- European Working Group on Echocardiography. The European cooperative study on the clinical significance of right heart thrombi. Eur Heart J. 1989 Dec;10(12):1046–1059.
- Wible BC, Buckley JR, Cho KH, et al. Safety and Efficacy of Acute Pulmonary Embolism Treated via Large-Bore Aspiration Mechanical Thrombectomy Using the Inari FlowTriever Device. J Vasc Interv Radiol. 2019 Sep;30(9):1370–1375. doi:10.1016/j.jvir.2019.05.024. Epub 2019 Jul 30. PMID: 31375449.